Abstract
Objective: To compare active (AM) with borderline (BM) myocarditis to verify whether the pathological distinction between the two forms may help to identify patients with different clinical and haemodynamic characteristics and to aid prognosis.
Materials: Myocarditis was diagnosed in 56 patients on endomyocardial biopsy (EMB) within one year from clinical onset of the disease between 1991 and 1998. Fourteen patients were excluded because of a lack of adequate and complete information. EMBs and clinical records of the 42 remaining patients were reviewed. Immunohistochemistry on bioptic samples was regularly performed. Polymerase chain reaction (PCR) for a panel of viruses was performed in 23 patients (55%). Clinicopathological correlations were calculated.
Results: The histological diagnosis was AM in 26 patients (62%) and BM in 16 (38%). Significant differences were found in the following parameters: presence of left bundle branch block on ECG (AM 2 (8%) v BM 5 (31%), p = 0.05); left ventricular volume on echocardiogram (mean (SD) AM 90 (42) ml/m2 v BM 128 (50) ml/m2, p = 0.002); mass to volume ratio (AM 1.0 (0) v BM 0.8 (0.1), p = 0.03); time interval between clinical onset of the disease and EMB (AM 40 (55) v BM 90 (93) days, p = 0.04); and degree of inflammatory infiltrates, scored on a scale of 0 to 3 (AM 1.65 (0.8) v BM 0.85 (0.3), p = 0.004). In 6 of 15 patients (40%) with AM and in 2 of 8 (25%) with BM, a viral genome was detected by PCR (NS). At follow up, no differences in death or heart transplantation were detected between the two forms (AM 4 patients (15%) v BM 2 patients (12.5%)). Three of eight PCR positive patients (37.5%) and 1 of 15 virus negative patients (7%) died or underwent heart transplantation.
Conclusions: BM seems to encompass inflammatory forms with a less aggressive inflammatory infiltrate evolving towards left ventricular dilatation. The term “chronic myocarditis” seems to be more appropriate. The absence of myocyte necrosis does not predict a more favourable prognosis, whereas the absence of a viral genome seems to predict it.
Keywords: myocarditis, endomyocardial biopsy, histopathology
Endomyocardial biopsy (EMB) is the only tool that may provide a definitive diagnosis of myocarditis, but interpretation of EMB is controversial.1–4
The Dallas criteria for histological diagnosis were introduced in 1984 to gain higher interobserver concordance. On the basis of these criteria myocarditis is defined as active (AM) or borderline (BM) according to the presence or absence, respectively, of myocardial necrosis or degeneration.5
Practical experience shows that residual interobserver variability and overall low sensitivity are serious limitations of the Dallas criteria.6, 7 These limitations could have contributed to the failure of the myocarditis treatment trial, in which the diagnosis of myocarditis was based entirely on histology.8–10 Quantitative criteria for inflammatory infiltrate, immunohistochemical, and molecular biology techniques are now deemed compulsory.11 Despite much criticism, however, the Dallas criteria are the only widely accepted guidelines for the histological diagnosis of myocarditis.
In our study, we analysed whether the histological distinction between AM and BM identifies different forms of the disease in terms of clinical features and outcome.
MATERIALS AND METHODS
Between January 1991 and December 1998, myocarditis was diagnosed on EMB in 56 patients referred from the cardiology clinic within one year of clinical onset of the disease. We reviewed the medical records of all of these patients. Fourteen patients were excluded because of a lack of adequate and complete information. Thus, the retrospective study was carried out in 42 patients. Follow up EMB were excluded from the study.
Clinical and haemodynamic study
The following data were collected: age, sex, family or personal history of heart disease or of autoimmune disorders, prodromal symptoms and signs, New York Heart Association (NYHA) functional class on admission, ECG findings, and echocardiograms. Clinical outcome and NYHA class were determined in the follow up examination.
Diagnostic right and left heart catheterisation was carried out by the standard technique. Pressures were measured in all the cardiac chambers and cardiac output was determined according to the Fick method. Selective coronary angiography and right ventricular EMB were performed.
Endomyocardial biopsy
By means of a disposable Cordis bioptome four bioptic specimens from the right side of the interventricular septum were obtained in each patient, using the long sheath technique and the femoral vein approach. In a limited number of patients a sample of frozen tissue was also stored. Tissues were fixed in a microwave. Each specimen ranged from 1–2 mm2, assuring that a total surface area of 4–8 mm2 was evaluated.
Four micrometre thick paraffin embedded serial sections were cut and routinely stained with haematoxylin and eosin and the trichrome Heidenhein technique.
The histopathological study was performed as follows. We evaluated the degree of inflammatory infiltration and the extension of fibrosis with a semiquantitative method, on a scale of 0 to 3 (a score of 0 is absence of infiltrate or fibrosis, a score of 1 is mild inflammation or fibrosis, a score of 2 is moderate inflammation or fibrosis, and a score of 3 is severe inflammation or fibrosis). The composition of the infiltrate (lymphocytes, macrophages, monocytes, eosinophils, neutrophils, and multinucleated giant cells) was also evaluated. The extension and distribution (focal, multifocal, and diffuse) of myocyte necrosis were evaluated as well. Particular attention was paid to the possible coexisting presence of histopathological features suggestive of dilated cardiomyopathy (hyperchromic, dysmetric, bizarre nuclei, and perinuclear halos of myocytes).
All samples underwent immunohistochemical analysis to characterise inflammatory cell infiltration by using the following antibodies: CD45 (Dako 1:20), CD43 (Dako 1:40), CD45RO (Dako 1:100), CD20 (Dako 1:100), CD68 (Dako 1:50), and factor VIII (Dako 1:100). The positivity of the antigen–antibody reaction was tested with the avidin-biotin peroxidase complex method. Sections of formalin fixed lymph nodes served as positive controls. Immunohistochemically stained sections were independently evaluated by two pathologists. Type and severity of infiltrate were assessed.
According to the Dallas criteria, myocarditis was classified as active if myocardial necrosis or degeneration (vacuolisation, irregular cellular outlines, and cellular disruption with lymphocytes closely applied to the sarcolemma) associated with inflammatory infiltration was present. Myocarditis was classified as borderline if necrosis and degeneration were absent.5 We took degenerative changes into consideration only when they were associated with inflammatory infiltrate, ruling out artefactual contraction bands. However, in addition to the Dallas criteria, in all cases we used immunohistochemistry to evaluate more correctly the presence or absence of myocardial inflammatory infiltrates.
Molecular analysis using polymerase chain reaction (PCR) or reverse transcriptase PCR was carried out in 23 of the 42 patients (55%) to detect the main cardiotropic viral genomes (enteric, adeno-, herpes- (Epstein-Barr, cytomegalo-, varicella-zoster), influenza, and mumps viruses). Total RNA and genomic DNA were isolated simultaneously from fresh frozen (when possible) or formalin fixed paraffin embedded myocardial samples by using Tris saturated phenol (pH 6.6) RNAzol solution as previously described.12 The final product of nucleic acid extraction was checked by using glyceraldehyde-3-phosphate dehydrogenase primers for RNA and β globin primers for DNA.13, 14 Primer pairs that had previously been designed to detect the main cardiotropic viruses were used for nucleic acid amplification.15–21
Statistical analysis
Data are expressed as mean (SD). The χ2 and Student's t test were used for parametric data and Kruskal-Wallis analysis was applied to non-parametric data. Probability values of p ≤ 0.05 were considered significant.
RESULTS
AM was diagnosed in 26 patients (62%) and BM in 16 (38%) (fig 1 and 2). Table 1 summarises clinical data. Mean age was similar in the two groups. Both AM and BM predominated slightly in men. A family history of dilated cardiomyopathy was present in none of the patients with AM and in three of 16 with BM (0 v 19%, p = 0.02). A history of immunological disorders was present in patients with AM (one with Churg-Strauss syndrome, one with ulcerative colitis, and one with multiple sclerosis) and BM (one with Hashimoto's thyroiditis and one with systemic lupus erythematosus). Peripartum onset occurred in both AM and BM (2 of 26 patients (8%) and 2 of 16 (12%), respectively). The frequency of flu like prodromal symptoms was similar. Clinical features at onset were different: in AM, congestive heart failure, chest pain with ST segment elevation on ECG mimicking acute myocardial infarction, and ventricular arrhythmias were seen in equal frequency (8 (31%), 8 (31%), and 7 (27%), respectively); cardiogenic shock was present in three patients. In BM, congestive heart failure was seen in 11 (68.7%) patients, mimicked AMI in 3 (18.7%), and arrhythmias in 2 (12.5%). Mean NYHA class on admission was similar: 2.4 in AM and 2.2 in BM. At a mean follow up of 24 months (28 (30) for AM and 18 (8) for BM, NS), no differences were found regarding survival: 4 patients with AM (15%) and 2 with BM (12.5%) died or underwent cardiac transplantation. NYHA class of the survivors was also similar (1.3 (0.6) v 1.1 (0.4)).
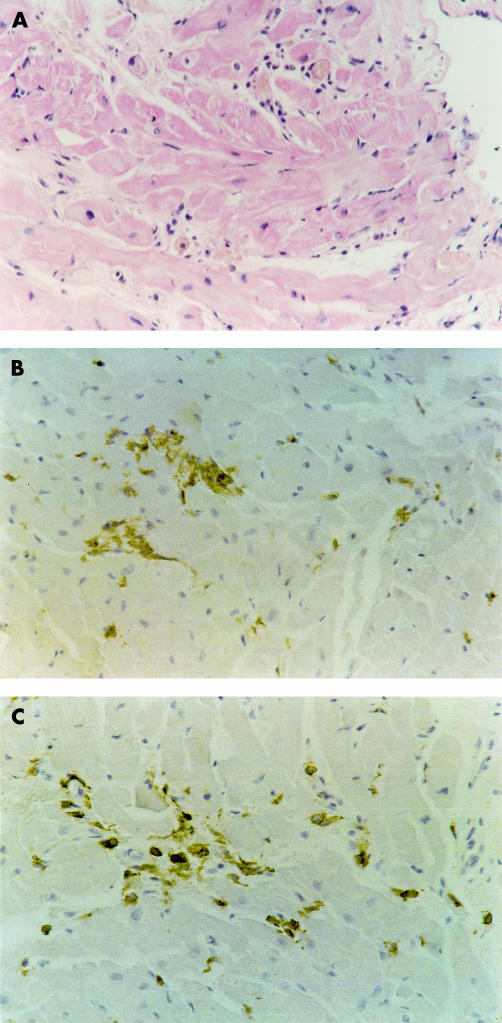
Figure 1.
Active myocarditis. (A) Haematoxylin and eosin staining shows focal myocyte necrosis and inflammatory infiltrates. Immunohistochemical staining for (B) CD45 (leucocytes) and (C) CD43 (T lymphocytes) confirms the presence of inflammatory cells within the myocardium. Original magnification ×240.
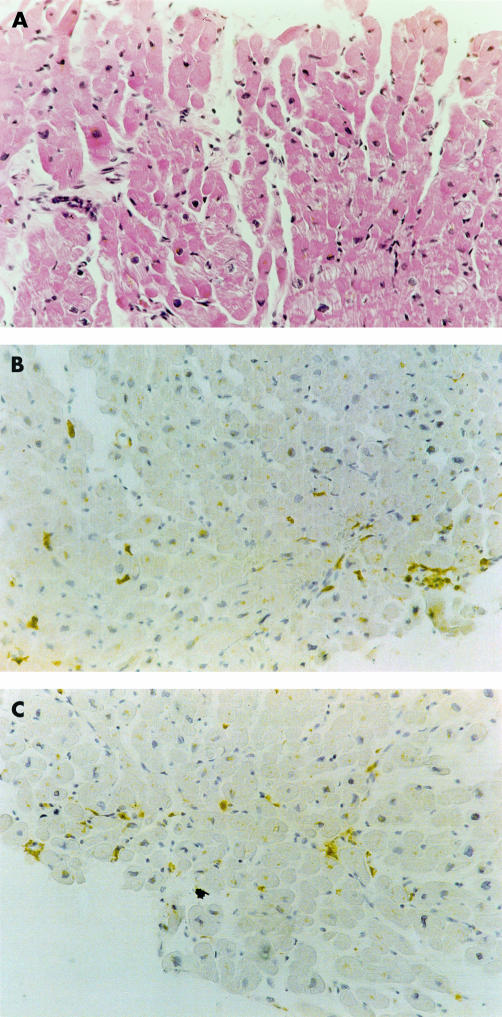
Figure 2.
Borderline myocarditis. (A) Haematoxylin and eosin staining shows absence of myocardial necrosis and a few inflammatory cell infiltrates. Immunohistochemical staining for (B) CD43 (T leucocytes) and (C) CD68 (macrophages) shows the presence of leucocytes within the myocardium. Original magnification ×180.
Table 1.
Comparison between active and borderline myocarditis: clinical data
| Active | Borderline | |
| Number of patients | 26 (62%) | 16 (38%) |
| Age (years) | 37 (15) (range 14–77) | 40 (13) (range 20–61) |
| Men/women | 16/10 | 9/7 |
| Familial history of dilated cardiomyopathy | 0 (0%) | 3 (19%)* |
| Peripartum onset | 2 (8%) | 2 (12.%) |
| Personal history of autoimmune disease | 2 (8%) | 3 (19%) |
| Flu like syndrome | 10 (38%) | 6 (37.%) |
| Clinical features at onset | ||
| CHF | 8 (31%) | 11 (68.7%) |
| AMI-like | 8 (31%) | 3 (18.7%) |
| VT | 7 (27%) | 2 (12.5%) |
| Cardiogenic shock | 3 (11%) | 0 (0%) |
| NYHA functional class at onset | 2.4 (1.3) | 2.2 (1.2) |
| Follow up | ||
| NYHA functional class | 1.3 (0.6) | 1.1 (0.4) |
| Death/CT | 4 (15%) | 2 (12.5%) |
Data are mean (SD).
*p = 0.02.
AMI, acute myocardial infarction; CHF, congestive heart failure; CT, cardiac transplantation; NYHA, New York Heart Association; VT, symptomatic ventricular tachycardia.
Table 2 summarises ECG, echocardiographic, and haemodynamic data on admission. Left bundle branch block was present in two patients with AM (8%) and in five with BM (31%) (p = 0.05). Pathological QS waves were seen in one patient with AM (8%) and in one with BM (6%). The echocardiogram showed depressed left ventricular performance in both AM and BM, as expressed by ejection fraction and contractility index. The left ventricle was more dilated in BM than in AM (mean left ventricular end diastolic volume 128 (50) v 90 (42) ml/m2, p = 0.002). Hypertrophy was inadequate in BM but not in AM (mean mass volume ratio 0.8 (0.1) v 1.0 (0), p = 0.03). Haemodynamic study showed a mean normal cardiac index in both AM and BM (3 (0.7) v 2.9 (1) l/min/m2). Mean pulmonary capillary and mean pulmonary artery pressures were also similar and within normal ranges.
Table 2.
Comparison between borderline and active myocarditis: instrumental data
| Active | Borderline | |
| Electrocardiogram | ||
| LBBB | 2 (8%) | 5 (31%)* |
| Necrosis | 2 (8%) | 1 (6%) |
| AV conduction disturbances | 2 (8%) | 0 (0%) |
| Echocardiogram | ||
| LVEDV(ml/m2) | 90 (42) | 128 (50)*** |
| LVEF (%) | 34 (12) | 34 (15) |
| M:V | 1 (0) | 0.8 (0.1)** |
| P:ESV | 2.21 (1.46) | 1.7 (0.5) |
| Haemodynamic study | ||
| CI (l/min/m2) | 3 (0.7) | 2.9 (1) |
| PCWP (mm Hg) | 10.6 (6.7) | 9.85 (6.7) |
| PAP | 17 (7.4) | 14.7 (7) |
Data are mean (SD).
*p = 0.05; **p = 0.03; ***p = 0.002.
AV, atrioventricular; CI, cardiac index; LBBB, left bundle branch block; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; M:V, mass to volume ratio; PAP, mean pulmonary artery pressure; P:ESV, pressure end systolic volume ratio; PCWP, pulmonary capillary wedge pressure.
Table 3 summarises EMB findings. The time interval between the onset of symptoms and EMB was significantly shorter in AM than in BM (40 (55) v 90 (93) days, p = 0.04; range 1–180 for AM and 5–335 for BM). Patients in whom AMI was mimicked underwent EMB earlier than those with arrhythmias (5 (4) v 15 (9) days, p = 0.05) or congestive heart failure (5 (4) v 46 (59) days, p = 0.001).
Table 3.
Comparison between borderline and active myocarditis: pathological data from the endomyocardial biopsy (EMB)
| Active | Borderline | |
| Number of patients | 26 (62%) | 16 (38%) |
| Days from onset to EMB | 40 (55) (range 1–180) | 90 (93) (range 5–335)* |
| Inflammatory infiltrate on HE | ||
| Quantification (0-3) | 1.65 (0.8) | 0.85 (0.3)** |
| Composition | ||
| Lymphomonocytes | 18 (69%) | 16 (100%) |
| Polymorphic | 8 (31%) | 0 (0%) |
| Fibrosis | ||
| Present | 18 (69%) | 11 (69%) |
| Quantification (0–3) | 1.1 (1) | 0.93 (0.7) |
| Histological aspects of DCM | 5 (19%) | 6 (37.5%) |
| Immunohistochemistry | ||
| Mainly CD43 | 11 (38%) | 6 (37.5%) |
| CD43 and CD68 | 7 (27%) | 6 (37.5%) |
| Mainly CD68 | 7 (27%) | 3 (18.7%) |
| Only CD45 | 1 (4%) | 1 (6.3%) |
| PCR positive | 6/15 (40%) | 2/8 (25%) |
| Enterovirus | 3 | 0 |
| Herpesvirus | 2 | 1 |
| Adenovirus | 0 | 1 |
| Influenza virus | 1 | 0 |
| Mumps virus | 0 | 0 |
Data are mean (SD).
*p = 0.04; **p = 0.004.
DCM, dilated cardiomyopathy; EMB, endomyocardial biopsy; HE, haematoxylin and eosin stain; PCR: polymerase chain reaction.
On haematoxylin and eosin staining, the inflammatory infiltrate score was significantly higher in AM than in BM (1.65 (0.8) v 0.85 (0.3), p = 0.004). The infiltrate was composed of lymphomonocytes in all samples from patients with BM and in 18 with AM (69%). In the remaining eight patients with AM (31%), the infiltrate had different features: in five it consisted of lymphomonocytes, eosinophils, and neutrophils, in one sample it was granulomatous, and in two it was composed mainly of multinucleated giant cells without granulomas. No differences in terms of severity of fibrosis were detected between the two groups. Histological aspects in keeping with dilated cardiomyopathy were present in five patients with AM (19%) and in six with BM (37.5%). On immunohistochemical analysis, the composition of the inflammatory infiltrate was similar: in 11 (38%) with AM and six (37.5%) with BM it was composed mainly of lymphocytes (CD43 positive); in seven (27%) with AM and six (37.5%) with BM of an equal amount of lymphocytes and macrophages; and in seven (27%) with AM and three (18.7%) with BM mainly of macrophages (CD68 positive).
As shown in table 4, AM was significantly more frequent within 30 days from clinical onset, whereas BM was more frequent after 30 days.
Table 4.
Occurrence of active and borderline myocarditis according to biopsy timing
| EMB timing (days) | |||
| ≤ 7 | 8–30 | ≥ 31 | |
| Myocarditis | 11 | 13 | 18 |
| Active | 9 (82%)* | 9 (69%) | 8 (44%)* |
| Borderline | 2 (18%) | 4 (31%) | 10 (56%) |
*p = 0.05.
PCR results
PCR was performed in 23 patients (55%). It was positive in 6 of 15 (40%) in the AM group and in 2 of 8 (25%) in the BM group (fig 3). Timing of EMB in relation to clinical onset was not different between PCR positive and negative groups (31 (52) v 47 (70) days, respectively). At follow up (20 (22) months for PCR positive and 19 (17) months for PCR negative patients, NS), 3 of 8 virus positive patients (37.5%) died or received a transplant, whereas only 1 of 15 (7%) virus negative patients died (NS).
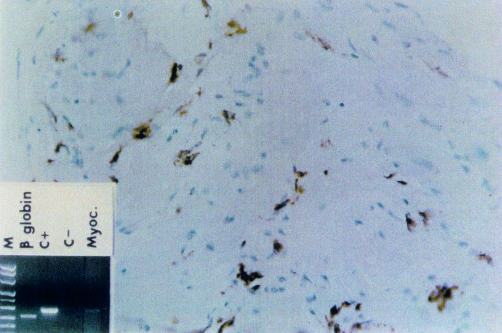
Figure 3.
Borderline myocarditis. Immunohistochemistry shows interstitial inflammatory cell infiltrate composed of CD68 positive cells (macrophages). Polymerase chain reaction performed on endomyocardial biopsy was positive for adenovirus (M, marker lane; C+, positive control lane; C−, negative control lane; Myoc, adenovirus myocarditis).
DISCUSSION
Myocarditis is a disease characterised by a polymorphic clinical presentation. Among patients in whom hospitalisation is required, a high mortality rate at follow up is reported. It is the second most frequent EMB diagnosis at our centre, after dilated cardiomyopathy. This is in keeping with other reported surveys.22, 23 EMB has been considered to be the yardstick for the in vivo diagnosis of myocarditis and the Dallas criteria are internationally accepted.2, 5
The presence of inflammatory cells infiltrates in the interstitium of “normal” myocardium is still a controversial issue. This is partly due to the difficulties in recruiting myocardium not affected by a disease. Previously reported studies examined myocardial tissue from patients with disease other than myocarditis or from donor hearts and found very few lymphocytes in the interstitium in normal conditions (table 5).9, 24–29 A cut off of five lymphocytes per high power field has been proposed for positive inflammatory cell infiltrate.26 The Marburg group suggested 14 cells/mm2 at immunohistochemisty as a quantitative criterion for inflammation.29, 30 Others considered 17 cells/mm2 in the interstitium of donor hearts to be the cut off.28 Immunohistochemistry increases specificity for inflammatory cells detection and decreases interobserver variability.11 In addition, it can provide information potentially relevant to immunopathogenesis and treatment.11, 28, 31–35 In our study, immunohistochemistry was therefore used as a fundamental tool for EMB interpretation.
Table 5.
Histological quantitative criteria for inflammatory infiltrate in myocarditis from other studies
| Authors | Specimen | Number of patients | Patient age (years) | Cells per field | Magnifying power | IHC |
| Yutani et al (1981)25 | Kawasaki | 201 | < 18 | 10–20 | 200× | No |
| Edwards et al (1982)26 | Heart disease | 170 | 29 | 400× (20 fields counted) | No | |
| Linder et al (1985)24 | Normal | 20 | 61 | 25–30/mm2 | 500× (20 fields counted) | No |
| Tazelaar et al (1986)9 | Heart transplant donors | 86 | 23 | Focus of at least 5 cells | 80× | No |
| Kuhl et al (1995)27 | DCM | 176 | > 2 (> 7 cells/mm2) | 400× (10 fields counted) | Yes | |
| Holzinger et al (1995)28 | Heart transplant donors | 7 | ≥ 17 cells/mm2 | 400× | Yes | |
| Maisch et al (1998)29 | – | 56 | > 14 cells/mm2 | 400× | Yes |
IHC, immunohistochemistry (No: not used, Yes: used).
Our investigation was meant to compare the clinical and pathological profiles of AM and BM. To obtain homogeneous data, we considered only those patients who had undergone EMB within one year from the onset of symptoms.
The clinical profile of the two groups was different. Patients with BM were slightly older—though not significantly—and on average underwent EMB later than did patients with AM. Nineteen per cent of patients with BM had a family history of dilated cardiomyopathy. These patients may have a peculiar familial form of dilated cardiomyopathy, which has been reported to be associated with inflammatory cell infiltrate in the interstitium, a feature considered to be a non-specific finding by some authors.27, 36 Patients with BM presented more often than AM patients with congestive heart failure, with a more heterogeneous clinical presentation, including cardiogenic shock.
One third of the patients with BM had a left bundle branch block at ECG compared with two (8%) patients with AM. This finding is in keeping with the echocardiographic data, which show that in BM the left ventricles are more dilated with a decreased mass to volume index. In these patients, the cell infiltrate was less severe in the pathological substrate and myocytes often showed the histological features of dilated cardiomyopathy. BM seems to have forms with a chronic evolution, in which inflammation is ongoing but the pattern of idiopathic dilated cardiomyopathy is already present. Fibroustissue was present in both AM and BM and it was mild in both groups.
The time interval between onset of symptoms and EMB correlates with the histological diagnosis: the likelihood of diagnosing BM increases with time. This finding is strengthened by the observation that EMB is performed earlier and the diagnosis is most often AM (81% in cases mimicking acute myocardial infarction and 100% in cardiogenic shock) when the clinical presentation is acute.
Despite the clinical differences, follow up of AM and BM did not differ in terms of NYHA class and outcome (death or heart transplantation). The inadequacy of the Dallas criteria needs to be considered. Myocarditis is classified as active only on the basis of the presence of necrosis and inflammation. Other morphological aspects of myocytes and interstitium that are highly suggestive of an evolution towards a chronic form have not been considered. The presence of myocyte necrosis is not an unfavourable prognostic indicator, as one could expect. Histological diagnosis, namely AM versus BM, does not influence the prognosis, while the presence of a viral genome seems to do so. In active forms viral genomes can be detected more frequently in the myocardium, although not significantly. This is in keeping with some previous reports.12 Larger studies are required to confirm this first observation.
Conclusion
BM seems to encompass ongoing forms with a less aggressive inflammatory infiltrate. The absence of myocyte necrosis does not predict a more favourable outcome in terms of death or heart transplantation, whereas the presence of viral genome seems to carry a more powerful negative prognostic value. The Dallas criteria have many ambiguities and are not of real value in identifying patients with a worse outcome.
Acknowledgments
This study was supported by MURST, Rome, Italy.
Abbreviations
AM, active myocarditis
BM, borderline myocarditis
EMB, endomyocardial biopsy
NYHA, New York Heart Association
PCR, polymerase chain reaction
REFERENCES
- 1.Pisani B, Taylor DO, Mason JW. Inflammatory myocardial disease and cardiomyopathies. Am J Med 1997;102:459–69. [DOI] [PubMed] [Google Scholar]
- 2.Billingham ME. Acute myocarditis: a diagnostic dilemma. Br Heart J 1987;58:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lie JT. Myocarditis and endomyocardial biopsy: a diagnosis in search of a disease. Ann Intern Med 1988;109:525–8. [DOI] [PubMed] [Google Scholar]
- 4.Peters NS, Poole-Wilson PA. Myocarditis, continuing clinical and pathological confusion. Am Heart J 1991;121:942–6. [DOI] [PubMed] [Google Scholar]
- 5.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis: a histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1:3–14. [PubMed] [Google Scholar]
- 6.McKenna WJ, Davies MJ. Immunosuppression for myocarditis [editorial]. N Engl J Med 1995;333:312–3. [DOI] [PubMed] [Google Scholar]
- 7.Maisch B, Herzum M, Hufnagel G, et al. Immunosuppressive and immunomodulatory treatment for myocarditis. Curr Opin Cardiol 1996;11:310–24. [DOI] [PubMed] [Google Scholar]
- 8.Mason JW, O'connell JB, Herskowitz A, et al, and the Myocarditis Treatment Trial investigators. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269–75. [DOI] [PubMed] [Google Scholar]
- 9.Tazelaar HD, Billingham ME. Myocardial lymphocytes: fact, fancy, or myocarditis? Am J Cardiovasc Pathol 1987;1:47–50. [PubMed] [Google Scholar]
- 10.Davies MJ, Ward DE. How can myocarditis be diagnosed and should it be treated [editorial]? Br Heart J 1992;68:346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart 2000;84:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin AB, Webber S, Fricker FJ, et al. Acute myocarditis: rapid diagnosis by PCR in children. Circulation 1994;90;330–9. [DOI] [PubMed] [Google Scholar]
- 13.Ercolani L, Florence B, Denaro M, et al. Isolation and complete sequence of a functional human glyceraldehyde-3-phospate dehydrogenase gene. J Biol Chem 1988;263:15335–41. [PubMed] [Google Scholar]
- 14.Saiki RK, Scharf, Falooma F, et al. Enzymatic amplification of beta globin genomic sequences and restriction site analysis for diagnosis of sickle-cell anemia. Science 1985;230:1350–4. [DOI] [PubMed] [Google Scholar]
- 15.Ni J, Bowles NE, Kim YH, et al. Viral infection of the myocardium in endocardial fibroelastosis: molecular evidence for the role of mumps virus as an etiologic agent. Circulation 1997;95:133–9. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese F, Valente M, Thiene G, et al. Enteroviral genome in native hearts may influence outcome of patients who undergo cardiac transplantation. Diagn Mol Pathol 1999;8:39–46. [DOI] [PubMed] [Google Scholar]
- 17.Davison AJ. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J 1983;2:2203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claas ECJ, Sprenger MJW, Kleter GEM, et al. Type-specific identification of influenza viruses A, B and C by the polymerase chain reaction. J Virol Methods 1992;39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozinski GM, Davis GG, Krous HF, et al. Adenovirus myocarditis: retrospective diagnosis by gene amplification from formalin-fixed, paraffin-embedded tissues. Hum Pathol 1994;25:831–4. [DOI] [PubMed] [Google Scholar]
- 20.Hebert MM, Yu C, Towbin JA, et al. Fatal Epstein-Barr virus myocarditis in a child with repetitive myocarditis. Ped Pathol Lab Med 1995;15:805–12. [DOI] [PubMed] [Google Scholar]
- 21.Piiparinen H, Vaheri A. Genotyping of herpes simplex viruses by polymerase chain reaction. Arch Virol 1991;119:275–83. [DOI] [PubMed] [Google Scholar]
- 22.Nippoldt TB, Edwards WD, Holmes D, et al. Right ventricular endomyocardial biopsy: clinicopathologic correlates in 100 consecutive patients. Mayo Clin Proc 1982;57:407–18. [PubMed] [Google Scholar]
- 23.Parrillo JE, Aretz TH, Palacios I, et al. The result of transvenous endomyocardial biopsy can frequently be used to diagnose myocardial diseases in patients with idiopathic heart failure. Circulation 1984;69:93–101. [DOI] [PubMed] [Google Scholar]
- 24.Linder J, Cassling RS, Rogler WC, et al. Immunohistochemical characterisation of lymphocytes in uninflamed ventricular myocardium: implications for myocarditis. Arch Pathol Lab Med 1985;109:917–20. [PubMed] [Google Scholar]
- 25.Yutani C, Go S, Kamiya T, et al. Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med 1981;105:470–3. [PubMed] [Google Scholar]
- 26.Edwards WD, Holmes RH, Reeder GS. Diagnosis of active lymphocytic myocarditis by endomyocardial biopsy: quantitative criteria for light microscopy. Mayo Clin Proc 1982;57:419–25. [PubMed] [Google Scholar]
- 27.Kuhl U, Noutsias M, Shultheiss HP. Immunohistochemistry in dilated cardiomyopathy. Eur Heart J 1995;16(suppl O):100–6. [DOI] [PubMed] [Google Scholar]
- 28.Holzinger S, Schollhammer A, Imhof M, et al. Phenotypic patterns of mononuclear cells in dilated cardiomyopathy. Circulation 1995;92:2876. [DOI] [PubMed] [Google Scholar]
- 29.Maisch B, Bultmann B, Factor S, et al. Dilated cardiomyopathy with inflammation or chronic myocarditis: variability and consensus in the diagnosis [abstract]. Eur Heart J 1998;19(suppl):647(P3625).9597415 [Google Scholar]
- 30.Maisch B, Hufnagel G, Schonian U, et al, for the ESETCID investigators. The European study of epidemiology and treatment of cardiac inflammatory disease. Eur Heart J 1995;16(suppl O):173–5. [DOI] [PubMed] [Google Scholar]
- 31.Schnitt SJ, Ciano PS, Schoen FJ. Quantitation of lymphocytes in endomyocardial biopsies: use and limitations of antibodies to leukocyte common antigen. Hum Pathol 1987;18:796–800. [DOI] [PubMed] [Google Scholar]
- 32.Chow LH, Ye Y, Linder J, et al. Phenotypic analysis of infiltrating cells in human myocarditis: an immunohistochemical study in paraffin-embedded tissue. Arch Pathol Lab Med 1989;113:1357–62. [PubMed] [Google Scholar]
- 33.Milei J, Bortman G, Fernandez-Alonso G, et al. Immunohistochemical staining of lymphocytes for the reliable diagnosis of myocarditis in endomyocardial biopsies. Cardiology 1990;77:77–85. [DOI] [PubMed] [Google Scholar]
- 34.Tarantini G, Menti L, Angelini A, et al. Life-threatening ventricular arrhythmias associated with giant cell myocarditis (possibly sarcoidosis). Am J Cardiol 2000;85:1280–2. [DOI] [PubMed] [Google Scholar]
- 35.Baratella MC, Menti L, Angelini A, et al. An unusual case of myocarditis. Int J Cardiol 1998;65:305–10. [DOI] [PubMed] [Google Scholar]
- 36.Mestroni L, Rocco C, Gregori D, et al, and the Heart Muscle Disease Study Group. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. J Am Coll Cardiol 1999;34:181–90. [DOI] [PubMed] [Google Scholar]