Abstract
Objective: To evaluate predictors of long term prognosis in infective endocarditis.
Design: Retrospective cohort study.
Setting: Tertiary care centre.
Patients: 212 consecutive patients with infective endocarditis between 1980 and 1995
Main outcome measures: Overall and cardiac mortality; event-free survival; and the following events: recurrence, need for late valve surgery, bleeding and embolic complications, cerebral dysfunction, congestive heart failure.
Results: During a mean follow up period of 89 months (range 1–244 months), 56% of patients died. In 180 hospital survivors, overall and cardiac mortality amounted to 45% and 24%, respectively. By multivariate analysis, early surgical treatment, infection by streptococci, age < 55 years, absence of congestive heart failure, and > 6 symptoms or signs of endocarditis during active infection were predictive of improved overall long term survival. Independent determinants of event-free survival were infection by streptococci and age < 55 years. Event-free survival was 17% at the end of follow up both in medically–surgically treated patients and in medically treated patients.
Conclusions: Long term survival following infective endocarditis is 50% after 10 years and is predicted by early surgical treatment, age < 55 years, lack of congestive heart failure, and the initial presence of more symptoms of endocarditis.
Keywords: infective endocarditis, late cardiac surgery, long term follow up, outcome
Although modern antibiotic and surgical treatments have substantially improved outcome in recent decades, infective endocarditis remains a life threatening disease. As mortality during the active phase of the infection has declined, long term morbidity and mortality caused by late sequelae such as congestive heart failure, valve incompetence, and predisposition to recurrent infective endocarditis are becoming more important. Moreover, the focus has shifted away from infections of native valves to endocarditis of prosthetic valves in the elderly and to endocarditis in users of injected drugs.
Evidence is growing that combined medical–surgical treatment leads to reduced short term mortality in both prosthetic and native valve infective endocarditis.1–3
Many studies have addressed the question of short term survival, whereas long term prognosis has been investigated less thoroughly. Data on direct comparisons between medical–surgical and medical treatments, as well as studies on the outcome of early valve surgery, are scarce.4–7
The objectives of this study were to evaluate predictors of long term survival and morbidity in a large cohort of patients with infective endocarditis. In particular, the study focused on comparing combined medical and early surgical treatment with medical treatment alone, on separately assessing the overall population and hospital survivors, and on determining the outcome in the more homogeneous subgroups of prosthetic and native valve endocarditis exclusive of users of injected drugs.
PATIENTS AND METHODS
Study design
In a European 1050 bed tertiary referral centre for a population of 1.2 million, data on all patients hospitalised with possible or definite infective endocarditis (fulfilling the Duke criteria 8) between 1 January 1980 and 31 December 1995 were retrospectively reviewed. Patients were followed up until death or 30 September 2000.
Data collection was based on the systematic review of all patient charts, all reports from the echocardiography laboratory, and all valve surgery reports during the study period. Furthermore, follow up data were obtained by written standardised questionnaires sent to patients' treating physician(s) and by telephone interviews with patients or their families.
Data analysis and definitions
Demographic data, risk factors for endocarditis, clinical variables at study entry, and clinical, echocardiographic, and outcome data were evaluated. Predefined end points and events were overall and cardiac mortality, recurrent infective endocarditis, valve surgery, valve prosthesis dysfunction, embolic and bleeding complications, cerebral dysfunction, and congestive heart failure. Morbidity was defined in accordance with the guidelines published by Clark and colleagues.9
Data were analysed for the entire study population and for hospital survivors only. Subgroup analysis was carried out of patients with native valve infective endocarditis exclusive of intravenous drug users and of those with prosthetic valve infective endocarditis.
Early and late surgical treatments were defined as valve repair or replacement during and after the active phase (that is, during antibiotic treatment) of infective endocarditis, respectively. Medical–surgical group and medical group refer to patients undergoing and not undergoing early surgical treatment, respectively.
Complete follow up was defined as availability of information up to the end of the study (30 September 2000) or death. Follow up was defined as incomplete where information could be gathered for a time after the index admission but not up to the end of the study or death.
Statistical analysis
Categorical and continuous variables were compared by the χ2 test and the unpaired Student t test, respectively. For multivariate analysis (Cox regression analysed by Stata Statistical Software 5.0, Stata Corporation, College Station, Texas, USA) and actuarial survival analysis (Mantel-Haenszel test analysed by StatView 4.57, Abacus Concepts, Berkeley, California, USA), significance was defined as p < 0.05. Data are reported as mean (SD).
RESULTS
Patient characteristics and clinical data
During the observation period, 212 patients were treated for infective endocarditis, of whom 73% and 17% had native and prosthetic valve infections, respectively; the remaining 10% were injected drug users. Isolated aortic, mitral, and tricuspid valve infections occurred in 39%, 21%, and 2%, respectively. Both the mitral and aortic valves were involved in 14%, and in 2% more than two valves were affected. In 22% the precise location of endocarditis could not be identified with certainty. Mean age was 53 years (range 17–90 years) with a predominance of male to female patients (3:1).
Responsible organisms were Streptococcus viridans (3%), other streptococci (10%), Staphylococcus aureus (23%), coagulase negative staphylococci (10%), other organisms (11%), polymicrobial (5%), and unknown (9%).
Baseline characteristics of the groups with and without early surgery differed only in factors that are indications for valve surgery during the active phase (table 1).
Table 1.
Characteristics of medically–surgically treated (MST) versus medically treated (MT) patients
| MST group (n=81) | MT group (n=131) | p Value | |
| Mean (SD) age (years) | 52 (16) | 53 (19) | NS |
| Cardiac risk factors | 53 (65) | 80 (61) | NS |
| Prior infective endocarditis | 3 (4) | 6 (5) | NS |
| Pre-existing valvar heart disease | 34 (42) | 58 (44) | NS |
| Pre-existing prosthetic valve | 19 (23) | 17 (13) | NS |
| Congenital heart disease | 3 (4) | 5 (4) | NS |
| Non-cardiac risk factors | 29 (36) | 56 (43) | NS |
| Injected drug use | 7 (9) | 15 (11) | NS |
| Renal insufficiency | 16 (20) | 21 (16) | NS |
| Diabetes mellitus | 10 (12) | 17(13) | NS |
| Cancer | 6 (7) | 16 (12) | NS |
| Causative organisms: staphylococci | 35 (43) | 41 (31) | NS |
| Causative organisms: streptococci | 33 (41) | 69 (53) | NS |
| Infection of aortic valve | 67 (83) | 49 (37) | <0.001 |
| Congestive heart failure during endocarditis | 37 (46) | 51 (39) | NS |
| Systemic emboli during endocarditis | 36 (44) | 35 (27) | <0.01 |
| Mean (SD) symptoms/signs of endocarditis | 7 (2) | 6 (2) | <0.02 |
| Mean (SD) duration of hospital stay (days) | 43 (28) | 32 (22) | <0.002 |
| Cerebral dysfunction at discharge | 7 (9) | 8 (6) | NS |
| Long term mortality | 36 (44) | 83 (63) | <0.01 |
Data are n (%) unless otherwise indicated.
A detailed study of the clinical, echocardiographic, and autoptic analyses during hospitalisation has been reported previously.10
Valve surgery, in addition to standard medical treatment, was performed in 81 patients after a mean of 19 (52) days following admission. Mitral, aortic, tricuspid, and combined mitral–aortic valve surgery was performed in 15%, 72%, 1%, and 12%, respectively.
Indications for surgery during active infective endocarditis were congestive heart failure (37 patients), persistent signs of septicaemia despite antibiotic treatment (7), recurrent embolisation (24), paravalvar involvement (4), vegetation size > 10 mm (13), and dysfunction of valve prosthesis (13).
All patients were followed up. Follow up was complete in 94% (mean 89 (60) months, range 1–244 months).
Mortality
Long term and in-hospital mortality
Overall mortality was 24%, 42%, 50%, and 56% after 1, 5, 10, and 20 years, respectively. For survivors of the active phase of infective endocarditis, mortality was 9%, 28%, 37%, and 45% after 1, 5, 10, and 20 years, respectively. The respective proportions for native valve infections were 22%, 38%, 46%, and 54% overall and 16%, 29%, 39%, and 47% for hospital survivors; those for prosthetic valve infections after 1, 5, and 10 years were 31%, 53%, and 64% overall and 14%, 41%, and 55% for hospital survivors (table 2).
Table 2.
Factors associated with long term survival: univariate analysis
| No death (n=93) | Death (n=119) | p Value | |
| Mean (SD) age (years) | 45 (17) | 59 (50) | <0.001 |
| Male sex | 70 (75) | 90 (76) | NS |
| Cardiac and non-cardiac risk factors | 70 (75) | 103 (87) | <0.04 |
| Cardiac risk factors | 60 (65) | 73 (61) | NS |
| Pre-existing valvar heart disease | 44 (47) | 48 (40) | NS |
| Pre-existing prosthetic valve | 12 (13) | 24 (20) | NS |
| Congenital heart disease | 6 (6) | 2 (2) | NS |
| Non-cardiac risk factors | 21 (23) | 64 (54) | <0.001 |
| Injected drug use | 9 (10) | 13 (11) | NS |
| Renal insufficiency | 10 (11) | 27 (23) | <0.02 |
| Diabetes mellitus | 8 (9) | 19 (16) | NS |
| Cancer | 3 (3) | 19 (16) | <0.003 |
| Mean (SD) symptoms/signs on admission | 6 (2) | 5 (2) | <0.05 |
| Symptoms/signs during endocarditis | |||
| Fatigue | 71 (76) | 68 (57) | <0.004 |
| Arthralgia | 34 (37) | 20 (17) | <0.001 |
| Congestive heart failure | 31 (33) | 57 (48) | <0.03 |
| Peripheral arterial embolism | 38 (41) | 33 (28) | <0.04 |
| Cough | 52 (56) | 44 (37) | <0.006 |
| Vascular phenomena | 57 (61) | 56 (47) | <0.04 |
| Mean (SD) C reactive protein (mg/l) | 83 (60) | 140 (80) | <0.001 |
| MT patients | 48 (52) | 83 (70) | <0.02 |
| Late valve surgery | 5 (5) | 11 (9) | NS |
| MST patients | 45 (48) | 36 (30) | <0.02 |
| Valve surgery during follow up | 2 (2) | 3 (3) | NS |
| Anticoagulant treatment endocarditis | 59 (63) | 65 (55) | NS |
| Causative organisms: staphylococci | 29 (31) | 47 (39) | NS |
| Causative organisms: streptococci | 53 (57) | 49 (41) | NS |
Data are n (%) unless otherwise indicated.
In-hospital mortality after the first admission amounted to 15% overall and was 22% and 14% for the groups with native and prosthetic valve infections, respectively.
During follow up, 48% of the survivors of the acute phase died after a mean of 61 (55) months, with cardiac causes of death in 30% (23% of the medical–surgical group and 37% of the medical group). Congestive heart failure (50%), sudden death (38%), infective endocarditis (8%), and myocardial infarction (4%) were similarly distributed between patients with and without early valve surgery.
Factors associated with long term mortality
By univariate analysis various risk factors for endocarditis such as pre-existing renal failure (serum creatinine > 200 μmol/l) and cancer, as well as a higher number of symptoms and signs of endocarditis, were directly associated with long term mortality (table 2). These associations persisted when applied to the group of native valve infections and to the respective groups of hospital survivors.
The presence of an abscess was not predictive of long term survival, neither for the overall population (mortality in patients with versus those without abscess was 63% v 52%, p = 0.43) nor for hospital survivors (40% v 46%, p = 0.72).
For prosthetic valve infections (overall and hospital survivors), age was the only factor associated with long term mortality (p < 0.02).
By multivariate analysis, the following factors were predictive of overall long term mortality: absence of early surgical treatment (odds ratio (OR) 1.86, 95% confidence interval (CI) 1.25 to 2.75, p < 0.002), organisms other than streptococci (OR 1.73, 95% CI 1.19 to 2.51, p < 0.004), fewer symptoms or signs of endocarditis on admission (OR 1.79, 95% CI 1.20 to 2.68, p < 0.004), congestive heart failure during active endocarditis (OR 1.76, 95% CI 1.20 to 2.59, p < 0.004), and age > 55 years (OR 2.57, 95% CI 1.72 to 3.83, p < 0.0001). The same association was found for hospital survivors and for overall long term mortality in native valve infections. For survivors of native valve infections, this was true only for no early surgical treatment, fewer symptoms on admission, and age > 55 years.
Effects of medical–surgical versus medical treatment on mortality
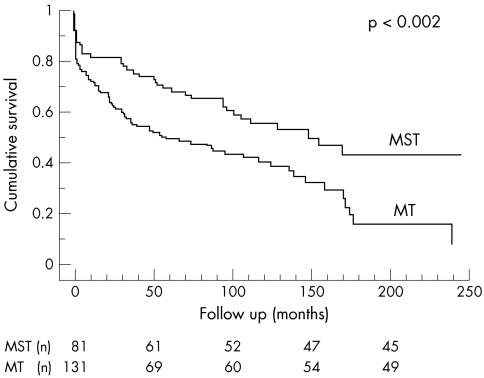
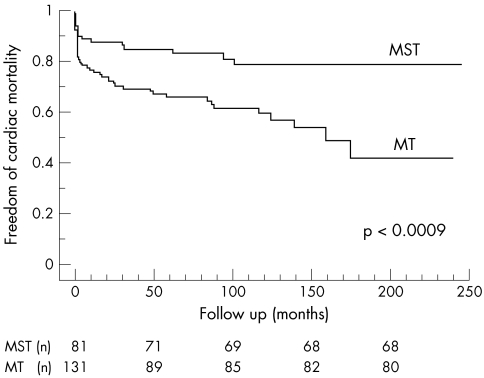
Both long term overall and cardiac mortalities were significantly lower in patients treated medically and surgically in the acute phase of the disease than in medically treated patients (figs 1 and 2).
Figure 1.
Overall survival in patients treated both medically and surgically (MST) versus those treated medically only (MT).
Figure 2.
Cardiac mortality in the medically and surgically (MST) treated group versus those treated medically only (MT).
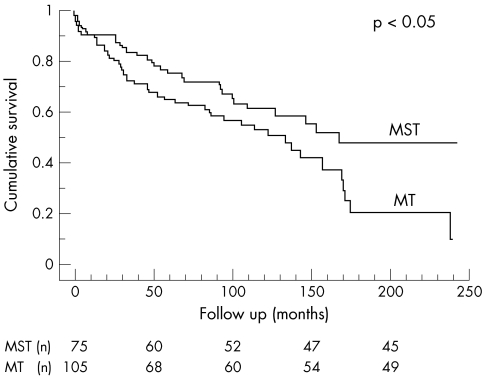
Even after this analysis was applied to the survivors of the initial hospitalisation, the same benefit persisted (fig 3).
Figure 3.
Overall survival of hospital survivors.
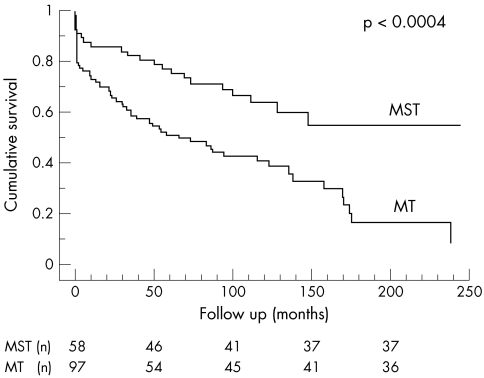
Furthermore, a comparison between the surgically–medically and medically treated patients in the subgroup of native valve infections showed the same pattern (fig 4). Differences in baseline characteristics were comparable with those of the total population (mortality, p = 0.0008; systemic emboli, p < 0.006; detection of vegetation, p < 0.0001; infection of the aortic valve, p < 0.003).
Figure 4.
Overall survival in native valve endocarditis
Patients with prosthetic valve endocarditis underwent surgery more often if they had an abscess (p < 0.008) or vascular symptoms on admission (p < 0.05). These patients had a higher likelihood of reoperations (p < 0.05) but did not have higher long term mortality.
Morbidity
Recurrence of infective endocarditis
During follow up, one third of patients who presented with recurrent infective endocarditis underwent valve surgery (table 3). The mean duration of hospitalisation was 26 (15) days, which was similar to the duration of stay for the initial episodes of infective endocarditis. Outcome was fatal in two patients who had received antibiotic treatment only initially. No factors predictive of recurrence were identified.
Table 3.
Morbidity following infective endocarditis
| Incidence (% per year) | MST group (n=75) | MT group (n=105) | p Value | |
| Recurrent endocarditis | 1.12 | 3 (4) | 12 (11) | 0.02 |
| Late valve surgery | 1.57 | 5 (7) | 16 (15) | 0.06 |
| Bleeding complications | 1.27 | 9 (12) | 8 (8) | NS |
| Cerebral dysfunction | 1.12 | 7 (9) | 8 (8) | NS |
| Peripheral arterial embolism | 0.15 | 0 (0) | 2 (2) | NS |
| Congestive heart failure | 2.62 | 13 (17) | 22 (21) | NS |
| Event-free survival | NA | 13 (17) | 18 (17) | NS |
Data are n (%) unless otherwise indicated. NA, not applicable.
Late valve surgery
Late valve surgery was performed in 11% of patients at a mean interval of 49 (51) months after the initial admission for infective endocarditis.
In patients with native valve endocarditis, severe valvar regurgitation (aortic valve in 11, mitral valve in three, combined aortic and mitral valve in two patients) was the indication for surgical treatment.
Pre-existing aortic valve stenosis (p < 0.005) and congestive heart failure during follow up (p = 0.003) predicted the need for late valve surgery.
Bleeding complications
During long term follow up, 56% of patients were taking oral anticoagulants for a mean of 91 (63) months. Although patients with prosthetic valve disease (p < 0.008) or with early valve surgery (p < 0.04) were more frequently taking oral anticoagulation, bleeding complications were not more frequent in these groups over the long term. Factors associated with bleeding complications were known diabetes mellitus (p < 0.01) and weight (p < 0.02) at the time of active endocarditis, but not oral anticoagulation.
Bleeding complications leading to hospital admission were intracranial haemorrhage and gastrointestinal bleeding (five cases each), as well as bleeding into the lung, spleen, gluteal muscle, breast, sternal wound, and pericardium (one case each). Seven patients died from bleeding complications (all intracranial and two gastrointestinal bleeding episodes).
Cerebral dysfunction
Cerebral dysfunction persisted on hospital discharge after the index admission in 8% of patients (9% in the medical–surgical and 8% in the medical group, NS). For native and prosthetic valve infections the proportions were 8% and 11%, respectively (NS).
After discharge, cerebral function deteriorated acutely in 7% of patients, 77% of whom were taking oral anticoagulants.
Age > 55 years was predictive of cerebral dysfunction after discharge (p < 0.05), but early valve replacement during the active phase of infective endocarditis was not.
Peripheral arterial embolism
Peripheral arterial embolism was observed in two patients (1%) with prosthetic valves on first admission who had been treated medically during the initial infection. Mesenteric infarction was present in one of these patients and embolism to the central artery of the retina in the other.
Congestive heart failure
During follow up, 19% of patients presented with congestive heart failure. The frequency was similar in the groups with (17%) versus those without (21%) early surgical treatment (NS) and with native (18%) versus prosthetic (16%) valve disease (NS).
A predictor of heart failure during long term follow up was age > 55 years (p < 0.003). However, there was no correlation between the severity of congestive heart failure before and that after active infective endocarditis.
Predictors of event-free survival
At the end of follow up (89 (60) months), 17% of patients both in the medical–surgical and in the medical group were alive and free of late complications.
Independent predictors of long term event free survival at the time of first hospital admission were infection by streptococci (OR 1.54, 95% CI 1.13 to 2.08, p < 0.006) and age < 55 years (OR 2.08, 95% CI 1.51 to 2.86, p < 0.0001). The same association was found for survivors of the initial episode and for native valve infections. For hospital survivors of native valve infection, the predictive parameters were age < 55 years and freedom from cerebral dysfunction at the time of hospital discharge.
DISCUSSION
This observational study in a large patient population with infective endocarditis, followed up for an average of 7.5 years, documented a high mortality and even greater morbidity: at 10 years of follow up, 50% of patients with infective endocarditis were dead and 85% of them had suffered morbid events such as recurrent endocarditis, bleeding and embolic complications, cerebral dysfunction, and congestive heart failure or they have required valve surgery for various reasons. Aside from a young age at the onset of the disease and streptococci as the causing organism, which reduced both mortality and morbidity, early valve surgery appears to improve the prognosis of infective endocarditis.
Mortality
Survival rates observed in this study are at the lower end of the range of other reports (survival at 10 years 52–73%), although direct comparison is difficult because of variable characteristics of the respective populations.11–15
Cardiac causes were responsible for one third of deaths during follow up and—as in previous studies16,17—were mostly related to congestive heart failure and sudden death in similar frequency. Mortality was highest during the first months after discharge and stable thereafter.14,17,18
Similar to previous reports, we found no difference in long term survival between mitral and aortic valve disease.14,19,20
Delahaye and colleagues18 found absent permanent pyrexia, male sex, valve prosthesis, older age, congestive heart failure during follow up, absence of early surgery, staphylococci or organisms other than streptococci and staphylococci, and acute or complicated onset to be independent predictors of fatal long term outcome. The latter four factors were equally predictive in our population.
Morbidity
As in other reports, event-free long term survival was rare. Congestive heart failure and the need for late valve surgery were the most frequent causes of morbidity.14,21,22 Recurrence of infective endocarditis was rare but the incidence was still higher than that of infective endocarditis in the general population (that is, < 0.003% per year14,21–25). As in our analysis, others found a similar predominance of recurrent endocarditis in medically compared with medically–surgically treated patients.2,15
The need for late valve surgery was more common in patients treated solely medically during active endocarditis. Although this pattern is consistent with most previously published data, the necessity for late valve surgery after medically treated infection was considerably lower in our series.14,15 A possible explanation is a more aggressive attitude towards early operative intervention in patients likely to develop persistent haemodynamic dysfunction.
Peripheral embolisation was a rare complication in spite of the prevalence of predisposing conditions such as prosthetic heart valves and a thrombogenically altered valve surface after healed endocarditis. These findings are in accordance with earlier reports2,14,26 and document the lower risk of such complications in recent years, which is caused, in part, by the drop in the use of prosthesis with high thrombogenicity (caged ball valves) and better adjustment of anticoagulation.27,28
To our knowledge there are no comparable data in the literature concerning the frequency of bleeding episodes and cerebral dysfunction in the long term after healed infective endocarditis, which in our population were rare.
While there are some early case reports of the occurrence of neurological complications during late follow up,29,30 Salgado and colleagues31 found no increased rate of stroke during a mean of 48 months after healed native valve endocarditis. However, they reported an incidence of 13% of cerebrovascular complications in 90 patients with prosthetic valves implanted either before, during or < 6 weeks after completion of antibiotic treatment. These data are not easily comparable with the present results because of the lack of data about the type of valve prosthesis and the anticoagulation regimens used, among other reasons. In a large meta-analysis of patients with mechanical heart valve prostheses treated with coumarin, Cannegieter and colleagues27 found an annual incidence of valve thrombosis of 2%, of major thromboembolism of 1%, and of major bleeding episodes of 1.4%.
Medical or medical–surgical treatment?
Effects on mortality
Since the time of the first successful implantation of a prosthetic valve during active infective endocarditis more than 35 years ago, one of the eminent practical questions in patients with acute infective endocarditis is whether they would benefit from early valve surgery.32 Potential risks of surgery in the active phase of the infection have to be weighed against the potentially unfavourable course of medical treatment only (early mortality caused by uncontrollable infection or late decision for surgery, haemodynamically important sequelae caused by impaired valve function).
Operative mortality in infective endocarditis has been declining steadily from 9–37% some decades ago33 to 2–12%34–35 in recent years.
Tornos et al14, Malquarti et al,20 and Castillo et al21 did not find a better survival rate following early surgery in their populations with native valve endocarditis. Studies by Vlessis et al36 and Olaison et al2 showed an association between early surgery and improved survival over five years, although this was no longer significant after multivariate analysis in the latter study. Other studies found better long term survival after surgery in specific subgroups such as aortic and prosthetic valve disease.1,37
In our analysis, long term mortality was significantly lower in patients undergoing surgery during the active phase of the disease. This was also true for patients with native valve infection, a condition commonly judged to be less indicative for early surgery.38,39
Effects on morbidity
Only a few studies have examined the possible impact of early surgery on long term morbidity compared with medical treatment.2,14 Even after successful medical treatment during the active phase, the necessity for valve replacement during the early follow up years is considerable.14
In one study only 13% of medically treated patients with native valve disease were alive without late valve replacement after a mean follow up of 7.3 years but reoperation was indicated in only 3% of patients with early valve surgery.15
In the study by Tornos and colleagues,14 valve surgery became necessary during early follow up in 50% of the medically treated episodes of native valve infection (38% survived valve prosthesis-free to the end of the 68 month follow up), while reoperation was necessary in only 6.5% of patients who underwent early surgery.14
In a recent series from the Mayo Clinic, 28% of surgically treated patients with infective endocarditis had to undergo reoperation after a mean of 4.1 years.12 In our patients this risk was fairly low at 4% over the whole 7.5 years of follow up.
Bleeding episodes, cerebral dysfunction, congestive heart failure, and event-free survival were similar in both groups, while recurrence of infective endocarditis and the need for late valve surgery were lower in patients undergoing early surgery.
Thus, our data do not support the rationale of refraining from early surgery because of more frequent reinfections or other complications in prosthetic valves.38,40
Study limitations
This analysis should be interpreted with regard to its retrospective design, especially concerning the comparison between patients with and those without early surgery. However, because of the nature of the disease, controlled studies are not feasible.
Our study population was heterogeneous, including in terms of native and prosthetic valve diseases, as is typical in a tertiary hospital setting. Nevertheless, analysis showed that relevant subgroups were similar to those found in the overall population. Furthermore, evaluation of a great variety of baseline characteristics in patients with and without early surgery showed differences only in factors that are indications for early surgical intervention (table 1).
In addition, patients with possible and definite infective endocarditis by the Duke criteria were included in our study. Although a bias is possible, direct comparison of both groups concerning relevant findings of our study showed no significant difference between them.
However, our findings are applicable to different populations only with caution. Firstly, because of the relatively low incidence of infective endocarditis, patients were included over a relatively long period of time of 15 years, during which medical and surgical methods improved considerably. This problem cannot be overcome, since adequate numbers of patients can hardly be collected by a single centre. Secondly, not all similar studies were based on the relatively strict Duke criteria for endocarditis.8 Thirdly, referral rates and access to surgery may differ between institutions.
Conclusion
Our study documented that even after successful treatment of an episode of infective endocarditis, long term mortality and long term morbidity remain high. Factors predictive of long term mortality are age > 55 years, congestive heart failure, and the initial presence of few symptoms of endocarditis. Moreover, early valve replacement has the potential to improve long term survival in a wide range of patients with infective endocarditis.
REFERENCES
- 1.Croft CH, Woodward W, Elliott A, et al. Analysis of surgical versus medical therapy in active complicated native valve infective endocarditis. Am J Cardiol 1983;51:1650–5. [DOI] [PubMed] [Google Scholar]
- 2.Olaison L, Hogevik H, Myken P, et al. Early surgery in infective endocarditis. Q J Med 1996;89:267–78. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JV, Karp RB, Kirklin JW, et al. Treatment of infective endocarditis: a 10-year comparative analysis. Circulation 1978;58:589–97. [DOI] [PubMed] [Google Scholar]
- 4.Nissen H, Nielsen PF, Frederiksen M, et al. Native valve infective endocarditis in the general population: a 10-year survey of the clinical picture during the 1980s. Eur Heart J 1992;13:872–7. [DOI] [PubMed] [Google Scholar]
- 5.Watanakunakorn C, Burkert T. Infective endocarditis at a large community teaching hospital, 1980–1990: a review of 210 episodes. Medicine (Baltimore) 1993;72:90–102. [DOI] [PubMed] [Google Scholar]
- 6.Sandre RM, Shafran SD. Infective endocarditis: review of 135 cases over 9 years. Clin Infect Dis 1996;22:276–86. [DOI] [PubMed] [Google Scholar]
- 7.Schon HR, Fuchs CJ, Schomig A, et al. [Changes in infectious endocarditis: analysis of a disease picture in the last decade]. Z Kardiol 1994;83:31–7. [PubMed] [Google Scholar]
- 8.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200–9. [DOI] [PubMed] [Google Scholar]
- 9.Clark RE, Edmunds LH Jr, Cohn LH, et al. Guidelines for reporting morbidity and mortality after cardiac valvular operations. Eur J Cardiothorac Surg 1988;2:293–5. [DOI] [PubMed] [Google Scholar]
- 10.Netzer RO, Zollinger E, Seiler C, et al. Infective endocarditis: clinical spectrum, presentation and outcome. An analysis of 212 cases 1980–1995. Heart 2000;84:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexiou C, Langley SM, Stafford H, et al. Surgical treatment of infective mitral valve endocarditis: predictors of early and late outcome. J Heart Valve Dis 2000;9:327–34. [PubMed] [Google Scholar]
- 12.Mullany CJ, Chua YL, Schaff HV, et al. Early and late survival after surgical treatment of culture-positive active endocarditis. Mayo Clin Proc 1995;70:517–25. [DOI] [PubMed] [Google Scholar]
- 13.d'Udekem Y, David TE, Feindel CM, et al. Long-term results of surgery for active infective endocarditis. Eur J Cardiothorac Surg 1997;11:46–52. [DOI] [PubMed] [Google Scholar]
- 14.Tornos MP, Permanyer-Miralda G, Olona M, et al. Long-term complications of native valve infective endocarditis in non-addicts: a 15-year follow-up study. Ann Intern Med 1992;117:567–72. [DOI] [PubMed] [Google Scholar]
- 15.Verheul HA, van den Brink RB, van Vreeland T, et al. Effects of changes in management of active infective endocarditis on outcome in a 25-year period. Am J Cardiol 1993;72:682–7. [DOI] [PubMed] [Google Scholar]
- 16.Cates JE, Christie RV. A review of 442 patients treated in 14 centres appointed by the penicilline trials committee of the MRC. Q J Med 1951;20:90–130. [PubMed] [Google Scholar]
- 17.Kaye D. Cure rates and long-term prognosis. In: Kaye D, ed. Infective endocarditis. Baltimore: University Park Press, 1976:201–11.
- 18.Delahaye F, Ecochard R, de Gevigney G, et al. The long term prognosis of infective endocarditis. Eur Heart J 1995;16(suppl B):48–53. [DOI] [PubMed] [Google Scholar]
- 19.Ormiston JA, Neutze JM, Agnew TM, et al. Infective endocarditis: a lethal disease. Aust NZ J Med 1981;11:620–9. [DOI] [PubMed] [Google Scholar]
- 20.Malquarti V, Saradarian W, Etienne J, et al. Prognosis of native valve infective endocarditis: a review of 253 cases. Eur Heart J 1984;5(suppl C):11–20. [DOI] [PubMed] [Google Scholar]
- 21.Castillo JC, Anguita MP, Ramirez A, et al. Long term outcome of infective endocarditis in patients who were not drug addicts: a 10 year study. Heart 2000;83:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tornos P, Almirante B, Olona M, et al. Clinical outcome and long-term prognosis of late prosthetic valve endocarditis: a 20-year experience. Clin Infect Dis 1997;24:381–6. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer JT, Thompson J, Valkenburg HA, et al. Epidemiology of bacterial endocarditis in the Netherlands. I. Patient characteristics. Arch Intern Med 1992;152:1863–8. [DOI] [PubMed] [Google Scholar]
- 24.Benn M, Hagelskjaer LH, Tvede M. Infective endocarditis, 1984 through 1993: a clinical and microbiological. J Intern Med 1997;242:15–22. [DOI] [PubMed] [Google Scholar]
- 25.Langley SM, Alexiou C, Stafford HM, et al. Aortic valve replacement for endocarditis: determinants of early and late outcome. J Heart Valve Dis 2000;9:697–704. [PubMed] [Google Scholar]
- 26.Steckelberg JM, Murphy JG, Ballard D, et al. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med 1991;114:635–40. [DOI] [PubMed] [Google Scholar]
- 27.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994;89:635–41. [DOI] [PubMed] [Google Scholar]
- 28.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian study on complications of oral anticoagulant therapy. Lancet 1996;348:423–8. [DOI] [PubMed] [Google Scholar]
- 29.Morgan WL, Bland EF. Bacterial endocarditis in the antibiotic era. Circulation 1959;19:753–65. [DOI] [PubMed] [Google Scholar]
- 30.Alajouanine T, Castaigne P, Lhermitte F, et al. The cerebral arteritis of bacterial endocarditis: its late complications. JAMA 1959;170:1858. [Google Scholar]
- 31.Salgado AV, Furlan AJ, Keys TF, et al. Neurologic complications of endocarditis: a 12-year experience. Neurology 1989;39:173–8. [DOI] [PubMed] [Google Scholar]
- 32.Wallace AG, Young GW, Osterhout S. Treatment of acute bacterial endocarditis by valve excision and replacement. Circulation 1965;31:450–3. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier LC, Baillot R, Auger P, et al. Early valve replacement in active infective endocarditis. Can J Surg 1984;27:383–6. [PubMed] [Google Scholar]
- 34.Peric M, Vuk F, Huskic R, et al. Active infective endocarditis: low mortality associated with early surgical treatment. Cardiovasc Surg 2000;8:208–13. [DOI] [PubMed] [Google Scholar]
- 35.Alexiou C, Langley SM, Stafford H, et al. Surgery for active culture-positive endocarditis: determinants of early and late outcome. Ann Thorac Surg 2000;69:1448–54. [DOI] [PubMed] [Google Scholar]
- 36.Vlessis AA, Hovaguimian H, Jaggers J, et al. Infective endocarditis: ten-year review of medical and surgical therapy. Ann Thorac Surg 1996;61:1217–22. [DOI] [PubMed] [Google Scholar]
- 37.Yu VL, Fang GD, Keys TF, et al. Prosthetic valve endocarditis: superiority of surgical valve replacement versus medical therapy only. Ann Thorac Surg 1994;58:1073–7. [DOI] [PubMed] [Google Scholar]
- 38.Aranki SF, Santini F, Adams DH, et al. Aortic valve endocarditis: determinants of early survival and late morbidity. Circulation 1994;90:II175–82. [PubMed] [Google Scholar]
- 39.Larbalestier RI, Kinchla NM, Aranki SF, et al. Acute bacterial endocarditis: optimizing surgical results. Circulation 1992;86(5 suppl):II68–74. [PubMed] [Google Scholar]
- 40.Grover FL, Cohen DJ, Oprian C, et al. Determinants of the occurrence of and survival from prosthetic valve endocarditis: experience of the Veterans Affairs cooperative study on valvular heart disease. J Thorac Cardiovasc Surg 1994;108:207–14. [PubMed] [Google Scholar]