Abstract
Objective: To assess the prevalence of myocardial viability by technetium-99m (Tc-99m)-tetrofosmin/fluorine-18-fluorodeoxyglucose (FDG) single photon emission computed tomography (SPECT) in patients with ischaemic cardiomyopathy.
Design: A retrospective observational study.
Setting: Thoraxcenter Rotterdam (a tertiary referral centre).
Patients: 104 patients with chronic coronary artery disease and severely depressed left ventricular function presenting with heart failure symptoms.
Main outcome measures: Prevalence of myocardial viability as evaluated by Tc-99m-tetrofosmin/FDG SPECT imaging. Two strategies for assessing viability in dysfunctional myocardium were used: perfusion imaging alone, and the combination of perfusion and metabolic imaging.
Results: On perfusion imaging alone, 56 patients (54%) had a significant amount of viable myocardium, whereas 48 patients (46%) did not. Among the 48 patients with no significant viability by perfusion imaging alone, seven additional patients (15%) had significantly viable myocardium on combined perfusion and metabolic imaging. Thus with a combination of perfusion and metabolic imaging, 63 patients (61%) had viable myocardium and 41 (39%) did not.
Conclusions: On the basis of the presence of viable dysfunctional myocardium, 61% of patients with chronic coronary artery disease and depressed left ventricular ejection fraction presenting with heart failure symptoms may be considered for coronary revascularisation. The combination of perfusion and metabolic imaging identified more patients with significant viability than myocardial perfusion imaging alone.
Keywords: myocardial viability, left ventricular dysfunction, heart failure
Chronic coronary artery disease is the most important cause of left ventricular dysfunction leading to heart failure. When this occurs it has an extremely poor prognosis.1,2 In nearly 70% of the patients in 13 major heart failure trials, coronary artery disease was the underlying cause of the heart failure.3 The management of these patients remains difficult, while both the incidence and the prevalence of chronic heart failure have been increasing in recent years.4 Medical treatment is still suboptimal in these patients, although significant improvements in survival have been achieved with angiotensin converting enzyme (ACE) inhibition, blockade of aldosterone receptors by spironolactone, and β adrenergic blockade.5–7 Other therapeutic options are heart transplantation and coronary revascularisation. The possibilities of heart transplantation are limited by the availability of donor hearts, but coronary revascularisation could be an effective alternative. In a subset of patients with heart failure secondary to chronic coronary artery disease, revascularisation has been shown to improve left ventricular ejection fraction (LVEF), heart failure symptoms, and survival.8,9 However, revascularisation in such patients is also associated with increased periprocedural morbidity and mortality.8,9
The concept of myocardial viability was proposed to explain the improvement of LVEF and heart failure symptoms after revascularisation.10,11 Dysfunctional but viable myocardium is likely to regain contractile function after coronary revascularisation.12 On the other hand, dysfunctional non-viable myocardium (scar tissue) clearly will not improve. Bonow estimated that between 25–40% of patients with heart failure caused by ischaemic heart disease have the potential for functional improvement after revascularisation.13 Quantification of myocardial viability in patients with chronic coronary artery disease, depressed left ventricular function, and heart failure is at present limited, but this information is important as such patients may benefit from revascularisation.14,15 Our aim in this study was therefore to evaluate myocardial viability in a consecutive series of 104 patients with chronic coronary artery disease and depressed LVEF presenting with heart failure symptoms.
METHODS
Patient population, study protocol
The study population consisted of 104 consecutive patients with ischaemic cardiomyopathy (chronic coronary artery disease as assessed by angiography and an LVEF of ≤ 35%), who presented with heart failure as the predominant symptom and were referred for evaluation of myocardial viability. Patients with primary cardiomyopathy or concomitant significant valvar disease were not included.
All patients underwent resting echocardiography to identify dysfunctional myocardial tissue, and dual isotope simultaneous acquisition myocardial single photon emission computed tomography (SPECT), including Tc-99m tetrofosmin and fluorodeoxyglucose (FDG) to assess myocardial perfusion and glucose utilisation, respectively.16,17 LVEF was assessed by radionuclide ventriculography.
The local medical ethics committee approved the protocol and all patients gave informed consent.
Assessment of contractile function
A Hewlett-Packard Sonos-5500 imaging system (Hewlett-Packard Inc, Andover, Massachusetts, USA), equipped with a 1.8 MHz transducer using second harmonic imaging to optimise endocardial border visualisation, was used to record cross sectional echocardiograms. Four standard views (apical two and four chamber views, and parasternal short and long axis views) were stored on tape.
Off-line interpretation was undertaken using a computer system that digitised the taped images and displayed them in a cineloop format. Two experienced reviewers blinded to the SPECT data scored the digitised echocardiograms visually. In case of disagreement, a majority decision was achieved by a third reviewer. The left ventricle was divided according to the standard 16 segment model described by the American Society of Echocardiography (six basal, six distal, and four apical segments).18 Regional wall motion and systolic wall thickening were scored using a five point grading scale: 1, normal (normal endocardial excursion and systolic wall thickening); 2, mildly hypokinetic (mildly reduced excursion and wall thickening); 3, severely hypokinetic (severely reduced excursion and thickening); 4, akinetic (absent excursion and wall thickening); 5, dyskinetic (paradoxical systolic outward wall motion). Myocardial segments were considered normal if the regional wall motion was normal or mildly hypokinetic. Only segments with severe hypokinesia, akinesia, or dyskinesia were evaluated for myocardial viability.
SPECT data acquisition
After a light breakfast, the patients received an intravenous injection of Tc-99m-tetrofosmin (600 MBq) to evaluate resting perfusion. FDG imaging to evaluate glucose utilisation was done after the administration of acipimox (500 mg oral dose) in all patients. Acipimox enhances myocardial FDG uptake by reducing the plasma concentration of free fatty acids. Following acipimox administration, the patients received a low fat, carbohydrate rich meal. This small meal further enhances myocardial FDG uptake by stimulating endogenous insulin release. Several studies have shown excellent imaging using acipimox.19
Patients with diabetes mellitus were asked to continue their antidiabetic drug regimen. Plasma glucose concentrations were measured immediately before the study. In patients with a plasma glucose concentration of more than 8 mmol/l, insulin was given intravenously to enhance myocardial FDG uptake. The plasma glucose concentration was then measured again and additional intravenous insulin given if necessary.
Sixty minutes after the meal, FDG (185 MBq) was injected, and after an a further 45 minutes to allow cardiac FDG uptake, dual isotope simultaneous acquisition SPECT was performed. Perfusion and metabolic imaging were both done at rest without stressors. Patients continued their normal cardiac drug treatment during the SPECT study.
A triple head gamma camera system (Picker Prism 3000XP, Cleveland, Ohio, USA) was used. The camera system was equipped with high energy 511 keV collimators. The energies were centred on the 140 keV photon peak of technetium-99m tetrofosmin with a 15% window and on the 511 keV photon peak of FDG with a 15% window. Data acquisition was done in the supine position, over 360° (120 sectors of 3°). Total imaging time was 32 minutes. Data were stored in a 64 × 64, 16 bit matrix.
SPECT data reconstruction and analysis
From the raw scintigraphic data, 6 mm thick (1 pixel) transaxial slices were reconstructed by filtered back projection using a Butterworth filter (cut off frequency at 0.17 cycle/pixel of order 3.5). Attenuation correction was not applied. Further reconstruction yielded standard short and long axis projections perpendicular to the heart axis. The Tc-99m-tetrofosmin and the FDG data were reconstructed simultaneously. This approach permits exact alignment of the perfusion and FDG images.
The perfusion and FDG short axis slices were adjusted to peak myocardial activity (100%). The left ventricle was divided into 16 segments matching the echocardiographic segments.20 Both Tc-99m-tetrofosmin and FDG uptake defects were graded semiquantitatively on a four point scale: 0, normal (≥ 75%–100%); 1, mildly reduced (≥ 50%–75%; 2, moderately reduced (≥ 25%–50%); 3, severely reduced or absent (≤ 25% uptake). Dysfunctional segments (identified by resting echocardiography) were subsequently evaluated for viability.
Viable myocardium was defined using two approaches: perfusion criteria alone, and a combination of perfusion and metabolic criteria. Using perfusion alone, myocardium with a To-99m-tetrofosmin uptake score of ≤ 1 was considered viable, while myocardium with a score of ≥ 2 was considered non-viable. Using the combination of perfusion and metabolism, myocardium with a Tc-99m-tetrofosmin uptake score of ≤ 1 or with a reduction in Tc-99m-tetrofosmin uptake score that was more severe than the reduction in FDG activity by ≥ 1 point (perfusion–metabolism mismatch pattern) was considered viable. Dysfunctional myocardium with a proportionate reduction in both Tc-99m-tetrofosmin and FDG uptake (perfusion–metabolism match pattern) was considered non-viable.
A patient with four or more dysfunctional but viable segments was considered to have a functionally significant amount of viable myocardium. This definition is based on previous work using receiver operating characteristic (ROC) curve analysis showing that improvement in LVEF after revascularisation can be anticipated when more than 25% of the left ventricle is viable (four or more segments in a 16 segment model).21
Assessment of LVEF
To assess LVEF at the time of the viability testing, we performed radionuclide ventriculography at rest in all patients. A small field of view gamma camera system (Orbiter, Siemens, Erlangen, Germany) was used, oriented in a 45° left anterior oblique position with a 5–10° caudal tilt. After injection of To-99m (740 MBq), radionuclide ventriculography was done at rest with the patient in the supine position. The LVEF was calculated by standard methods (Odyssey VP, Picker, Cleveland, Ohio, USA).
Statistical analysis
All continuous data are expressed as mean (SD). Percentages are rounded. Comparisons were made using the Student t test for unpaired samples. A probability value of p < 0.05 was considered significant.
RESULTS
Patient characteristics
Clinical characteristics of the 104 patients (87 men, 17 women; mean (SD) age, 60 (9) years) are summarised in table 1. All patients presented with symptoms of heart failure. The mean New York Heart Association (NYHA) functional class was 2.5 (0.9); 67 patients were in NYHA class III or IV. The majority (93%) of the patients had suffered a previous myocardial infarct (all occurring more than one month before viability assessment). LVEF assessed by radionuclide ventriculography averaged 25 (7)% (range 9–35%). In 23 patients the LVEF was 20% or less, in 54 it was between 21% and 30%, and in 27 it was more than 30%.
Table 1.
Clinical characteristics of 104 patients undergoing dual isotope simultaneous acquisition single photon emission computed tomography
| Clinical features | Number of patients |
| LVEF ≤20% | 23 (22%) |
| LVEF >20%, ≤30% | 54 (52%) |
| LVEF >30%, ≤35% | 27 (26%) |
| Number of stenosed arteries (mean (SD)) | 2.1 (0.8) |
| Previous MI | 97 (93%) |
| Previous CABG | 16 (15%) |
| Previous PTCA | 13 (13%) |
| Diabetes mellitus | 15 (14%) |
| Drug treatment | |
| ACE inhibitors | 86 (83%) |
| β Blockers | 53 (51%) |
| Calcium antagonists | 29 (28%) |
| Nitrates | 74 (71%) |
| Diuretics | 66 (63%) |
Data are n (%) unless specified.
ACE, angiotensin converting enzyme; CABG, coronary artery bypass graft surgery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Segmental analysis
Contractile function
Cross sectional echocardiographic analysis of regional left ventricular function was done in 1664 segments, of which 189 were normal and 297 mild hypokinetic. Of 1178 dysfunctional segments (71%), 584 showed severe hypokinesia, 578 akinesia, and 16 dyskinesia. The mean (SD) number of dysfunctional segments per patient was 11.3 (4.2).
Perfusion imaging alone
Using myocardial perfusion as the only criterion of viability, 497 dysfunctional segments (42%) were classified as viable. Three hundred and sixteen dysfunctional segments (27%) had normal Tc-99m-tetrofosmin uptake, and 181 (15%) had a mildly reduced uptake. The remaining 681 dysfunctional segments (58%) had a Tc-99m tetrofosmin uptake score of 2 or 3 and were classified as non-viable.
Combined perfusion and metabolic imaging
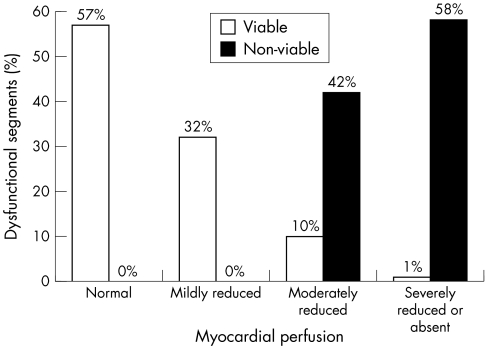
Using perfusion imaging in conjunction with FDG SPECT, 558 of the dysfunctional myocardial segments (47%) were classified as viable. Of these viable dysfunctional segments, 497 (42%) had normal or mildly reduced perfusion and 61 additional segments (5%) had a perfusion–metabolism mismatch pattern. Conversely, 620 dysfunctional segments (53%) were classified as non-viable (with a matching pattern of perfusion and metabolism). Figure 1 shows the myocardial perfusion in the dysfunctional segments that were classified as viable and non-viable by combined perfusion and metabolic imaging.
Figure 1.
Myocardial perfusion in viable and non-viable tissue. In viable myocardium, perfusion was normal in 57% of dysfunctional segments, mildly reduced in 32%, moderately reduced in 10%, and severely reduced or absent in 1%. Non-viable tissue had moderately reduced perfusion in 42% of dysfunctional segments and severely reduced or absent perfusion in 58%.
Patient analysis
Contractile function
Patients with an LVEF of 20% or less had more dysfunctional segments than those with an LVEF of 21–30% (14.1 (2.0) v 10.9 (4.5), p < 0.005) or those with an LVEF of more than 30% (14.1 (2.0) v 9.7 (3.9), p < 0.0001). The number of dysfunctional segments was not significantly different between patients with an LVEF of more than 30% and those with an LVEF of 21–30% (9.7 (3.9) v 10.9 (4.5); p = 0.2) (table 2).
Table 2.
Comparison of segment viability in three groups divided by left ventricular ejection fraction
| Characteristics of segments | LVEF ≤20% (23 patients) | LVEF 21–30% (54 patients) | LVEF >30% (27 patients) |
| Dysfunctional | 14.1 (2.0) | 10.9 (4.5)† | 9.7 (3.9) |
| Viable by Tc-99m | 6.4 (3.7) | 4.6 (3.8)* | 3.8 (3.2) |
| Viable by Tc-99m + FDG | 7.2 (4.0) | 5.1 (4.1)* | 4.2 (3.2) |
*p<0.05; †p<0.005 v LVEF ≤20%.
FDG, fluorodeoxyglucose; LVEF, left ventricular ejection fraction.
Perfusion imaging alone
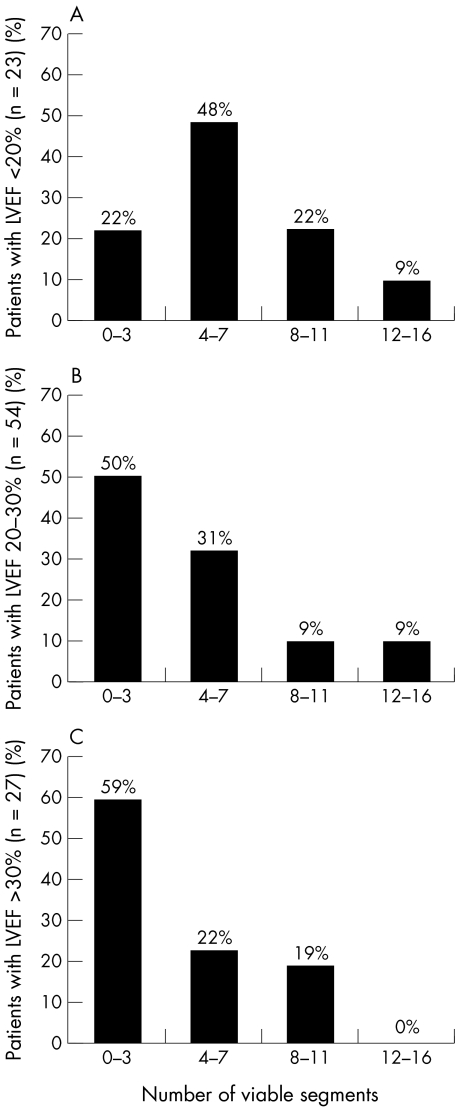
All patients had on average 4.8 (3.7) (range 0–14) dysfunctional but viable segments. When myocardial perfusion was used as the only criterion of viability, 56 patients (54%) had a significant amount of viable myocardium (on average 7.5 (2.9) segments) and 48 patients (46%) did not have significant viability (1.6 (1.2) segments). Figure 2 shows the number of viable segments per patient with perfusion imaging alone for the three LVEF groups.
Figure 2.
(A) Amount of viable myocardium indicated by the number of viable segments in 23 patients with a left ventricular ejection fraction (LVEF) ≤ 20%. Using perfusion as the only criterion of viability, 18 patients (78%) had significant viability (≥ 4 viable segments) and five (22%) did not have significant viability. (B) Amount of viable myocardium indicated by the number of viable segments in 54 patients with an LVEF > 20% but ≤ 30%. Using perfusion as the only criterion of viability, 27 patients (50%) had significant viability (≥ 4 viable segments), and 27 (50%) did not have significant viability. (C) Amount of viable myocardium indicated by the number of viable segments in 27 patients with an LVEF > 30% but ≤ 35%. Using perfusion as the only criterion of viability, 11 patients (41%) had significant viability (≥ 4 viable segments) and 16 (59%) did not have significant viability.
Combined perfusion and metabolic imaging
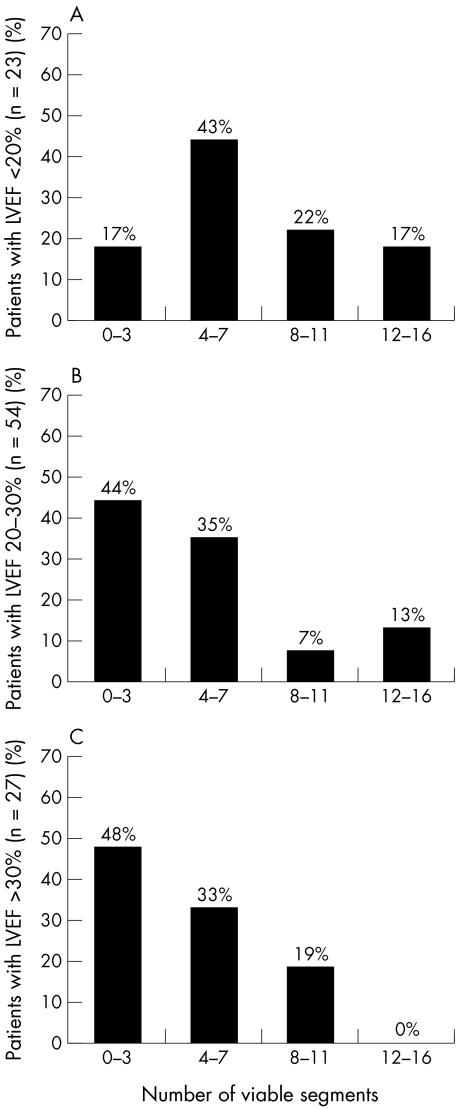
Among the 48 patients with no significant viability by perfusion imaging alone, seven additional patients (15%) had viable myocardium when assessed using a combination of perfusion and metabolic imaging. All 56 patients with significant viability when assessed by perfusion alone also had significant viability with combined perfusion and metabolic imaging (on average, 8.2 (3.2) viable segments). Thus perfusion imaging in combination with metabolic imaging showed that 63 patients (61%) had a significant amount of viable tissue (7.8 (3.2) segments). Forty one patients (39%) did not have significant residual viable dysfunctional tissue (on average, 1.6 (1.2) viable segments). Overall, the patients had 5.4 (4.0) viable dysfunctional segments. Figure 3 shows the number of viable dysfunctional segments for patients in the three different LVEF categories.
Figure 3.
(A) Amount of viable myocardium indicated by the number of viable segments in 23 patients with a left ventricular ejection fraction (LVEF) ≤ 20%. Using perfusion in combination with metabolic criteria for viability, 19 patients (83%) had significant viability (≥ 4 viable segments) and four (17%) did not. (B) Amount of viable myocardium indicated by the number of viable segments in 54 patients with an LVEF > 20% but ≤ 30%. Using perfusion in combination with metabolic criteria for viability, 30 patients (56%) had significant viability (≥ 4 viable segments) and 24 (44%) did not. (C) Amount of viable myocardium indicated by the number of viable segments in 27 patients an LVEF > 30% but ≤ 35%. Using perfusion in combination with metabolic criteria for viability, 14 patients (52%) had significant viability (≥ 4 viable segments) and 13 (48%) did not.
DISCUSSION
Viable myocardium
Heart failure is becoming a major problem in clinical cardiology. In recent years the incidence of patients with heart failure has increased despite progress in prevention and treatment.4 Chronic heart failure is predominantly caused by coronary artery disease and has a poor prognosis.3 In a subset of patients with heart failure resulting from coronary artery disease, coronary revascularisation may improve left ventricular function, heart failure symptoms, and survival,8,9 but the risks of revascularisation in such patients are substantial. Also, apart from the need to assess clinical variables such as target vessels and comorbid factors, it is important to evaluate myocardial viability as part of the complex selection process in candidates for revascularisation.
Previous estimates suggest that between 25–40% of the patients with heart failure caused by coronary artery disease may show useful functional improvement after revascularisation.13 In our hospital, patients with coronary artery disease, a depressed LVEF, and heart failure are always referred for assessment of myocardial viability. This approach allows improved risk stratification and results in optimal treatment for the individual patient. In the present study, we examined myocardial viability in a consecutive series of patients from our heart failure registry.
A few previous studies have examined myocardial viability on an individual patient basis. Using FDG positron emission tomography (PET), Pasquet and colleagues found that among 66 patients with severe left ventricular dysfunction, 28 (47%) had an improvement in LVEF of at least 5% after coronary revascularisation, while eight (14%) had an improvement of more than 10%.22 Al-Mohammad and colleagues studied the prevalence of hibernating myocardium in 27 patients using FDG PET.14 Fourteen of these had significant areas of hibernating myocardium on PET. Auerbach and associates, also using FDG PET, studied 283 patients with ischaemic cardiomyopathy and found that 156 (55%) had viable myocardium.15 In the present study, 54% of the patients showed significant viability when myocardial perfusion was used as the only index, while perfusion imaging in combination with metabolic imaging identified a further 7%; thus 61% of the patients in all had a significant amount of viable tissue. These observations are in close agreement with those of Al-Mohammad and Auerbach using FDG PET.14,15
In the present study patients with an LVEF of 20% or less had more dysfunctional but viable segments than those with an LVEF of 21–30% or with an LVEF of more than 30%. In line with our results, Fath-Ordoubadi and colleagues reported that in 47 patients with coronary artery disease and chronic left ventricular dysfunction, those with an LVEF of 30% or less had more dysfunctional but viable segments than those with an LVEF of more than 30%.23 Moreover, after revascularisation the LVEF improved in patients with an initial LVEF of 30% or less, whereas it remained unchanged in those with an LVEF of more than 30%. It seems that patients with the most severe left ventricular dysfunction benefit more from revascularisation than those with less severe dysfunction. These findings may have important clinical implications in relation to revascularisation, as severe left ventricular dysfunction has a very poor prognosis when treated medically.3
How much viable tissue is required to be clinically relevant?
In the present study a patient was considered to have a significant amount of viable myocardium if there were at least four or more dysfunctional viable segments in a 16 segment model. This definition was based on a previous study using ROC curve analysis showing that improvement in LVEF after revascularisation can be anticipated when 25% or more of the left ventricle is viable.21 Di Carli and colleagues reported that in patients with coronary artery disease and left ventricular dysfunction and with more than 5% viable tissue, the survival rate of revascularised patients was higher than in those who were treated medically (88% v 50%).24 These data suggest that revascularisation in patients with 5–25% of viable myocardial tissue, while being unlikely to result in functional recovery, may still improve prognosis. Moreover, five prognostic FDG PET studies have reported a high event rate (42%) in patients with viable tissue treated medically,25 though these studies were of retrospective design without randomised treatment. Nevertheless it appears that for functional improvement to occur more viable myocardium is needed than for improvement in prognosis. How much viable myocardium is needed to be clinically relevant remains to be established in randomised prospective studies focusing on both functional improvement and prognosis.
Perfusion alone versus perfusion and metabolism
Detection of reduced myocardial perfusion (without any additional information about metabolism) has been proposed as a method for differentiating viable from non-viable myocardium. Thallium-201 or technetium-99m labelled tracers have been used in various studies to assess myocardial viability.26 However, it appears that the diagnostic accuracy of perfusion imaging for detecting viable tissue is less than that of perfusion imaging combined with FDG metabolic imaging. Three studies have shown that metabolic evidence of viability may be present while perfusion is absent. Sawada and colleagues studied 20 patients with a previous myocardial infarction and reported that evidence of viability obtained by FDG PET was still present in 50% of segments which had technetium-99m activity of less than 40%.27 Soufer and associates, in a study of 37 patients with coronary artery disease, found that FDG PET accurately predicted functional improvement after revascularisation in segments that were non-viable with technetium-99m imaging but viable with FDG metabolic imaging.28 Altehoefer and colleagues studied 111 patients with coronary artery disease and showed that 5–11% of segments with technetium-99m activity of 30% or less were viable according to FDG imaging.29
Our study supports those findings. When only perfusion was used to indicate viable myocardium, 56 patients (54%) showed significant viability, while a combination of perfusion and FDG imaging identified 63 patients (61%) with significantly viable myocardium. Hence, of the 48 patients with no significant viability by perfusion imaging alone, seven additional patients (15%) had significantly viable myocardium when the information on metabolic imaging was added. This is of clinical relevance, as about one in seven patients initially thought not to have a significant amount of viable tissue on perfusion imaging were found to be eligible for revascularisation when the combined test was used. It thus appears that perfusion imaging alone underestimates viability, and the combination of perfusion imaging and FDG metabolic imaging is a better way of discriminating between viable and non-viable myocardium. Furthermore, combined imaging has been shown to provide better discrimination between segments with a relatively high and a relatively low likelihood of recovery of function after revascularisation.30
Study limitations
The centre where this study took place is a tertiary referral centre and thus a pre-existing patient selection bias may be present.
The SPECT imaging protocol did not include administration of nitrates before tracer injection. Nitrate administration may enhance the detection of myocardial viability with To-99m-tetrofosmin SPECT in patients with coronary artery disease and left ventricular dysfunction.31
During SPECT data reconstruction, no attenuation correction was applied and this may have influenced the accuracy of the results. Matsunari and colleagues reported that the use of attenuation corrected Tc-99m-tetrofosmin SPECT improved the detection of viable myocardium, mainly by decreasing the underestimation of viability in the inferior septal region.32
The findings in our study did not include the results of recovery of function after revascularisation. However, that was not a goal of the study. Our aim was specifically to quantify the presence of significant myocardial viability in patients with chronic coronary artery disease, depressed LVEF, and heart failure symptoms.
Conclusions
On the basis of the presence of viable dysfunctional myocardium, 61% of patients with chronic coronary artery disease and a depressed left ventricular ejection fraction presenting with heart failure symptoms may be considered for coronary revascularisation. The combination of perfusion and metabolic imaging identified more patients with viable myocardium than perfusion imaging alone.
Abbreviations
ACE, angiotensin converting enzyme
FDG, fluorodeoxyglucose
LVEF, left ventricular ejection fraction
PET, positron emission tomography
ROC, receiver operating characteristic
SPECT, single photon emission computed tomography
REFERENCES
- 1.Bourassa MG, Gurne O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The studies of left ventricular dysfunction (SOLVD) investigators. J Am Coll Cardiol 1993;22(suppl):14–19A. [DOI] [PubMed] [Google Scholar]
- 2.Hamer AW, Takayama M, Abraham M, et al. End-systolic volume and long-term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation 1994;90:2899–904. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Bonow RO. Chronic heart failure in the United States – a manifestation of coronary artery disease. Circulation 1998;97:282–9. [DOI] [PubMed] [Google Scholar]
- 4.Ghali JK, Cooper R, Ford E. Trends in hospitalization rates for heart failure in the United States, 1973–1986: evidence for increasing population prevalence. Arch Intern Med 1990;150:769–73. [PubMed] [Google Scholar]
- 5.Pfeffer MA, Braunwald E, Moye LA, et al, on behalf of the SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669–77. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Zannad F, Remme WJ, et al, for the randomized aldactone evaluation study investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 7.CIBIS-II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 8.Baker DW, Jones R, Hodges J, et al. Management of heart failure. III. The role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. JAMA 1994;272:1528–34. [DOI] [PubMed] [Google Scholar]
- 9.Elefteriades JA, Tolis G, Levi E, et al. Coronary artery bypass grafting in severe left ventricular dysfunction: excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol 1993;22:1411–17. [DOI] [PubMed] [Google Scholar]
- 10.Rahimtoola SH. The hibernating myocardium. Am Heart J 1989;117:211–21. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA. Hibernating myocardium. Am Heart J 1989;118:1361. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Cornel JH, Visser FC, et al. F18-fluorodesoxyglucose single-photon emission computed tomography predicts functional outcome of dyssynergic myocardium after surgical revascularization. J Nucl Cardiol 1997;4:302–8. [DOI] [PubMed] [Google Scholar]
- 13.Bonow RO. Identification of viable myocardium. Circulation 1996;94:2674–80. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mohammad A, Mahy IR, Norton MY, et al. Prevalence of hibernating myocardium in patients with severely impaired ischaemic left ventricles. Heart 1998;80:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auerbach MA, Schöf-der H, Hoh C, et al. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation 1999;99:2921–6. [DOI] [PubMed] [Google Scholar]
- 16.Sandler MP, Videlefsky S, Delbeke D, et al. Evaluation of myocardial ischemia using a rest metabolism/stress perfusion protocol with fluorine-18 deoxyglucose/technetium-99m MIBI and dual-isotope simultaneous-acquisition single-photon emission computed tomography. J Am Coll Cardiol 1995;26:870–80. [DOI] [PubMed] [Google Scholar]
- 17.Sandler MP, Bax JJ, Patton JA, et al. Fluorine-18-fluorodeoxyglucose cardiac imaging using a modified scintillation camera. J Nucl Med 1998;39:2035–43. [PubMed] [Google Scholar]
- 18.Bourdillon PD, Broderik TM, Sawada SG, et al. Regional wall motion index for infarct and non-infarct region after reperfusion in acute myocardial infarction: comparison with global wall motion index. J Am Soc Echocardiogr 1989;2:398–407. [DOI] [PubMed] [Google Scholar]
- 19.Nuutila P, Knuuti J, Raitakari M, et al. Effect of antilipolysis on heart and skeletal muscle glucose uptake in overnight fasted humans. Am J Physiol 1994;267:E941–6. [DOI] [PubMed] [Google Scholar]
- 20.Rambaldi R, Poldermans D, Bax JJ, et al. Dobutamine stress echocardiography and technetium-99m-tetrofosmin/fluorine 18-fluorodeoxyglucose single-photon emission computed tomography and influence of resting ejection fraction to assess myocardial viability in patients with severe left ventricular dysfunction and healed myocardial infarction. Am J Cardiol 1999;84:130–4. [DOI] [PubMed] [Google Scholar]
- 21.Bax JJ, Poldermans D, Elhendy A, et al. Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol 1999;34:163–9. [DOI] [PubMed] [Google Scholar]
- 22.Pasquet A, Lauer MS, Williams MJ, et al. Prediction of global left ventricular function after bypass surgery in patients with severe left ventricular dysfunction. Impact of pre-operative myocardial function, perfusion, and metabolism. Eur Heart J 2000;21:125–36. [DOI] [PubMed] [Google Scholar]
- 23.Fath-Ordoubadi F, Pagano D, Marinho NVS, et al. Coronary revascularization in the treatment of moderate and severe postischemic left ventricular dysfunction. Am J Cardiol 1998;82:26–31. [DOI] [PubMed] [Google Scholar]
- 24.Di Carli MF, Davidson M, Little R, et al. Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol 1994;73:527–33. [DOI] [PubMed] [Google Scholar]
- 25.Bax JJ, Wijns W. Fluorodeoxyglucose imaging to assess myocardial viability: PET, SPECT or gamma camera coincidence imaging? J Nucl Med 1999;40:1893–5. [PubMed] [Google Scholar]
- 26.Bax JJ, Wijns W, Cornel JH, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol 1997;30:1451–60. [DOI] [PubMed] [Google Scholar]
- 27.Sawada SG, Allman KC, Muzik O, et al. Positron emission tomography detects evidence of viability in rest technetium-99m sestamibi defects. J Am Coll Cardiol 1994;23:92–8. [DOI] [PubMed] [Google Scholar]
- 28.Soufer R, Dey HM, Ng CK, et al. Comparison of sestamibi single-photon emission computed tomography with positron emission tomography for estimating left ventricular myocardial viability. Am J Cardiol 1995;75:1214–19. [DOI] [PubMed] [Google Scholar]
- 29.Altehoefer C, Vom Dahl J, Biedermann M, et al. Significance of defect severity in technetium-99m-MIBI SPECT at rest to assess myocardial viability: comparison with fluorine-18-FDG PET. J Nucl Med 1994;35:569–74. [PubMed] [Google Scholar]
- 30.Bax JJ, Visser FC, Elhendy A, et al. Prediction of improvement of regional left ventricular function after revascularization using different perfusion–metabolism criteria. J Nucl Med 1999;40:1866–73. [PubMed] [Google Scholar]
- 31.Flotats A, Carrio I, Estorch M, et al. Nitrate administration to enhance the detection of myocardial viability by technetium-99m tetrofosmin single-photon emission tomography. Eur J Nucl Med 1997;24:767–73. [DOI] [PubMed] [Google Scholar]
- 32.Matsunari I, Boning G, Ziegler SI, et al. Attenuation-corrected 99mTc-tetrofosmin single-photon emission computed tomography in the detection of viable myocardium: comparison with positron emission tomography using 18F-fluorodeoxyglucose. J Am Coll Cardiol 1998;32:926–35. [DOI] [PubMed] [Google Scholar]