Abstract
Familial amyotrophic lateral sclerosis-linked mutations in copper-zinc superoxide dismutase cause motor neuron death through one or more acquired toxic properties. An early event in the mechanism of toxicity from such mutants is now demonstrated to be activation of caspase-1. Neuronal death, however, follows only after months of chronic caspase-1 activation concomitantly with activation of the executioner caspase-3 as the final step in the toxic cascade. Thus, a common toxicity of mutant SOD1 is a sequential activation of at least two caspases, caspase-1 that acts slowly as a chronic initiator and caspase-3 acting as the final effector of cell death.
Amyotrophic lateral sclerosis (ALS) is a paralytic disorder caused by selective death of motor neurons. From 2 to 3% of cases are caused by mutations in the gene encoding the free radical scavenging enzyme, Cu,Zn superoxide dismutase (SOD1) (1). More than 70 different mutations in SOD1 are known. That mutant SOD1 triggers the disease through one or more toxic properties (2) is suggested by the dominant inheritance pattern in familial ALS and the observation that SOD1-null mice do not develop motor neuron disease (3). Moreover, transgenic mice expressing three different familial ALS-linked mutants in SOD1 develop motor neuron disease despite elevated (4, 5) or unchanged (6) SOD1 activity, whereas neither onset age nor rapidity of disease progression correlates with SOD1 activity in patients (7).
One possible element in SOD1-mediated neuronal death is apoptosis, or programmed cell death, in which the cell activates a preprogrammed, intracellular suicide machinery. The central components in this pathway are caspases, cysteine proteases with aspartate specificity (8). These proteases are translated as inactive pro-enzymes that are activated after cleavage at specific aspartate residues. Once activated, they cleave other selected intracellular targets including caspases, leading to an amplified cell death cascade.
Caspases are divided into two major subgroups: upstream (initiator) caspases that initiate the proteolytic cascade and downstream (effector) caspases, such as caspase-3, that kill the cell by cleaving specific intracellular targets (9). After a death stimulus, upstream caspases are directly recruited by ligand-bound death receptors (e.g., fas, tumor necrosis factor receptor) or, as in the case of caspase-9, activated intracellularly by a caspase-activating factor (9). These events trigger activation of downstream caspases, processing of the effector caspases and cell death.
For neuronal cells in the high oxygen environment of cell culture, mutant SOD1 protein is pro-apoptotic after withdrawal of trophic support (10–12) in contrast to native wild-type (wt) SOD1, which is anti-apoptotic (10, 13, 14). In mice that develop motor neuron disease from expression of one ALS-linked SOD1 mutant, postnatal elevation of the anti-apoptotic gene Bcl-2 delays onset of motor neuron disease and prolongs survival without affecting disease duration (15). Expression of a mutant caspase-1 that dominantly inhibits caspase-1 activity also slows disease progression (16). Recently, Li et al. (17) demonstrated activation of caspases-1 and -3 in the G93A mice and showed that treatment with the broad caspase inhibitor zVAD-fmk (z-benzyloxycarbonyl, fmk = fluoromethyl ketone) delays disease onset.
Altered expression of some members of the Bcl-2 family has been found in affected regions of symptomatic G93A mice (18). In human ALS tissues, Bcl-2 and BAX mRNA levels were generally decreased and increased respectively (19, 20). Further, in spinal cords of transgenic ALS mice carrying the G37R and G85R mutations, caspase-1 is proteolytically processed (21), although how this relates to the timing of disease onset or progression is unknown. Additionally, in differentiated mouse neuroblastoma N2a cells, all three SOD1 mutants examined provoked caspase-1 activation and release of active IL-1β following oxidative challenge (21).
Despite this evidence, doubt about an apoptotic cell death mechanism in the G93A mice has emerged (22). Furthermore, the slow cell death process in ALS mouse models, where pathology occurs months before motor neuron loss, contrasts strikingly with the very rapid caspase-dependent cell suicide pathways in other contexts. We now demonstrate that lines of mice that develop ALS-like disease by expressing any of three SOD1 mutants (including G93A) activate caspase-1 as an early event in the motor neuron death. This is temporally followed by activation of caspase-3 specifically in affected regions simultaneously with the appearance of apoptotic neurons and astrocytes. These findings indicate that a toxic cascade common to ALS-mutant SOD1 proteins is the sequential activation of at least two caspases, caspase-1 that acts slowly as a chronic initiator, and caspase-3 acting as the final effector of cell death.
Materials and Methods
N2a Cell Culture.
N2a cell cultures were grown as described (21). At 6 days after differentiation, cells were incubated with xanthine/xanthine-oxidase (X/XO; 100 μM-10 milliunits/ml) as described (21). When Ac-YVAD-CMK and Ac-YVAD-CHO (Bachem; CMK = chloromethylketone, CHO = aldehyde derivative) were used, the cells were preincubated for 1 h with the inhibitor before X/XO addition.
Western Immunoblots.
N2a cells or tissues from transgenic mice were lysed in Hepes buffer (pH 7.6) containing 40 mM KCl, 5 mM MgCl2, 1% sodium lauryl sulfate salt (SDS), 1 mM EGTA, and 1 mM EDTA with protease inhibitors. Proteins (30 μg/lane) were electrophoresed and blotted to poly(vinyldene difluoride) membrane. Blots were probed with anti-human SOD1 (Calbiochem), rabbit anti-caspase-1 active fragment (Santa Cruz or Biosource), or a rabbit anti-activated caspase-3 p20 (CM1; IDUN Pharmaceuticals, La Jolla, CA) antibodies and visualized by using chemilumenescence detection (Amersham).
Caspase Activity Assay.
N2a cells were lysed in buffer containing 10 mM Tris⋅HCl, 10 mM NaH2PO4/NaHPO4 (pH 7.5), 130 mM NaCl, 1% Triton X-100, and 10 mM NaPPi with protease inhibitors. Equal amounts of lysates were incubated for 1 h at 37°C with 200 μl of Hepes buffer with either Ac-YVAD-AMC or Ac-DEVD-AMC (PharMingen; AMC = 7-amino-4-methylcoumarin) to measure caspase-1 and -3, respectively. Fuorescence of the free AMC fluorophore was measured using a Fluo-star BMG fluorometer (excitation and emission wavelengths of 380 nm and 420 nm).
Immunofluorescence and Immunohistochemistry.
Mice were transcardially perfused and postfixed overnight with 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.6). Paraffin-embedded spinal cord sections were deparaffinzed and incubated with the anti-activated caspase-3 (CM1), anti-glial fibrillary acidic protein (GFAP; Research Diagnostics, Flanders, NJ), or the anti-nonphosphorylated neurofilament (SMI32, Sternberger Monoclonals, Baltimore, MD) antibodies. The peroxidase-antiperoxidase method (Vector Laboratories) was used to visualize immunoreactivity. For double immunofluorescence, deparaffinized spinal cord sections were incubated in primary antibodies and incubated with either FITC or Texas Red conjugated secondary antisera. DAPI was used to stain nuclei. Deconvolution microscopy was performed using Applied Precision delta vision digital deconvolution software.
Tissue Preparation for Axon Counting and Electron Microscopy.
Mice were perfused and postfixed overnight in 0.1 M sodium phosphate (pH 7.6). Samples were treated with 2% osmium tetroxide, washed, dehydrated, and embedded in Epon-araldite resin. Thick sections (0.75 μm) for light microscopy were stained with toluidine blue. Axons were counted from the L5 ventrals root of three to five mice from each genotype and age. Thin sections (70 nm) for electron microscopy were stained with uranyl acetate/lead citrate.
Results
Caspase-1 Activation Is a Very Early Event in Mutant SOD1 Toxicity.
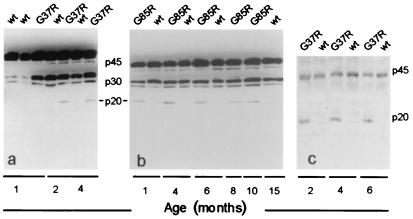
To extend our earlier evidence for caspase-1 cleavage in spinal cords of SOD1 mutant mice at endstage disease (21), we sought to determine the timing of caspase-1 activation by assaying for cleavage of this caspase in G37R (line 42) and G85R (line 148) mice at different stages of disease. Immunoblotting revealed early appearance of the caspase-1 p20 active fragment in spinal cord extracts of both lines of mice. In the G37R mice (Fig. 1 a and c), the fragment was seen by 2 months, approximately 3–4 months before disease onset. In the G85R mice (Fig. 1b), this fragment appeared as early as 1 month, ≈10–11 months before clinical disease and before neuronal death could be detected in spinal cord sections or motor roots (6). In both lines of mice, the caspase-1 cleavage product was present throughout the disease, whereas no active fragment was detected in mice overexpressing wt hSOD1 by approximately sixfold (Fig. 1c), even late in life (15 months, Fig. 1b). Thus, caspase-1 activation is abundant long before neuronal death and/or phenotypic onset.
Figure 1.
Caspase-1 cleavage appears early in the lifespan of G37R- and G85R-transgenic mice. Immunoblots of spinal cord extracts from G37R (a) and G85R (b) mice probed with a polyclonal antibody against both the inactive (p45) pro-enzyme and the active (p20) caspase-1. In mice over-expressing the wt human SOD1 (hSOD1) protein, the antibody only recognized the p45 inactive and the p30 intermediate form. (c) Caspase-1 cleavage in G37R mice detected with the polyclonal antibody used for the cell culture experiments. wt indicates mice overexpressing wt hSOD1.
Caspase-3 Is Activated in Motor Neurons Late in the Course of Mouse ALS.
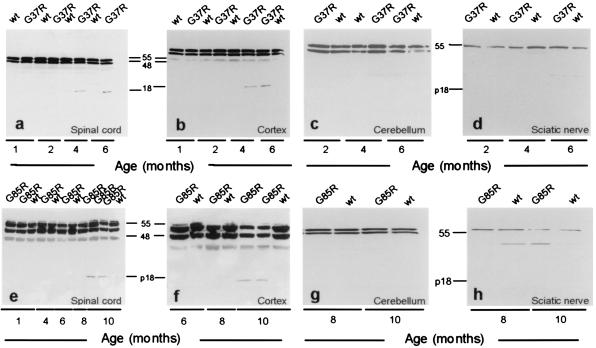
That the timing of caspase-1 activation is not closely correlated with the phenotypic onset of disease in SOD1 mutant mice suggested that either caspase-1 activation is not sufficient to induce motor neuron cell death or that within motor neurons chronic caspase-1 activation represents only an initial phase of a cell death response whose kinetics are much slower than that observed in the typical developmental or in vitro cell culture models. To test whether cell death arose from sequential activation of an initiator caspase (caspase-1) ultimately triggering activation of the executioner caspase-3, extracts from spinal cords of G37R, G85R, and littermate control mice were immunoblotted for caspase-3 activation by using the CM1 antibody specific for the p20-p18 active fragment (23–25). Activation of caspase-3 was detected in the spinal cords and brain cortices of both mouse lines ≈2 months before the onset of hindlimb weakness (Fig. 2 a, b, e, and f). In the G37R mice (Fig. 2 a and b), activated caspase-3 first appeared at 4 months, increasing in abundance by 6 months, concomitant with the onset of clinical disease. In the G85R mice for which no pathology is observable before 10 months (6), the active caspase-3 fragment was not detected on immunoblots before 10 months but was observable at later ages (Fig. 2 e and f) when weakness began. No active fragments were seen in the nontransgenic littermate controls at any age. This activation of caspase-3 was selective for regions undergoing neurodegeneration: the p18 fragment was detected in spinal cord and cortex (Fig. 2 a, b, e, and f) but not cerebellum or sciatic nerve (Fig. 2 c, d, g, and h).
Figure 2.
Caspase-3 is activated late in mutant SOD1-transgenic mouse spinal cord and cortex but not in cerebellum or sciatic nerve. Immunoblots of spinal cord and cortex extracts from G37R (a and b) and G85R (e and f) mice probed with the CM1 antibody. No immunostaining is detected early in life. Staining for the p18 fragment appears at 4 months in the G37R mice and at 10 months in the G85R mice but not in littermate nontransgenic controls (wt). Activation of caspase-3 is tissue-specific; no active fragment appears in cerebellum or sciatic nerve of either G37R (c and d) or G85R (g and h) mice. Each gel was loaded with 30 μg/lane.
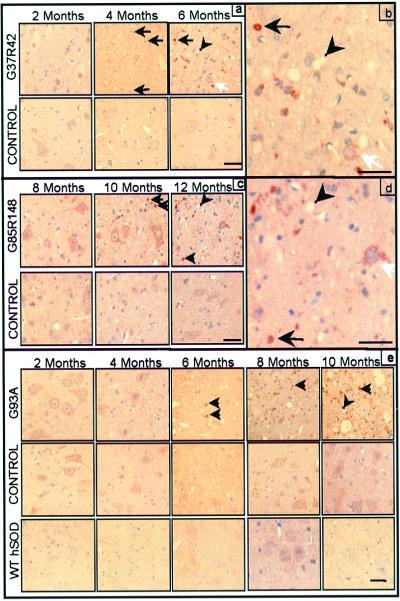
Immunocytochemistry with the CM1 antibody defined the identities of cells activating caspase-3 in mutant G37R and G85R mouse spinal cords. At 2 months, G37R mutant spinal cords contained vacuoles but no detectable activated caspase-3 (Fig. 3a). By 4 months of age, caspase-3 immunoreactivity was present as small dots, at a time that degenerating axons were visible by examination of motor roots (≈2 months before the onset of weakness). By 6 months of age, when most motor neurons have been lost and the mice are clinically affected, caspase-3 activation was greatly increased in remaining neurons and in small cells with caspase-3-positive, round inclusions. In addition, caspase-3-positive debris not associated with cells became more abundant (Fig. 3b), probably representing degenerating cells or phagocytic cells that have engulfed cell remnants. Similarly, G85R mutant spinal cords displayed no activated caspase-3 through 8 months, but by 10 months (≈2 months before onset of clinical disease), several small caspase-3-positive bodies were observed (Fig. 3c). By endstage, more activated caspase-3-positive debris was present and the few surviving neurons contained cleaved caspase-3 (Fig. 3d). Activated caspase-3 was not detected in nontransgenic littermate control mice from any line or in age-matched mice overexpressing wt hSOD1 (Fig. 3 a, c, and e).
Figure 3.
Caspase-3 immunoreactivity appears ≈2 months before onset and increases with age in the spinal cord anterior horn of all mutant SOD1 mouse lines tested. Immunostaining for active caspase-3 (brown precipitate) in G37R (a and b) and G85R (c and d) mice and their nontransgenic littermate controls. (b and d) Increased magnification of a and c (end stage). (e) Active caspase-3 immunoreactivity in G93A mouse, nontransgenic littermate control, and wt hSOD1-expressing mouse spinal cord. Black arrows indicate active caspase-3-positive inclusions. White arrows indicate caspase-3-positive motor neurons. Arrowheads indicate vacuoles. (Bars = 25 μm.)
To further test the generality of caspase-3 activation just before disease onset, spinal cords from mice that develop motor neuron disease from another ALS-linked SOD1 mutation G93A (low expressing line; ref. 26) also were examined with the anti-activated caspase-3 antibody. We could not confirm an earlier report of appearance of caspase-3 activation diffusely within the cytoplasm of most motor neurons before phenotypic onset in a G93A line with earlier disease onset (17). Rather, caspase-3 activation was first visible in a few motor neurons at 6 months of age, contemporaneous with phenotypic onset, and the proportion of motor neurons stained for caspase-3 increased by 10 months (Fig. 3e). Activated caspase-3 was not uniform throughout the neuronal cytoplasm but rather was characterized by a mixture of diffuse cytoplasmic staining in a few neurons as well as positive inclusions in others and/or surrounding debris as seen in the G37R and G85R mice. Overall, the temporal pattern of specific caspase-3 activation detected in all three of the SOD1 mouse models of ALS matched the timing of motor neuron loss and phenotypic onset.
Caspase-1 Activation Precedes and Caspase-3 Activation Coincides with Loss of Large Motor Axons in Mice with Different SOD1 Mutations.
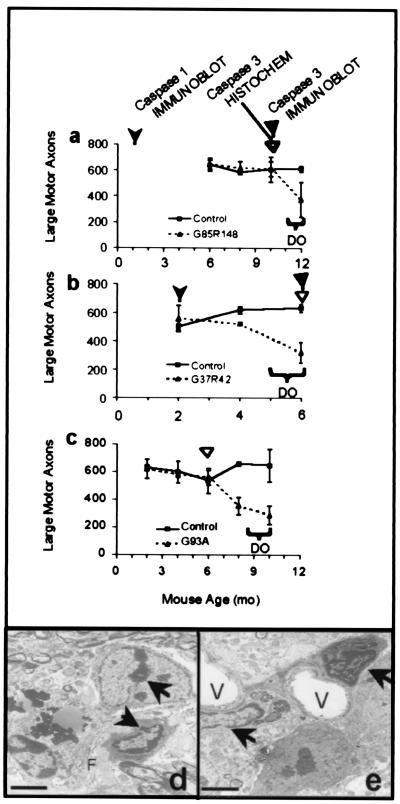
To determine how the timing of caspase-1 and- 3 activation relates to the death of motor neurons, L5 motor axons were counted from SOD1 mutant mice at several times. This analysis, in combination with immunoblotting and immunocytochemistry assays, revealed that the onset of motor axon loss occurred much later than the appearance of activated caspase-1 and was coincident with or just after the earliest detected activated caspase-3. For G85R mice that developed clinical weakness only in the last 2–4 weeks of life, loss of large motor axons could not be detected before 12 months of age (Fig. 4a). By contrast, caspase-1 activation was found by immunoblotting as early as 1 month (Fig. 1b), and caspase-3 activation was detectable at 10 months [by immunohistochemistry and immunoblot (Figs. 3c and 2e)].
Figure 4.
Caspase-1 and caspase-3 activation precedes loss of large motor axons and the appearance of apoptotic morphology in all mutant SOD1 mouse lines tested. Axon counts from the L5 motor root of the spinal cord for G85R (a), G37R (b), and G93A (c) mice (dotted lines) and their littermate nontransgenic controls (solid lines) at various ages. The appearance of activated caspase-1 in immunoblot is indicated by the leftmost arrowhead in a and b. The appearance of activated caspase-3 in immunoblot is indicated by solid triangles. The appearance of activated caspase-3 in immunohistochemistry is indicated by open triangles in a, b, and c. Axon counts are averages from three to five animals of each genotype and age. Horizontal brackets indicate the age of disease onset (DO). Error bars are the SD of the data. (d and e) Electron microscopy images show apoptotic changes within the ventral horn of 8-mo-old G93A-mutant mice. Arrows indicate condensed chromatin within nuclei. Vacuoles are marked with a V. Filaments within neurons are indicated with an F. (Bars in d and e = 2 μm.)
The G37R mice experienced a significant loss of large motor axons by 4 months, 2 months after caspase-1 activation and concomitant with caspase-3 activation (Fig. 4b). Mice with the G93A mutation first exhibited caspase-3 activation at 6 months, whereas a significant loss of large motor axons was not evident until 8 months (Fig. 4c). These data are consistent with a sequence involving early activation of caspase-1 followed much later by caspase-3 activation that occurs just before or coincident with significant axon loss and clinical disease.
Electron microscopy of tissues demonstrated that caspase-3 activation was accompanied by ultrastructural evidence of apoptosis. The anterior horn region of spinal cords from G85R, G37R, and G93A mice all contained cells with classic apoptotic features (shown in Fig. 4 d and e for G93A) including chromatin condensation (arrows) and cell shrinkage with relative preservation of subcellular organelles (27). Chromatin condensation was evident in cells at least as early as 10 months in G85R mice, 4 months in G37R, and 8 months in G93A mice, ages when caspase-3 is activated and motor axons begin to die in the respective lines. Nontransgenic littermate control mice did not contain observable apoptotic cells.
Activated Caspase-3 Appears Within Neurons and Glia of SOD1 Mutant Mouse Spinal Cords.
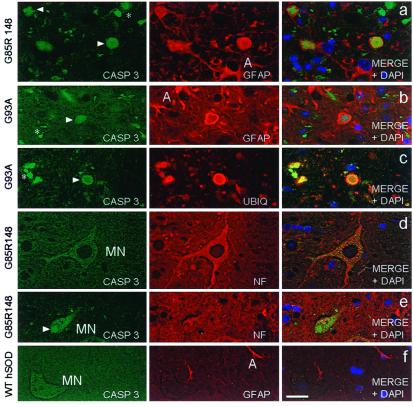
To determine the identity of cells containing activated caspase-3, double-immunofluorescence was used to stain simultaneously for the active fragment of caspase-3 and markers of either neurons (anti-neurofilament) or astrocytes (GFAP). This revealed that in the anterior horn region of G85R (Fig. 5a) and G93A mutant mice (Fig. 5b), activated caspase-3 was present within inclusions circumscribed by GFAP, demonstrating either that the inclusion is within glial cells (28) or that glial processes envelop extracellular inclusions. These round inclusions (Fig. 5c) were identical to the Lewy-body-like, ubiquitin- and SOD1-positive inclusions described previously both in G85R (6, 28) and G93A mice (26) and in some examples of SOD1-mutant mediated human ALS (28, 35). Because electron microscopy of these earlier examples has demonstrated inclusions clearly within astrocyte cell bodies in mice and humans (6, 35), we infer that at least some of these caspase-3 aggregates must be within dying astroctyes. Several of the few motor neurons remaining at endstage disease contained activated caspase-3 (Fig. 5 d and e). The caspase-3 staining appeared in a punctate pattern within the cell body and proximal processes of motor neurons and stained neuronal inclusions intensely (Fig. 5e). A significant proportion of activated caspase-3 was found in smaller, irregular patches that did not colocalize with GFAP or anti-neurofilament (Fig. 5 a, b, c, and e). This immunoreactivity did not appear to be associated with any cell and may represent extracellular aggregates or debris within phagocytic cells. For G37R mice, which lack prominent Lewy body-like astrocytic inclusions, caspase-3 activation solely localized within neurons and debris (not shown). In spinal cords of nontransgenic littermate controls or in mice expressing wt hSOD1 (Fig. 5f), activated caspase-3 was not detected.
Figure 5.
Activated caspase-3 appears within the neuronal and glial inclusions present in mutant SOD1 mouse spinal cords. Double immunofluorescence of spinal cord anterior horn using CM1 antibody (Casp 3 in the Left panels) and either GFAP antibody to stain astrocytes, ubiquitin antibody (UBIQ) to stain inclusions, or an antibody recognizing nonphosphorylated neurofilaments (NF) to visualize neurons. Right panels depict the merged image from the first two channels with DAPI to show the position of nuclei. Activated caspase-3-positive inclusions (arrowheads) within astrocytes (A) and/or extracellular debris (*) are common features within mutant G85R (a) and G93A-mutant (b) spinal cords. (c) Activated caspase-3 colocalizes with ubiquitin-positive inclusions in G93A-mutant mice. Activated caspase-3 immunoreactivity is present within motor neurons (MN) (d) and motor neuron inclusions (e) (arrow) of mutant G85R mice. (f) Activated caspase-3 is absent from 10-mo-old wt hSOD1-transgenic mice whereas astrocytic GFAP staining is normal. (Bar = 12.5 μm.)
Thus, from the timing of appearance of active caspase-3 and its known properties as an executioner protease (9), activation of caspase-3 appears to be the final step in the toxicity of familial ALS mutants of SOD1.
An in Vitro Temporal Cascade of Caspase-1 and -3 from Mutant SOD1.
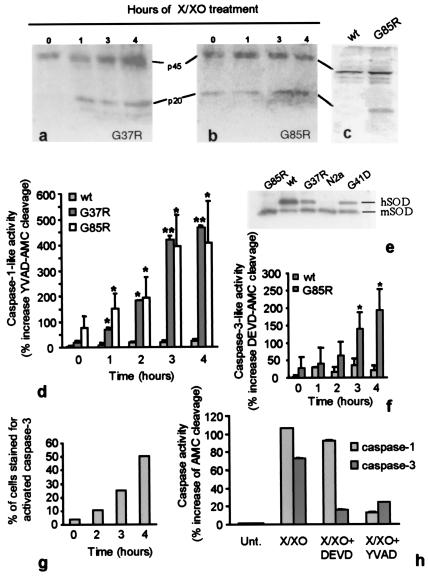
Caspase-1 has previously been shown to be activated in neuroblastoma cells expressing human mutant but not wt hSOD1 following differentiation into neurons induced by serum withdrawal and an additional oxidative stress from treatment with X/XO (21). To test if neuronal death arose from a cascade of caspase-1 activation followed by caspase-3 activation, the activities of both were determined in a set of N2a cells stably expressing wt hSOD1 (three times the endogenous mouse SOD1 level) or any of three mutants (G37R, G41D, or G85R) to levels equivalent to mouse SOD1 (Fig. 6e). Every hour for 4 h, cells were harvested and caspase-1 activation detected by protein immunoblot using the antibody recognizing the p20 active fragment of caspase-1. Activation was seen within 1 h after X/XO treatment in G37R-expressing cells (Fig. 6a), whereas in the G85R expressing cells, the active p20 fragment was chronically present even without X/XO treatment (Fig. 6b). In a third line of cells expressing the ALS related G41D mutation, the cleaved p20 fragment also was detected before X/XO treatment and increased within 1 h (not shown). By contrast, cells expressing the wt hSOD1 protein did not show evidence of the p20 product even after 4 h of X/XO treatment (Fig. 6c).
Figure 6.
Caspase-1 is activated early in differentiated mouse neuroblastoma N2a cells. Immunoblots showing caspase-1 activation in differentiated G37R (a) or G85R (b) positive N2a exposed to X/XO for 0, 1, 3, or 4 h. (c) Immunoblot of lysates from wt hSOD1-expressing cells (left) or G85R-positive cells (right) 4 h after X/XO treatment. (d) YVAD-AMC cleavage was measured as described and is reported relative to the cleavage induced by lysates of untreated (time 0) wt hSOD1-positive cultures. Data are the mean ± SD of three independent experiments for the G85R-positive cells and of two independent experiments assayed in duplicate for the G37R and wt hSOD1 expressing cells. Asterisks (*, P < 0.05; **, P < 0.01) indicate significant differences between groups. (e) Immunoblot showing levels of wt and mutant SODs in N2a cell lines by using an antibody that recognizes human and mouse SOD1 equally. The hSOD1 protein migrates more slowly than the mouse, only the G85R mutant comigrates with the mouse SOD1. (f) Caspase-3 activity recorded as DEVD-AMC cleavage by cell lysates relative to the cleavage induced by lysates of untreated wt hSOD1-positive cells. Data are the mean ± SD of three independent experiments. Asterisks (*, P < 0.05) indicate a statistical difference between groups. (g) Percentage of cells that stained positive for activated caspase-3 at different times after X/XO treatment. (h) YVAD-AMC cleavage and DEVD-AMC cleavage measured in lysates from G85R cells treated with X/XO in the presence of caspase-1-and-3 inhibitors. Data are the mean ± SD of six replicates. Fluorescence emitted from the fluorogenic substrate is expressed relative to that emitted from untreated cells.
Use of the substrate Ac-YVAD-AMC, which is preferentially cleaved by caspase-1 or caspase-1-like proteases (25) and which yields a fluorescent cleavage product, confirmed that the early and rapid appearance of the caspase-1 cleavage product was accompanied by a corresponding increase in caspase-1 like activity (Fig. 6d). For the G85R mutant, an elevated level was detected even without X/XO treatment. As early as 1 h after treatment with X/XO, there was a significant increase in caspase-1 activity in both mutant expressing cells with maximal increase detected by 3 h of X/XO treatment, whereas no changes in caspase-1 activity were detected in wt hSOD1-expressing cells (Fig. 6d).
Caspase-3-like activity in the G85R-expressing cells was monitored with the caspase-3-like protease specific Ac-DEVD-AMC fluorogenic substrate. Significant increases in caspase-3 activity were detected by 3 h of X/XO treatment, 2 h after the increase in caspase-1 activation was first observed in the previous experiment (Fig. 6f). The caspase-3 inhibitor Ac-DEVD-CHO completely blocked the X/XO-mediated DEVDase activity (not shown). This was confirmed by immunocytochemistry: despite chronic, partial caspase-1 activation (Fig. 6 b and d), the proportion of G85R-expressing N2a cells with detectable activated caspase-3 escalated markedly after imposition of oxidative stress (Fig. 6g). Counting the number of cells containing activated caspase-3 revealed a sizable loss of cells over the 4-h period after X/XO treatment, with ≈50% of those cells remaining contained the active caspase-3 fragment (Fig. 6g). Similar results were obtained for the G37R and G41D-expressing cells. In contrast, activated caspase-3 was always absent in the wt hSOD1-expressing cells.
The selective caspase-3 inhibitor Ac-DEVD-CHO (100 μM) yielded almost complete blockage of caspase-3 activity after imposition of oxidative stress, with no effect on caspase-1 (Fig. 6h). The caspase-1 and caspase-1-like protease inhibitor Ac-YVAD-CMK blocked activation of both caspases, as expected (Fig. 6h). These results confirm that caspase-1 activation is independent of caspase-3 activity, whereas the sequential order of caspase activation suggests that mutant SOD1-dependent increases in caspase-3 activity depend on previous activation of caspase-1.
Discussion
This study provides evidence for a sequential cascade of caspase activation in mutant SOD1-mediated ALS both in an in vitro cell culture model and in transgenic mouse models of ALS. In mutant SOD1 mice, caspase-1 is activated early, months before neuronal death, and in vitro, activation occurs soon after oxidative stress. Caspase-3 activation occurs after caspase-1 activation both in vitro and in vivo. In all three lines of SOD1 mutant expressing mice, caspase-3 activation appears at or just before the onset of motor axon loss and the appearance of apoptotic morphology. This sequential caspase activation only occurs in regions affected by neurodegeneration in ALS. This proteolytic cascade is shared by SOD1 mutants that provoke quite diverse pathologies in the various ALS mouse models. Li et al. (17), reported that both caspase-1 and -3 are activated in one line of ALS transgenic mice and that intrathecal administration of the pan-caspase inhibitor zVAD-fmk prolongs the life of these mice by ≈25%. Our data extend those findings in four important directions.
First, whereas Li et al. (17) did not resolve the timing of activation of the two caspases, we have clearly established that caspase-1 is activated months before activation of caspase-3. The protracted time course of caspase-1 activity, essentially throughout the adult life of these mice, raises several hypotheses concerning mechanisms of caspase-1-dependent motor neuron toxicity. These include a possible interplay between inflammation and apoptosis, and a role for caspase-1 as an early mediator of cell death, because caspase-1 is responsible for the activation of executioner caspases during the progression of apoptosis. Each mechanism can now be assessed.
Second, we also have documented that the sequential activation of caspase-1 and -3 is induced by oxidative stress in a mutant SOD1-dependent manner in vitro. Within a time course that is compressed in vitro, this strikingly recapitulates the activation pattern in the mouse spinal cord. These observations suggest that specific upstream caspases activate other downstream effector caspase family members within a preexisting apoptotic caspase signaling pathway.
Third, the close temporal relationship between caspase-3 activation and cell death that we demonstrate in vivo and in vitro firmly argues that this downstream executioner caspase mediates the death of motor neurons in ALS. Indeed, this is supported by prior evidence that caspase-3 is one of the key effector caspases in mammalian apoptosis. Caspase-3-deficient mice have distinctive developmental abnormalities, such as brain enlargement, that arise because neuronal apoptotic death is defective in the absence of caspase-3 (29). Unlike the other major effector caspase, caspase-7 (30), caspase-3 is prominently expressed in multiple regions of the brain (25).
Fourth, caspase-3 activation is especially prominent within astrocytes of mutant G85R and G93A mice. In light of the dependence of motor neurons on astrocytes to prevent excitotoxicity by rapid recovery of synaptic glutamate by the EAAT2 glutamate transporter (31, 32), it is possible that astrocytes are themselves direct targets for SOD1-mediated toxicity and that the resulting impairment of astrocyte function hastens disease progression. It may be relevant that astrogliosis is common in ALS spinal cord and that glial inclusions containing wt and mutant SOD1 are found in G93A mice (28), G85R mice (6), and some examples of SOD1-mediated human disease.
How might caspase-1 participate in this disease? In several systems, caspase-1 is essential in inflammation and/or in pathological apoptotic death, in the latter instances acting as both activator and effector caspase depending on the cell death stimulus. One possibility is that caspase-1 activation contributes to an inflammatory pathway causing early astrocytosis in mutant SOD1 mice. Beyond this, caspase-1 in vitro directly cleaves and activates the effector pro-caspases-3 and -7, and to a lesser extent pro-caspase-6 (23, 33), whereas in Fas-induced apoptosis, caspase-3 activation may depend on caspase-1-like activity (34). A key question in the ALS mice is whether caspases-1 and -3 are activated within the same cells. In our mutant SOD1 mouse model of ALS, caspase-1 activation occurs before activation of caspase-3 and active caspase-3 is found both in motor neurons and astrocytes, strongly suggesting that in at least one of these cell types the proteolytic cascade of caspase activation occurs within a single cell. However, using G93A, G37R, or G85R mice, we have been unable to confirm a report that an antibody selectively recognizing activated caspase-1 colocalizes with activated caspase-3 (17). Thus, our best current evidence for a temporal cascade within the same cells arises from our neuronal cell culture model, in which caspase-1 is clearly activated before activation of caspase-3 and the resultant cell death follows sequential activation of the caspases within the same cells. It remains to be established whether caspase-3 is directly cleaved by caspase-1.
The appearance of activated caspase-1 in these ALS-mutant mice months before neuronal loss suggests there is a graded response: some threshold of caspase-1 activity is required to trigger the downstream death cascade. Consistent with this view, we have demonstrated that chronic, partial activation of caspase-1 in N2a cells expressing the G85R mutation does not provoke rapid cell death. Thus, unlike developmental apoptosis in which cell death ensues rapidly (within hours) after initial caspase activation, in spinal cords with mutant SOD1 there is a preapoptotic state in which caspase-1 remains activated for months before activation of the effector caspase-3. Traditional concepts of the mechanisms and timing of apoptosis may therefore require revision in the context of a complex neurodegenerative death process evolving over months or years. A detailed analysis of these mechanisms may have broad importance for understanding the biology of neural cell death.
Acknowledgments
We thank Dr. Borchelt for the N2a cells, Dr. Feramisco for help with deconvolution microscopy, Ms. Folmer for EM sectioning, Mr. Ward for some of the mice, Dr. Srinivasan for CM1 antibody, and Drs. Przedborski and Trotti for discussions. This work was supported by the Amyotrophic Lateral Sclerosis Association, the Pierre L. Bourgknecht ALS Foundation and Project ALS (to R.H.B.), Muscular Dystrophy Association (to R.H.B. and D.W.C.), National Institutes of Health Grants 1PO1NS31248–02 and 5F32H10064 (to R.H.B.) and NS R01 27036 (to D.W.C.), and Telethon Italia (to P.P.).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD1
CU,Zn superoxide dismutase
- wt
wild-type
- GFAP
glial fibrillary acidic protein
- hSOD1
human SOD1
- fmk
fluoromethyl ketone
- CMK
chloromethyl ketone
- CHO
aldehyde derivative
- AMC
7-amino-4-methylcoumarin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240305897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240305897
References
- 1.Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan J P, Deng H X, et al. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Cleveland D W. Neuron. 1999;24:515–20. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 3.Reaume A, Elliott J, Hoffman E, Kowall N, Ferrante R, Siwek D, Wilcox H, Flood D, Beal M, Brown, et al. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 4.Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H-X, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 5.Wong P, Pardo C, Borchelt D, Lee M, Copeland N, Jenkins N, Sisodia S, Cleveland D, Price D. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 6.Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, et al. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 7.Cleveland D W, Laing N, Hurse P V, Brown R H., Jr Nature (London) 1995;378:342–343. doi: 10.1038/378342a0. [DOI] [PubMed] [Google Scholar]
- 8.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 9.Cryns V, Yuan J. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 10.Rabizadeh S, Gralla E, Borchelt D, Gwinn R, Valentine J, Sisodia S, Wong P, Lee M, Hahn H, Bredesen D. Proc Natl Acad Sci USA. 1995;92:3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghadge G, Lee J, Bindokas V, Jordan J, Ma L, Miller R, Roos R. J Neurosci. 1997;17:8756–8766. doi: 10.1523/JNEUROSCI.17-22-08756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durham H, Roy J, Dong L, Figlewicz D. J Neuropathol Exp Neurol. 1997;56:523–530. doi: 10.1097/00005072-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Greenlund L, Deckwerth T, Johnson E. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 14.Jordan J, Ghadge G, Prehn J, Toth P, Roos R, Miller R. Mol Pharmacol. 1995;47:1095–1100. [PubMed] [Google Scholar]
- 15.Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- 16.Friedlander R M, Brown R H, Gagliardini V, Wang J, Wang J. Nature (London) 1997;388:31. doi: 10.1038/40299. (lett.). [DOI] [PubMed] [Google Scholar]
- 17.Li M, Ona V, Guegan C, Chen M, Jackson-Lewis V, Andrews L, Olszewski A, Stieg P, Lee J-P, Przedborski S, et al. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 18.Vucosavic S, Dubois-Dauphin M, Romero N, Przedborski S. J Neurochem. 1999;73:2460–2468. doi: 10.1046/j.1471-4159.1999.0732460.x. [DOI] [PubMed] [Google Scholar]
- 19.Mu X, He J, Anderson D, Trojanowski J, Springer J. Ann Neurol. 1996;40:379–386. doi: 10.1002/ana.410400307. [DOI] [PubMed] [Google Scholar]
- 20.Tews D, Goebel H, Meinck H. Acta Neurol Scand. 1997;96:380–386. doi: 10.1111/j.1600-0404.1997.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 21.Pasinelli P, Borchelt D R, Houseweart M K, Cleveland D W, Brown R H., Jr Proc Natl Acad Sci USA. 1998;95:15763–15768. doi: 10.1073/pnas.95.26.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, Bendotti C. Nat Med. 1999;5:966–967. doi: 10.1038/12381. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson D, Ali A, Thornberry N, Vaillancourt J, Ding C, Gallant M, Gareau Y, Griffin P, Labelle M, Lazebnik Y, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 24.Erhardt P, Cooper G M. J Biol Chem. 1996;271:17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 25.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli K J, Yuan J, Moskowitz M A. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Canto M C, Gurney M E. Acta Neuropathol. 1997;93:537–550. doi: 10.1007/s004010050650. [DOI] [PubMed] [Google Scholar]
- 27.Desjardins P, Ledoux S. Metab Brain Dis. 1998;13:79–96. doi: 10.1023/a:1020605112755. [DOI] [PubMed] [Google Scholar]
- 28.Brujin L I, Williamson T L, Becher M W, Price D L, Cleveland D W. In: Amyotrophic Lateral Sclerosis. Brown R H, Swash M, Meininger V, editors. London: Dunitz; 1999. pp. 341–362. [Google Scholar]
- 29.Kuida K, Zheng T S, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 30.Juan T S, McNiece I K, Argento J M, Jenkins N A, Gilbert D J, Copeland N G, Fletcher F A. Genomics. 1997;40:86–93. doi: 10.1006/geno.1996.4548. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein J, Kammen M, Levey A, Martin L, Kuncl R. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 32.Trotti D, Rolfs A, Danbolt N C, Brown R H, Jr, Hediger M A. Nat Neurosci. 1999;2:848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- 33.de Craen D. Cell Death Differ. 1999;6:1117–1124. doi: 10.1038/sj.cdd.4400589. [DOI] [PubMed] [Google Scholar]
- 34.Enari T, Talanian R V, Wong W W, Nagata S. Nature (London) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 35.Kato S, Hayashi H, Nakashima K, Nanba E, Kato M, Hirano A, Nakano I, Asayama K, Ohama E. Am J Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]