Abstract
Objective: To examine retrospectively the changes in ECG parameters over time and their correlation with other quantitative right ventricular (RV) function parameters in patients with chronic RV pressure overload caused by congenital heart disease.
Methods: 48 patients with chronic RV pressure overload caused by the following congenital heart diseases were studied: nine with congenitally corrected transposition of the great arteries (TGA), 12 with surgically corrected TGA, and 27 with a subpulmonary pressure overloaded RV. QRS duration and dispersion were measured manually from standard ECG recorded twice within five years. RV end diastolic volume (EDV) and RV mass were determined by magnetic resonance imaging. Brain natriuretic peptide (BNP) plasma concentrations were measured.
Results: QRS duration and QRS dispersion increased in all patient groups during the follow up period. QRS duration increased significantly in the congenitally corrected TGA (p = 0.04) and the subpulmonary pressure overloaded RV groups (p = 0.01). QRS dispersion increased significantly in patients with surgically corrected TGA (p = 0.03) and in the subpulmonary pressure overloaded RV group (p = 0.02). A significant correlation was found between QRS duration and RVEDV (r = 0.71, p < 0.0001). RV mass was significantly correlated with QRS duration in patients with tetralogy of Fallot (r = 0.67, p = 0.01). Mean (SD) plasma brain natriuretic peptide concentrations (6.6 (5.4) pmol/l) were increased compared with normal reference values but no correlation was found with ECG parameters or RV systolic pressure. No malignant arrhythmia or sudden death occurred.
Conclusions: ECG parameters worsened gradually in asymptomatic or minimally symptomatic patients with chronic RV pressure overload, regardless of the nature of their congenital heart disease. In all patients, a significant positive correlation was found between QRS duration and RVEDV. In patients with tetralogy of Fallot there was also a correlation between QRS duration and RV mass.
Keywords: congenital heart disease, ECG parameters, magnetic resonance imaging, brain natriuretic peptide
Ventricular arrhythmia is an important cause of death in patients with a chronic pressure overloaded right ventricle (RV) caused by congenital heart disease.1, 2 Stratification of patients at risk of life threatening arrhythmias is therefore mandatory in clinical follow up, ideally by means of sensitive and specific predictive indices, which are easy to obtain in a non-invasive way. Several studies have shown that a QRS complex duration of > 180 ms and increased QRS dispersion on the surface ECG are associated with an increased risk of malignant ventricular arrhythmias in patients with tetralogy of Fallot.3–6 RV failure is another important cause of death in patients with chronic RV pressure overload. Only a few quantitative parameters for RV function are available. Novel techniques of volumetry such as magnetic resonance imaging (MRI) that may overcome the limitations of other imaging techniques for the assessment of RV volume and mass have become available.7, 8
Other quantitative parameters such as plasma neurohormone concentrations are gaining an important role in the early diagnosis of heart failure.9 Few studies have investigated the correlation between RV function and brain natriuretic peptide (BNP) concentrations,10, 11 although recent studies have shown that plasma concentrations of BNP are highly accurate for predicting and detecting left ventricular failure.12, 13 The aim of this study was to examine the clinical significance of ECG parameters and their correlation with other non-invasive quantitative RV parameters in adult patients with chronic RV pressure overload caused by congenital heart disease.
METHODS
Patient population
Forty eight patients with chronic RV pressure overload caused by congenital heart disease in a tertiary referral centre were examined in a retrospective study. The main inclusion criterion was chronic RV pressure overload (RV systolic pressure > 35 mm Hg assessed by echocardiography) caused by congenital heart disease without important additional haemodynamic lesions in asymptomatic or minimally symptomatic patients (New York Heart Association functional class I or II). Patients were divided into three groups according to the nature of the congenital disease. Group 1 comprised nine patients with congenitally corrected transposition of the great arteries (TGA). Group 2 comprised 12 patients with surgically corrected TGA by the Mustard or Senning procedures. Twenty seven patients with a subpulmonary pressure overloaded RV constituted group 3. Group 3 was divided into two subgroups: firstly, 13 patients with tetralogy of Fallot (10 of whom had significant pulmonary regurgitation ranging between 30–50 ml/stroke and three with < 10 ml/stroke) and, secondly, 14 patients with RV pressure overload caused by other congenital heart disease (table 1). The latter group consisted of nine patients with residual pulmonary valve stenosis after valvotomy, four patients with peripheral pulmonary stenosis, and one patient with primary pulmonary hypertension. Patients with irregular rhythm or pacemakers were not included in the study because of MRI technical features: an irregular heart rate has a negative effect on the quality of MRI causing blurred images; and pacemakers and leads can be damaged by the magnetic field or cause internal burns from the magnetic induction.
Table 1.
Clinical characteristics of the study population
| Subpulmonary pressure overloaded RV | |||||
| ccTGA | TGA | Total | TOF | Other* | |
| Number | 9 | 12 | 27 | 13 | 14 |
| Men/women | 4/5 | 7/5 | 13/14 | 5/8 | 8/6 |
| Age (years) | 25.9 (6.1) | 22.8 (3.4) | 28.3 (8.7) | 27.1 (9.8) | 29.4 (7.7) |
| RVSP (mm Hg) (range) | 113.9 (110–125) | 114.4 (90–134) | 56.1 (35–100) | 52.3 (35–76) | 59.8 (35–100) |
| NYHA class I/II | 5/4 | 3/9 | 21/6 | 10/3 | 11/3 |
| SVT/no SVT | 1/8 | 1/11 | 5/27 | 3/10 | 2/12 |
| VT/no VT | 2/7 | 0/12 | 2/27 | 0/13 | 2/12 |
Values are mean (SD).
ccTGA, congenitally corrected transposition of the great arteries; NYHA, New York Heart Association; RV, right ventricle; RVSP, right ventricular systolic pressure; SVT, supraventricular tachycardia; TGA, surgically corrected transposition of the great arteries; TOF, tetralogy of Fallot; VT, ventricular tachycardia.
*Nine patients with residual pulmonary valve stenosis, four with peripheral pulmonary stenosis, and one with primary pulmonary hypertension.
The study design was approved by the institutional ethics committee and informed consent was obtained from all study subjects. Table 1 shows patient population and clinical characteristics.
Electrocardiography
A standard (speed of 25 mm/s and 1 mV/cm standardisation) resting 12 lead ECG was obtained during the patient’s last visit to the outpatient clinic and compared with ECGs from a visit five years previously.
The ECG markers measured and analysed were QRS duration and its interlead dispersion marker. QRS duration was measured manually by one blinded observer and was defined as the maximal QRS length in any lead from the first inflection to the final sharp vector crossing the isoelectric line.3 QRS dispersion was defined as the difference between the maximum and minimum QRS interval occurring in any of the 12 leads.
Magnetic resonance imaging
MRI study was performed at the end of the five year follow up. Study subjects were placed supine in a 1.5 T MRI scanner with high power gradients (Vision, Siemens, Erlangen, Germany). MRI acquisition involved a standardised protocol. Imaging sessions were initiated with scout images to determine the position of the heart in the thoracic cavity. Based on these images, an ECG triggered T1 weighted turbo spin echo series of axial images was acquired. A gradient echo cine sequence was then constructed in a plane bisecting the mitral valve orifice and passing through the apex, visualising the long axis view to locate the atrioventricular valve plane. An ECG triggered ultrafast breath-hold gradient echo cine sequence with the following parameters was then used to acquire images in the short axis plane, in contiguous 10 mm slices encompassing the heart from the valve plane to the apex: repetition time = RR interval, time of echo of 4.8 ms, slice thickness of 10 mm, imaging matrix of 256 × 256, field of view of 350 mm, and flip angle of 20°. End systolic and end diastolic volumes (EDV) were calculated from this multislice multiphase image set.
Image analyses
A Unix workstation was used for analysis of the MRI images. MASS (Medis, Leiden, the Netherlands) image analysis software was used to display multislice, multiphase images individually and in a movie loop mode. EDV (maximal ventricular volume) frames were determined by manual outlining of a mid-ventricular slice in all phases. On end diastolic time frames, endocardial borders of the RV were outlined manually. Papillary muscles and the moderator band were not included in the ventricular volume. The enclosed RV cross sectional areas were measured by computer, multiplied by section thickness, and summed according to Simpson’s rule to provide RV volumes. Total MRI examination time was approximately 45 minutes.
Brain natriuretic peptide
Blood samples were obtained from the antecubital vein of all subjects after they had rested for at least 15 minutes. Blood was collected into chilled tubes containing EDTA and aprotinin (1.9 mg and 100 kIU/ml blood, respectively). The blood samples were promptly centrifuged (3000 rpm for 10 minutes) and stored at −70°C until final analysis. BNP concentrations were determined with immunoradiometric assay kits (Shionoria, Osaka, Japan). Details of our methods have been published previously.10
Rhythm measurements
Twenty four hour Holter recordings were analysed by an experienced cardiac technician. The number and duration of the occurrence of supraventricular (SVT) and ventricular tachycardias (VT) were recorded.
Statistical analyses
Group data are expressed as mean (SD). Student’s t tests were used to compare normally distributed variables. The relation between two factors was ascertained by plotting them against each other and obtaining a linear regression line. The coefficient of correlation (r) was obtained from the slope of this line. A probability value of p = 0.05 was considered significant.
RESULTS
By definition, the follow up period was five years. There were no significant differences in age or sex between the studied patient groups. Holter recording in seven patients showed SVT lasting less than three seconds (one patient with congenitally corrected TGA (six SVTs), one with TGA (one SVT), and five with subpulmonary pressure overloaded RV—three with tetralogy of Fallot (2, 2, and 34 SVTs, respectively) and two with other congenital heart diseases (both one SVT)). Four patients showed non-sustained VT lasting less than three seconds (two patients with congenitally corrected TGA patients (one and two VTs, respectively) and two patients with subpulmonary pressure overloaded RV—both in the other congenital heart disease subgroup (one and two VTs, respectively)) (table 1). Six patients had a QRS duration > 180 ms (one patient with congenitally corrected TGA, one with tetralogy of Fallot, and four in the subpulmonary other congenital heart disease subgroup). One patient with QRS > 180 ms had VT and five patients with QRS > 180 ms had no VT. No malignant arrhythmia or sudden death occurred during the study.
ECG markers
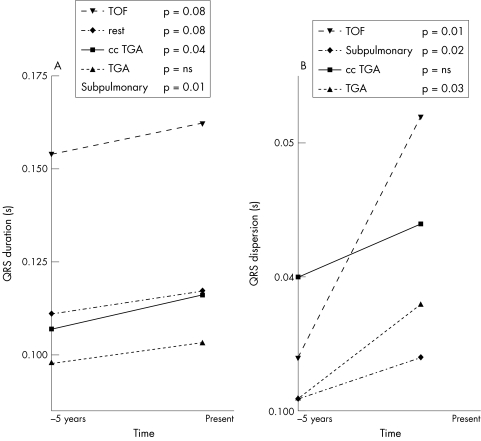
Mean QRS duration and QRS dispersion increased over time in all patient groups (fig 1).
Figure 1.
Changes in ECG markers during follow up in patients with chronic RV pressure overload. (A) Changes in QRS duration. (B) Changes in QRS dispersion. Rest: nine patients with residual pulmonary valve stenosis, four with peripheral pulmonary stenosis, one with primary pulmonary stenosis. Subpulmonary: all patients with tetralogy of Fallot and all patients in the rest group. ccTGA, congenitally corrected transposition of the great arteries; TGA, surgically corrected transposition of the great arteries; TOF, tetralogy of Fallot.
We examined the occurrence of significant changes during the follow up period in duration of QRS intervals or QRS dispersion. QRS duration and QRS dispersion increased in each of the groups during the five years. Significant increases in QRS duration during the follow up period were found in the congenitally corrected TGA group (0.107 (0.033) to 0.116 (0.039) ms, p = 0.04) and in the subpulmonary pressure overloaded RV group (0.130 (0.036) to 0.139 (0.036) ms, p = 0.01; fig 1A). The increase in QRS dispersion was significant in patients with TGA (0.031 (0.010) to 0.038 (0.016) ms, p = 0.03) and in the subpulmonary pressure overloaded RV group (0.032 (0.014) to 0.043 (0.018) ms, p = 0.02; fig 1B).
RVEDV and mass
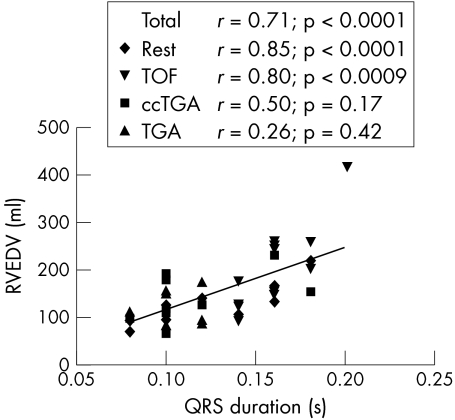
Table 2 summarises RVEDV and RV mass parameters. In all of the groups taken together, there was a significant correlation between QRS duration and RVEDV (r = 0.71, p = 0.0001; fig 2). In the groups taken separately, these factors were significantly correlated in those with tetralogy of Fallot (r = 0.80, p = 0.0009; fig 2) and in the subpulmonary group with other congenital heart diseases (r = 0.85, p < 0.0001; fig 2).
Table 2.
Quantitative parameters in the patient groups
| Subpulmonary pressure overloaded RV | ||||||
| Parameter | Reference value | ccTGA | TGA | Total | TOF | Other* |
| QRS duration (s) | <0.100† | 0.116 (0.033) | 0.103 (0.04) | 0.139 (0.036) | 0.162 (0.019) | 0.117 (0.036) |
| QRS dispersion (s) | 0.023 (0.005)† | 0.047 (0.02) | 0.038 (0.016) | 0.043 (0.018) | 0.052 (0.019) | 0.034 (0.012) |
| Change in QRS duration (s) | 0 | 0.017 (0.015) | 0.006 (0.013) | 0.005 (0.009) | 0.006 (0.010) | 0.005 (0.009) |
| Change in QRS dispersion (s) | 0 | 0.007 (0.024) | 0.013 (0.014) | 0.015 (0.018) | 0.018 (0.018) | 0.015 (0.013) |
| RVEDV (ml) | 141 (51) | 127 (33) | 164 (75) | 205 (84) | 126 (39) | |
| RV mass (g) | 23–68† | 139 (44) | 152 (38) | 83 (35) | 83 (33) | 82 (38) |
| BNP (pmol/l) | 2.3‡ | 8.96 (6.87) | 5.94 (4.03) | 6.09 (5.41) | 7.41 (4.34) | 4.51 (6.34) |
Values are mean (SD).
BNP, brain natriuretic peptide; EDV, end diastolic volume.
*Nine patients with residual pulmonary valve stenosis, four with peripheral pulmonary stenosis, and one with primary pulmonary hypertension;
†Lentner C, Geigy Scientific Tables, 5: Heart and Circulation. Basel: Ciba-Geigy; 1990:63–6, 148–67. ‡Data from reference 10.
Figure 2.
Correlation between ECG markers and right ventricular (RV) end diastolic volume (EDV) in patients with chronic RV pressure overload. Rest: nine patients with residual pulmonary valve stenosis, four with peripheral pulmonary stenosis, one with primary pulmonary stenosis. Subpulmonary: all patients with tetralogy of Fallot and all patients in the rest group. Total: all patient groups together. ccTGA, congenitally corrected transposition of the great arteries; TGA, surgically corrected transposition of the great arteries; TOF, tetralogy of Fallot.
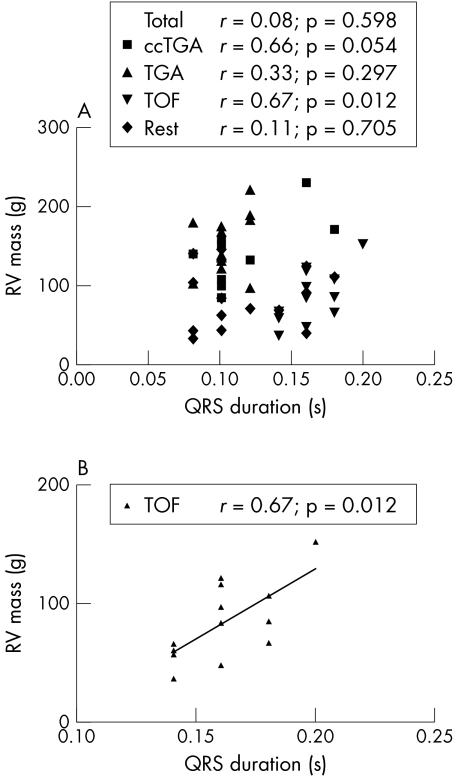
Examining all groups together, there was no correlation between QRS duration and RV mass (fig 3A). When the groups were analysed separately, QRS duration and RV mass were significantly correlated in the tetralogy of Fallot group (r = 0.67, p = 0.01; fig 3B). In patients with tetralogy of Fallot and congenitally corrected TGA, we found a significant correlation between RVEDV and RV mass (r = 0.91, p = 0.001 and r = 0.82, p = 0.007, respectively).
Figure 3.
Correlation between QRS duration and right ventricular (RV) mass in patients with chronic RV pressure overload. (A) All patient groups. (B) Patients with tetralogy of Fallot. Rest: nine patients with residual pulmonary valve stenosis, four with peripheral pulmonary stenosis, one with primary pulmonary stenosis. Total: all patients in the rest group. ccTGA, congenitally corrected transposition of the great arteries; TGA, surgically corrected transposition of the great arteries; TOF, tetralogy of Fallot.
Brain natriuretic peptide
BNP concentrations were increased in all patient groups (table 2) compared with reference values.10 No significant correlation between BNP plasma concentrations and ECG parameters was found. In line with our previous results,10 no correlation was found between BNP plasma concentrations and RV systolic pressure. A weak correlation was found between BNP and RVEDV (r = 0.59, p = 0.004).
DISCUSSION
Our data show a prolongation in QRS duration and QRS dispersion over time in patients with chronic RV pressure overload, regardless of the nature of congenital heart disease. This is the first study to explore changes in ECG markers over time in patients with chronic RV pressure overload caused by congenital heart disease. In the studied population, RVEDV, RV mass, and BNP were increased relative to the known reference values for healthy volunteers. A significant correlation was found between QRS duration and RVEDV in patients with a subpulmonary RV subjected to chronic pressure overload.1, 14 With this study, we showed a significant correlation between QRS duration and RV mass in patients with tetralogy of Fallot.
QRS duration
Several studies described the importance of QRS duration and dispersion as a predictor of dangerous tachycardias and sudden death in patients with congenital heart disease.3, 5, 6, 14–17 In patients with tetralogy of Fallot, QRS > 180 ms is a strong predictor of malignant VTs.5, 6 The exact length of QRS prolongation that predicts VT may be variable.5 Only six patients from our study had QRS > 180 ms. Four patients had non-sustained VTs (only one patient had QRS > 180 ms), all lasting less than three seconds. We observed an increase of QRS duration over a period of five years in all patient groups, which was significant in the congenitally corrected TGA and in the subpulmonary RV patient group. Our findings emphasise the need for consequent follow up of ECG parameters in these patients.
QRS dispersion
Over the five years, QRS dispersion increased significantly in patients with surgically corrected TGA and tetralogy of Fallot. Gatzoulis and colleagues5 introduced QRS dispersion as a marker to study inhomogeneity of ventricular depolarisation and found depolarisation abnormalities in patients with tetralogy of Fallot. These are significantly greater in patients with sustained VT and a QRS > 180 ms than in patients without VT and with a QRS < 180 ms.5 In our patient population with increased QRS dispersion (n = 17), we found only one patient with non-sustained VT and two with QRS > 180 ms. Most of the examined patients had QRS < 180 ms. However, according to the findings of Gatzoulis and colleagues,5 the significant increase in QRS dispersion in our patient groups with surgically corrected TGA and tetralogy of Fallot may have important clinical implications for the risk of development of malignant VTs.
ECG parameters and RVEDV
We found a significant correlation between QRS duration and RVEDV in our patient population. The strongest correlation was found in the group of patients with subpulmonary RV functioning under chronic pressure overload. An association has been reported between ventricular enlargement secondary to pulmonary regurgitation and a prolonged QRS duration on surface ECG as a predictor of ventricular arrhythmias.3 In that study, the authors used plain chest radiography for RV size determination. Similar findings have been reported by Abd El Rahman and colleagues14 using echocardiography for RVEDV determination in patients with tetralogy of Fallot. By applying newly available three dimensional imaging techniques such as MRI and three dimensional echocardiography, determination of RV volume in patients with congenital heart disease has become more reliable. Daliento and associates,1 using echocardiography in patients with tetralogy of Fallot, observed that RVEDV and QRS duration are significantly associated with SVT or ventricular fibrillation. The authors concluded that RVEDV is the most significant marker of malignant ventricular arrhythmias.
Our results suggest that RVEDV and QRS duration are correlated and may progressively increase over time. A close follow up of both parameters is therefore clinically important in these patients.
A significant correlation was found between RVEDV and RV mass in patients with tetralogy of Fallot and congenitally corrected TGA. A similar correlation has already been described by Grossman and colleagues18 for left ventricular eccentric hypertrophy.
ECG parameters and RV mass
For the first time, we have shown a significant correlation between RV mass and QRS duration in patients with tetralogy of Fallot; increased mass is most probably caused by RV hypertrophy. No correlation was found between RV mass and QRS duration in patients with surgically corrected TGA or congenitally corrected TGA. In these patients, the RV supports the systemic circulation from the earliest stages of cardiac development and the increase in RV mass is most probably the result of hyperplasia.19, 20
ECG parameters and BNP
The possible relation between plasma neurohormone BNP concentrations and ECG parameters was examined. Raised plasma concentrations of BNP have been reported previously in patients with left ventricular systolic dysfunction.12, 21, 22 Tsutamoto and colleagues23 stated that a high BNP concentration may predict mortality and morbidity in asymptomatic patients with left ventricular dysfunction. The relation between neurohumoral factors and RV function so far has received little attention.10, 11
The patients we studied were asymptomatic or slightly symptomatic. Although our patient population had higher mean BNP plasma concentrations than those in control healthy volunteers,10 we found no significant correlation between plasma BNP and ECG parameters. In these asymptomatic patients, ECG changes are probably related to anatomical changes such as ventricular volume and postoperative scars, while BNP plasma concentrations most probably are related to RV function.10 The clinical consequences of these results remain speculative as the variation in plasma neurohormone concentrations over time has yet to be studied.
Study limitations
Examination of small groups entails loss of statistical power and therefore the present results require confirmation in larger groups of patients. Furthermore, all patients were asymptomatic or slightly symptomatic. Follow up of these patients will be necessary to learn more about the prognostic significance of the quantitative determinants examined in this study.
Conclusions
Our study found a gradual worsening of ECG parameters in asymptomatic or minimally symptomatic patients with chronic RV pressure overload, regardless of the nature of congenital heart disease. In all patients, a positive significant correlation was found between QRS duration and RVEDV. In patients with tetralogy of Fallot, a significant correlation was found between RV mass and QRS duration. No correlation was found between QRS duration and plasma BNP concentrations.
Acknowledgments
I I Tulevski is supported by the Netherlands Heart Foundation (NHS) (grant 99207) and Interuniversity Cardiology Institute of the Netherlands (ICIN-KNAW). D J van Veldhuisen is a Clinical Established Investigator of the National Health Service.
Abbreviations
BNP, Brain natriuretic peptide
EDV, end diastolic volume
MRI, magnetic resonance imaging
RV, right ventricular
SVT, supraventricular tachycardia
TGA, transposition of the great arteries
VT, ventricular tachycardia
REFERENCES
- 1.Daliento L, Rizolli G, Menti L, et al. Accuracy of electrocardiographic and echocardiographic indices in predicting life threatening ventricular arrhythmias in patients operated for tetralogy of Fallot. Heart 1999;81:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chander JS, Wolff GS, Garson A, et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol 1990;65:655–61. [DOI] [PubMed] [Google Scholar]
- 3.Gatzoulis MA, Till JA, Somerville J, et al. Mechanoelectrical interaction in tetralogy of Fallot: QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995;92:231–7. [DOI] [PubMed] [Google Scholar]
- 4.Berul CI, Hill SL, Geggel RL, et al. Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. J Cardiovasc Electrophysiol 1997;8:1349–56. [DOI] [PubMed] [Google Scholar]
- 5.Gatzoulis MA, Till JA, Redington AN. Depolarization-repolarization inhomogeneity after repair of tetralogy of Fallot: the substrate for malignant ventricular tachycardia? Circulation 1997;95:401–4. [DOI] [PubMed] [Google Scholar]
- 6.Balaji S, Lau YR, Case CL, et al. QRS prolongation is associated with inducible ventricular tachycardia after repair of tetralogy of Fallot. Am J Cardiol 1997;80:160–3. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, Getberlet M, Dittrich S, et al. Comparison of transthoracic three dimensional echocardiography with magnetic resonance imaging in the assessment of right ventricular volume and mass. Heart 1997;78:127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papavassiliou DP, Parks WJ, Hopkins KL, et al. Three dimensional echocardiographic measurement of right ventricular volume in children with congenital heart disease validated by magnetic resonance imaging. J Am Soc Echocardiogr 1998;11:770–7. [DOI] [PubMed] [Google Scholar]
- 9.Troughton R, Frampton W, Yandle CM, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355:1126–30. [DOI] [PubMed] [Google Scholar]
- 10.Tulevski II, Groenink M, van der Wall EE, et al. Increased brain and atrial natriuretic peptides in patients with chronic right ventricualr pressure overload: correlation between plasma neurohormones and right ventricular dysfunction. Heart 2001:86:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaya N, Nishikimi T, Okano Y, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 1998;31:202–8. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9–13. [DOI] [PubMed] [Google Scholar]
- 13.Cowie MR. BNP: soon to become a routine measure in the care of patients with heart failure? Heart 2000;83:617–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abd El Rahman MY, Abdul-Khaliq H, Vogel M, et al. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 2000;84:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berul CI, Sweeten TL, Dubin AM, et al. Use of the rate-correced JT interval for prediction of repolarization abnormalities in children. Am J Cardiol 1994;74:1254–7. [DOI] [PubMed] [Google Scholar]
- 16.Therrien J, Siu SC, Harris L, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation 2001;103:2489–94. [DOI] [PubMed] [Google Scholar]
- 17.Gatzoulis MA, Walters J, McLaughlin PR, et al. Late arrhythmia in adults with the Mustard procedure for transposition of great arteries: a surrogate marker for right ventricular dysfunction? Heart 2000;84:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975;56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oparil S, Bishop SP, Clubb FJ Jr. Myocardial cell hypertrophy or hyperplasia. Hypertension 1984;6:III38–43. [DOI] [PubMed] [Google Scholar]
- 20.Perloff JK. Development and regression of increased ventricular mass. Am J Cardiol 1982;50:605–11. [DOI] [PubMed] [Google Scholar]
- 21.Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993;341:1105–9. [DOI] [PubMed] [Google Scholar]
- 22.Talwar S, Squire IB, Davies JE, et al. Plasma N-terminal pro-brain natriuretic peptide and the ECG in the assessment of left-ventricular systolic dysfunction in a high risk population. Eur Heart J 1999;20:1736–44. [DOI] [PubMed] [Google Scholar]
- 23.Tsutamoto T, Wada A, Maeda K, et al. Plasma brain natriuretic peptide level as a biochemical marker of morbidity and mortality in patients with asymptomatic or minimally symptomatic left ventricular dysfunction: comparison with plasma angiotensin II and endothelin-1. Eur Heart J 1999;20:1799–807. [DOI] [PubMed] [Google Scholar]