Abstract
Background: In patients with the tetralogy of Fallot, QRS prolongation predicts malignant ventricular arrhythmias. QRS prolongation may result from right ventricular dilatation. The relation of ECG markers to biventricular wall mass and volumes has not been assessed.
Objective: To investigate the relations of surface ECG markers of depolarisation and repolarisation to right and left ventricular volume and biventricular wall mass.
Methods: 37 Fallot patients (mean (SD) age 17 (9) years) were studied 14 (8) years after surgical repair; 34 had important pulmonary regurgitation. Left and right ventricular size was assessed from tomographic magnetic resonance imaging (MRI), and the amount of pulmonary regurgitation by velocity mapping MRI. QT, QRS, and JT duration and interlead dispersion markers were derived from a standard 12 lead ECG.
Results: Mean QRS duration was significantly prolonged (133 (31) v 91 (11) ms in controls), as were dispersion of QRS (36 (17) v 20 (6) ms), QT interval (87 (48) v 42 (20) ms), and JT interval (93 (48) v 42 (19) ms). Biventricular volumes were increased (right ventricular end diastolic volume, 129 (41) v 70 (9) ml/m2; left ventricular end diastolic volume, 83 (16) v 69 (10) ml/m2), as was right ventricular wall mass (24 (7) v 17 (2) g/m2). QRS duration correlated best with right ventricular mass (r = 0.55, p < 0.01).
Conclusions: In patients operated on for tetralogy of Fallot and with pulmonary regurgitation, ECG predictors of ventricular arrhythmias are influenced by several mechanical factors that may occur simultaneously. These include increased right ventricular volume, but also increases in left ventricular volume and in right and left ventricular wall mass.
Keywords: tetralogy of Fallot, ventricular arrhythmias, ventricular function, congenital heart disease
Tetralogy of Fallot is the most common type of cyanotic congenital heart disease, occurring in approximately 2700 newborns each year in the USA.1 Surgical correction now has a low mortality, and long term survival is good.1 Nevertheless, patients operated on for tetralogy of Fallot are at increased risk of sudden death, which is often attributed to the development of ventricular arrhythmias.2–4 Several risk factors for ventricular arrhythmias have been identified in these patients. These factors—which may be interrelated—include, among others, residual haemodynamic abnormalities (for example, diminished right ventricular function, increased right ventricular systolic pressure, and pulmonary regurgitation), residual electrophysiological abnormalities (inducible sustained ventricular tachycardia, conduction defects, abnormalities of dispersion of depolarisation and repolarisation), and surgical variables (transatrial v transventricular approach, age at operation).2,3,5–10
Despite these well recognised risk factors, the prediction of the occurrence of malignant ventricular arrhythmias has been cumbersome.3 Recently, prolongation (and prolongation rate) of the QRS duration—particularly a QRS duration of more than 170 ms in children and more than 180 ms in adults—has been established as a sensitive predictor of life threatening ventricular arrhythmias in patients operated on for tetralogy of Fallot.10–12 A direct relation between QRS duration and right ventricular size and function has been suggested. This relation would have various clinical implications, including the need for the development of strategies to prevent right ventricular dilatation after surgical repair of tetralogy of Fallot. From available studies it remains unclear whether QRS duration is an adequate marker of right ventricular dilatation. Furthermore, QRS duration may be influenced by factors other than right ventricular volume.13–15
Magnetic resonance imaging is an excellent non-invasive technique for assessing the size of geometrically complex heart chambers, particularly the right ventricle.16,17 In this study we aimed to assess the value of QRS prolongation as a marker of right ventricular dilatation, and also to assess the relation of surface ECG markers of depolarisation and repolarisation to left ventricular volume and biventricular wall mass.
METHODS
Study subjects
Magnetic resonance imaging (MRI) results from 37 patients who had undergone complete repair of the tetralogy of Fallot at a mean (SD) age of 17 (9) years (19 male, 18 female), and from 37 healthy volunteers matched for age and sex, were studied retrospectively (table 1). The adults in this group were consecutive patients referred for magnetic resonance evaluation of pulmonary regurgitation and ventricular function. The paediatric patients were randomly selected from the institutional database.17,18
Table 1.
Patient characteristics
| Patients | Healthy controls | |
| Male/female (n) | 19/18 | 19/18 |
| Age at study (years) | 16.8 (9), range 6–33 | 17.2 (9), range 6–33 |
| Children (age <18 years) (n=24) | 12 (3) | 12 (3) |
| Adults (n=13) | 29 (7) | 28 (9) |
| Body surface area (m2) | 1.7 (0.49) | 1.7 (0.42) |
| Age at operative repair (years) | 4.5 (3) | |
| Time of follow up (years) | 14 (7) | |
| Right ventriculotomy (n) | 10 | |
| Transannular patch (n) | 13 | |
| Restrictive RV filling pattern (n) | 13 | |
| Pulmonary stenosis (>30 mm Hg) (n) | 3 | |
| Pulmonary regurgitation (n) | 34 | |
| Functional state at time of study | ||
| NYHA class I (n) | 34 | |
| NYHA class II (n) | 3 |
NYHA, New York Heart Association functional class; RV, right ventricular.
Patients were included in the study only if recent ECG data were available (not more than two months from the time of the MRI study). Thirty four patients had residual pulmonary regurgitation, and three had residual pulmonary stenosis with Doppler gradients of 40, 70, and 75 mm Hg, respectively. We excluded patients with known residual intracardiac shunting.
The baseline characteristics of all the patients are given in table 1. Restrictive right ventricular diastolic function was considered to be present when late diastolic forward flow occurred in the pulmonary artery with velocity mapping MRI. This flow pattern has previously been shown to be a true marker of restrictive right ventricular physiology.11,18
Surgical correction had been performed at a mean age of 4.5 (3.0) years. Patients were studied at a mean of 14 (7) years postoperatively (range 5–28 years). Details of the operative procedures are given in table 1. All operations included cardiopulmonary bypass and profound hypothermia.
Three patients had documented episodes of supraventricular tachycardia. Drugs used in these patients were propranolol in two and sotalol in one. In two patients, episodes of monomorphic ventricular tachycardias had been documented. Both these patients were on sotalol. All subjects were in sinus rhythm during the MRI examination. None of the patients had used antiarrhythmic drugs within three days of the ECG examinations. Patients were studied without sedation.
The study protocol was approved by the committee on medical ethics of our institution and study subjects were entered in the study after informed consent had been obtained.
Electrocardiography
Standard 12 lead ECGs, recorded at 25 mm/s, were analysed by one observer (WAH), blinded to the clinical history and the results of MRI studies and the 24 hour ECGs. QT, QRS, and JT duration were measured manually on recordings of all 12 leads. Rate correction was performed with Bazett’s formula, for lead II and for maximum and mean QRS, QT, and JT measurements (irrespective of lead). Interlead dispersion was calculated as the maximum (QRS, QT, or JT) minus the minimum interval, derived from at least 11 recordings. The end of the T wave was defined as the point where it crossed the isoelectric baseline, or where the tangent of the downward slope of the T wave crossed the baseline. U waves, if present, were not included in the measurements. In case of a U wave the end point of the T wave was measured at the nadir of the T and U wave. The JT interval was defined as the interval from the end of the QRS complex to the end of the T wave.
ECG data of the patients were compared with those of healthy controls, matched for age and sex. None of the healthy controls was taking drugs affecting ECG parameters.
Magnetic resonance imaging
The MRI studies were done using a Gyroscan S15 or NT15 system (Philips Medical Systems, Best, Netherlands) operating at 1.5 tesla. Spin-echo localising views were used to determine the position of the ventricles and the orientation of the planes for velocity mapping, perpendicular to the direction of measured flows. Volume flow out of or back into the right ventricle was assessed with magnetic resonance velocity mapping of flow across the main pulmonary artery. Magnetic resonance velocity mapping is a modified gradient echo sequence that uses a velocity encoding magnetic field gradient in the direction of flow.19 The imaging plane was positioned half way between the presumed pulmonary valve “annulus” and the bifurcation of the main pulmonary artery. Velocity maps were acquired with a flip angle of 20°, an echo time of 12 ms, a section thickness of 8 mm, and a field of view of 300 × 300 mm. A 128 × 128 scan matrix was reconstructed to a display matrix of 256 × 256. Through-plane flow was encoded at a maximum velocity of 1.0–4.5 m/s for the pulmonary artery, anticipated from the flow velocity from previous echo Doppler measurements and cine loop display of gradient echo images. The cardiac cycle was divided into approximately 30 time frames, with temporal resolution ranging from 21–36 ms, depending on the heart rate of the patient.
Ventricular volumes were measured from a multisection gradient echo image set. Details of this technique have been reported previously.17 In brief, for both ventricles a transverse stack of 10–12 multiphase gradient echo magnetic resonance images was acquired with a flip angle of 50°, an echo time of 4–10 ms, and a section thickness of 8–10 mm with a 0.8–1.0 mm slice gap. Temporal resolution ranged from 22–35 ms. Magnetic resonance imaging was triggered to the R wave of the ECG. Retrospective ECG gating was used for the velocity mapping measurements.19
Volumetric flow measurements
Analysis of the magnetic resonance flow velocity studies was undertaken using the FLOW software package.20 Volume of flow was calculated by tracing a region of interest along the inner borders of the pulmonary arterial wall in each time frame of a velocity map series. For every time frame, instantaneous volumetric flow was calculated using a computer algorithm, by multiplying spatial average flow velocity by the area of the region of interest. Integration of all instantaneous volumetric flow data yielded total volume flow per cardiac cycle.
Ventricular volumes and mass
Ventricular volumes were calculated by summation of ventricular cavity areas, assessed by manual tracing of the endocardial border on a stack of gradient echo image sections of a specific time frame, and multiplied by section thickness with correction for the interslice gap. Papillary muscles and the moderator band were not included in the ventricular area. Epicardial contours were drawn on end systolic frames to determine ventricular wall mass. The interventricular septum was considered to be part of the left ventricular wall. Ventricular wall volume was calculated as myocardial area (epicardial minus endocardial area) multiplied by the sum of slice and interslice gap thickness. A specific gravity of 1.05 g/ml was used for calculating ventricular mass.
Stroke volume was defined as end diastolic (maximum ventricular) volume minus end systolic (minimum) volume. Ejection fraction was calculated as stroke volume divided by end diastolic volume. Ventricular volume indexes were calculated as volume divided by the body surface area.
Statistical analysis
Values are expressed as mean (SD). Linear regression analysis was used to assess the relation between ECG findings and magnetic resonance results, age, and body surface area. Comparison of results from patients with versus without restrictive right ventricular physiology was done using Student’s t test. ECG results of patients were compared with those of healthy controls, matched for age and sex. Magnetic resonance measurements from healthy controls obtained previously served as a comparison for the results from the patients. All analyses were performed using the SPSS-PC statistical software package. A probability value of p < 0.05 was considered to indicate significance.
RESULTS
Electrocardiography
The ECG results are given in table 2. Significant differences between patients and controls were noted for mean QRS duration, interlead QRS dispersion, QT dispersion, and JT dispersion. The rate corrected JT interval was significantly decreased. Two of the patients had documented episodes of ventricular tachycardia. Mean QRS durations in these patients were 163 ms and 187 ms, respectively. Thirty five patients had right bundle branch block.
Table 2.
ECG results
| Variable | Patients | Controls | p Value |
| VT (n) | 2 | ||
| SVT (n) | 3 | ||
| cRBBB (n) | 33 | ||
| QRS (ms) | 133 (36) (83–195*) | 91 (11) | <0.001 |
| QRS >180 ms (n) | 5 | ||
| QTc (ms) | 437 (28) | 421 (24) | NS |
| JTc (ms) | 292 (26) | 320 (22) | <0.001 |
| QRSd (ms) | 36 (17) | 20 (6) | <0.01 |
| QTd (ms) | 87 (48) | 42 (20) | <0.01 |
| JTd (ms) | 93 (48) | 42 (19) | <0.001 |
Values are mean (SD); *range.
cRBBB, complete right bundle branch block; JTc, rate corrected JT interval; JTd, JT dispersion; QRSd, QRS dispersion; QTd, QT dispersion; QTc, rate corrected QT interval; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
Magnetic resonance imaging
The MRI results are given in table 3. Compared with controls, right ventricular volumes were increased (p < 0.01/0.001). The right ventricular ejection fraction was decreased (p < 0.01/0.05) and right ventricular wall mass increased (p < 0.05). The left ventricle in the patients was also enlarged (p < 0.01), and the ejection fraction decreased (p < 0.01). Left ventricular wall mass of the patients did not differ from that of the controls. Among the 34 patients with pulmonary regurgitation, the regurgitant fraction ranged from 4% to 70%.
Table 3.
Results of magnetic resonance measurements
| Patients | Controls | p Value | |
| RV EDV (ml/m2) | 129 (41) | 70 (9) | <0.001 |
| RV ESV (ml/m2) | 64 (30) | 32 (6) | <0.01 |
| RV EF (%) | 49 (10) | 68 (6) | <0.01 |
| RV mass (g/m2) | 24 (7) | 17 (2) | <0.05 |
| LV EDV (ml/m2) | 83 (16) | 69 (10) | <0.01 |
| LV ESV (ml/m2) | 45 (15) | 20 (5) | <0.01 |
| LV EF (%) | 50 (11) | 70 (6) | <0.01 |
| LV mass (g/m2) | 51 (13) | 54 (11) | NS |
| PR (% RV SV) (range) | 39 (21) (0–70) | ||
EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; LV, left ventricular; PR, pulmonary regurgitation; RV, right ventricular; SV, stroke volume.
Correlations
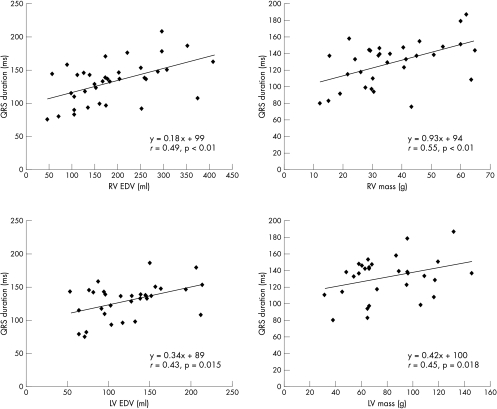
Correlations between ECG findings and MRI measurements in the Fallot patients are given in table 4 and fig 1. QRS duration correlated significantly with right ventricular end diastolic volume, end systolic volume, right ventricular wall mass, left ventricular volumes, left ventricular wall mass, percentage pulmonary regurgitation, age, and body surface area. The best correlation was with right ventricular wall mass (r = 0.55, p < 0.01). A significant correlation was also noted between rate corrected mean JT interval and biventricular volumes and wall mass. Correlation between QTc and MRI measurements, age, and body surface area did not reach significance; the same was noted for dispersion of QRS, QT, and JT.
Table 4.
Correlation (linear regression analysis) of ECG findings and magnetic resonance measurements
| RV EDV | RV ESV | RV mass | LV EDV | LV ESV | LV mass | PR | Age | BSA | ||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| QRS | 0.49 | <0.01 | 0.47 | <0.01 | 0.55 | <0.01 | 0.43 | 0.015 | 0.45 | <0.01 | 0.45 | 0.018 | 0.44 | 0.025 | 0.45 | <0.01 | 0.54 | <0.01 |
| JTc | −0.48 | <0.01 | −0.48 | <0.01 | −0.40 | 0.04 | −0.44 | <0.01 | −0.45 | <0.01 | −0.43 | 0.02 | 0.21 | NS | 0.32 | NS | 0.37 | NS |
BSA, body surface area; EDV, end diastolic volume; JTc, rate corrected JT interval; LV, left ventricular; PR, pulmonary regurgitation; RV, right ventricular.
Figure 1.
Graphs of linear regression analysis of QRS duration versus right ventricular end diastolic volume (RV EDV) (top left), left ventricular end diastolic volume (LV EDV) (bottom left), RV wall mass (top right), and LV wall mass (bottom right).
There was no significant difference between any of the ECG findings in the patients with restrictive right ventricular physiology and those without. Indices of ventricular size and function did not differ between those patients with and without restrictive right ventricles. Correlations with MRI calculations did not differ statistically between ECG measurements derived from lead II, or from maximum or mean ECG measurements.
DISCUSSION
Our study involved a relatively young group of patients operated on for tetralogy of Fallot. Most of the surgery had been done using recent techniques, avoiding right ventriculotomy. Thirty four patients had pulmonary regurgitation, and right ventricular size was increased. QRS duration encompassed the entire spectrum commonly found in these patients. We found a significant correlation between surface ECG QRS duration and right ventricular volumes. This correlation also exists between QRS duration and right ventricular wall mass. However, in the present group, in whom an increased left ventricular size and diminished left ventricular ejection fraction were observed, QRS duration also correlated with left ventricular size and wall mass.
The observed negative correlation between JTc duration and biventricular volumes is compatible with previous observations of decreased repolarisation duration with increased mechanical stress.21
Abnormalities of dispersion of depolarisation and repolarisation (increase in dispersion of QRS, QT, and JT) were present in our Fallot patients. These variables could not be related to ventricular size or wall mass. Restrictive right ventricular physiology has been related to a reduced risk of ventricular arrhythmias in adult Fallot patients.11 This has been explained on the basis of a decrease in right ventricular size caused by impairment of filling and a subsequent reduction in the amount of pulmonary regurgitation with restrictive right ventricular function.11 In our study, we could not relate ECG characteristics to restrictive right ventricular diastolic functional abnormalities.
Documented ventricular arrhythmias occurred in only two patients (5.4%). This low incidence of ventricular arrhythmias prevented us from assessing potentially “critical” ventricular volumes or wall mass that might predict the occurrence of malignant arrhythmias.
Previous studies of mechanoelectrical interaction in tetralogy of Fallot
Arrhythmias of ventricular origin occur with an incidence of 0.5–6% in patients who have undergone complete repair of tetralogy of Fallot.5 In these patients, delayed conduction (as evidenced by prolonged QRS duration) often occurring directly postoperatively, and increased inhomogeneity of repolarisation (increased dispersion markers) provide the basis for re-entry tachycardias, which increase the risk of sudden death.8,10,11 Re-entry circuits may include a ventricular septal defect patch, right ventricular outflow patches, and ventricular scars.4 Furthermore, residual haemodynamic problems, such as pulmonary stenosis or regurgitation and decreased right ventricular ejection fraction, have been associated with an increased risk of ventricular ectopic activity and with inducible ventricular arrhythmias.4–7,9,12,22
In many of the studies on this subject, accurate measurements of ventricular volumes or wall mass were not available. The relation between residual haemodynamic problems and ventricular arrhythmias has been explained by mechanoelectrical interaction, which has been defined as the development of potentially arrhythmogenic electrophysiological changes caused by alterations in the loading conditions of the cardiac chambers.23 Recently, studies by Gatzoulis and colleagues and Berul and associates showed an increased risk of ventricular tachycardias in Fallot patients with a prolonged QRS duration (> 170 ms in children10 or > 180 ms in adults,11,12 respectively). A significant correlation (r = 0.43) between QRS duration and echocardiographic right ventricular long axis length was established.11 This relation between QRS duration and right ventricular size was also demonstrated by Balaji and colleagues,9 but could not be confirmed in another echocardiographic study.10 Abd El Rahman and co-workers, using three dimensional echocardiography, established a correlation with QRS duration for right ventricular end diastolic volume of r = 0.61, and for end systolic volume of r = 0.51.24 In these studies, relations between QRS duration and other mechanical factors could not be established.9–12,24,25
Using MRI as the gold standard technique, we confirmed that QRS duration correlates with right ventricular volumes. However, it is unlikely that right ventricular dilatation alone is the cause of QRS prolongation or ventricular arrhythmias. Previous studies have shown a clear relation between QRS duration and left ventricular volumes and wall mass.13–15 Our observations confirm these findings. We showed that QRS duration also correlates with right ventricular and left ventricular wall mass in patients operated on for tetralogy of Fallot. The occurrence of ventricular arrhythmias is not increased by chronic ventricular dilatation alone, but when it is combined with regional differences in stretch and contractility.23 These regional differences may be promoted by myocardial hypertrophy, which results in areas of slow conduction or unidirectional block.26 It is conceivable that the combination of right ventricular volume overload and left ventricular function abnormalities—as may be present with important pulmonary regurgitation—may further enhance regional differences in stretch and contractility.27–29
These factors, particularly the influence of right ventricular wall mass, have been difficult to assess in previous studies of patients with tetralogy of Fallot3,4,6 using conventional imaging techniques. In the present study, we did not aim to assess a direct link between ventricular size, wall mass, and clinical arrhythmias. However, our results may call into question the concept that the increased risk of ventricular arrhythmias in patients with tetralogy of Fallot results from right ventricular dilatation alone. This is in accordance with results of a recent large multicentre study in adult patients,12 in which it was concluded from circumstantial evidence rather than direct measurements that chronic pulmonary regurgitation was associated with sustained ventricular arrhythmias. A QRS duration of more than 180 ms was a clear risk factor for ventricular tachycardia and sudden death,12 as was a rapid rate of lengthening of QRS duration. This is in accordance with a combined effect of ventricular size and enhancement of regional differences in stretch and contractility as risk factors for ventricular arrhythmias. Elaborating on this concept, Vogel and colleagues recently showed an association between regional right ventricular wall motion abnormalities and repolarisation–depolarisation disturbances in patients with tetralogy of Fallot.30 However, wall motion abnormalities could not be correlated with wall mass or the amount of pulmonary regurgitation.30
Clinical implications
The findings of our study show that ventricular depolarisation and repolarisation in patients operated on for tetralogy of Fallot and with pulmonary regurgitation are influenced by several mechanical factors that may occur simultaneously. These include not only increased right ventricular volumes but also increased left ventricular volumes and right ventricular and left ventricular wall mass. These factors may contribute to electrical inhomogeneity, thus promoting the substrate for re-entry tachycardias. At the same time, biventricular volumes and wall mass may also influence the haemodynamic response to ventricular tachycardias. Thus QRS prolongation should be considered a surrogate risk factor for ventricular arrhythmias, itself influenced by multiple factors. Which of these factors is most important is not evident. Of note, we were able to show a significant correlation between QRS and JTc duration with left ventricular mass, although left ventricular mass in the patients did not differ from that in the healthy controls.
Further evaluation, including assessment of dispersion markers and of haemodynamic factors such as right ventricular and left ventricular function and the severity of pulmonary regurgitation, is mandatory in the presence of important QRS prolongation after repair of tetralogy of Fallot. Such a strategy, which may have to be combined with electrophysiological studies, should help to distinguish those patients who require surgical relief of haemodynamic abnormalities from those who may be managed with antiarrhythmic drugs or an implantable cardioverter-defibrillator to prevent life threatening ventricular arrhythmias.4,31
Conclusions
In patients operated on for tetralogy of Fallot, ECG predictors of ventricular arrhythmias are influenced not only by right ventricular volumes but also by left ventricular volumes and wall mass.
REFERENCES
- 1.Murphy JG, Gersh BJG, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med 1993;329:593–9. [DOI] [PubMed] [Google Scholar]
- 2.Bricker JT. Sudden death and tetralogy of Fallot. Risks, markers and causes. Circulation 1995;92:162–3. [DOI] [PubMed] [Google Scholar]
- 3.Chandar JS, Wolff GS, Garson A, et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol 1990;65:655–61. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DA, Harris L, Siu SC, et al. Sustained ventricular tachycardia in adult patients late after repair of tetralogy of Fallot. J Am Coll Cardiol 1997;30:1368–73. [DOI] [PubMed] [Google Scholar]
- 5.Kugler JD. Predicting sudden death in patients who have undergone tetralogy of Fallot repair: is it really as simple as measuring ECG intervals? J Cardiovasc Electrophysiol 1998;9:103–6. [DOI] [PubMed] [Google Scholar]
- 6.Marie PY, Marçon F, Brunotte F, et al. Right ventricular overload and induced sustained ventricular tachycardia in operatively “repaired” tetralogy of Fallot. Am J Cardiol 1992;69:785–9. [DOI] [PubMed] [Google Scholar]
- 7.Garson A, Porter CJ, Gillette PC, et al. Induction of ventricular tachycardia during electrophysiologic study after repair of tetralogy of Fallot. J Am Coll Cardiol 1983;1:1493–502. [DOI] [PubMed] [Google Scholar]
- 8.Gatzoulis MA, Till JA, Redington AN. Depolarization-repolarization inhomogeneity after repair of tetralogy of Fallot. The substrate for malignant ventricular tachycardia? Circulation 1997;95:401–4. [DOI] [PubMed] [Google Scholar]
- 9.Balaji S, Lau YR, Case CL, et al. QRS prolongation is associated with inducible ventricular tachycardia after repair of tetralogy of Fallot. Am J Cardiol 1997;80:160–3. [DOI] [PubMed] [Google Scholar]
- 10.Berul CI, Hill SL, Geggel RL, et al. Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. J Cardiovasc Electrophysiol 1997;8:1349–56. [DOI] [PubMed] [Google Scholar]
- 11.Gatzoulis MA, Till JA, Somerville J, et al. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995;92:231–7. [DOI] [PubMed] [Google Scholar]
- 12.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356:975–81. [DOI] [PubMed] [Google Scholar]
- 13.Jauch W, Hicks MN, Cobbe SM. Effects of contraction-excitation feedback on electrophysiology and arrhythmogenesis in rabbits with experimental left ventricular hypertrophy. Cardiovasc Res 1994;28:1390–6. [DOI] [PubMed] [Google Scholar]
- 14.Dean JW, Lab MJ. Arrhythmia in heart failure: role of mechanically induced changes in electrophysiology. Lancet 1989;i:1309–11. [DOI] [PubMed] [Google Scholar]
- 15.Pye MP, Cobbe SM. Arrhythmogenesis in experimental models of heart failure: the role of increased load. Cardiovasc Res 1996;32:248–57. [DOI] [PubMed] [Google Scholar]
- 16.Boxt LM, Katz J, Kolb T, et al. Direct quantitation of right and left ventricular volumes with nuclear magnetic resonance imaging in patients with primary pulmonary hypertension. J Am Coll Cardiol 1992;19:1508–15. [DOI] [PubMed] [Google Scholar]
- 17.Helbing WA, Rebergen SA, Maliepaard C, et al. Quantitation of right ventricular function with magnetic resonance imaging in children with normal hearts and with congenital heart disease. Am Heart J 1995;130:828–37. [DOI] [PubMed] [Google Scholar]
- 18.Helbing WA, Niezen RA, LeCessie S, et al. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol 1996;28:1827–35. [DOI] [PubMed] [Google Scholar]
- 19.Rebergen SA, Van der Wall EE, Doornbos J, et al. Magnetic resonance measurement of velocity and flow: technique, validation, and cardiovascular applications. Am Heart J 1993;126:1439–56. [DOI] [PubMed] [Google Scholar]
- 20.van der Geest RJ, Buller VGM, Reiber JHC. Automated quantification of flow velocity and volume in the ascending and descending aorta using MR flow velocity mapping. Comp Cardiol 1995:29–32.
- 21.Levine JH, Guarnieri T, Kadish AH, et al. Changes in myocardial repolarization in patients undergoing balloon valvuloplasty for congenital pulmonary stenosis: evidence for contraction-excitation feedback in humans. Circulation 1988;77:70–7. [DOI] [PubMed] [Google Scholar]
- 22.Lucron H, Marçon, Bosser G, et al. Induction of sustained ventricular tachycardia after surgical repair of tetralogy of Fallot. Am J Cardiol 1999;83:1369–73. [DOI] [PubMed] [Google Scholar]
- 23.Franz MR, Cima R, Wang D, et al. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation 1992;86:968–78. [DOI] [PubMed] [Google Scholar]
- 24.Abd El Rahman MY, Abdul-Khaliq H, Vogel M, et al. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 2000;84:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Book WM, Parks WJ, Hopkins KL, et al. Electrocardiographic predictors of right ventricular volume measured by magnetic resonance imaging late after total repair of tetralogy of Fallot. Clin Cardiol 1999;22:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pye MP, Cobbe SM. Mechanisms of ventricular arrhythmias in cardiac failure and hypertrophy. Cardiovasc Res 1992;26:740–50. [DOI] [PubMed] [Google Scholar]
- 27.Niezen RA, Helbing WA, van der Wall EE, et al. Biventricular systolic function and mass studied with MR imaging in children with pulmonary regurgitation after repair for tetralogy of Fallot. Radiology 1996;201:135–40. [DOI] [PubMed] [Google Scholar]
- 28.Kondo C, Nakazawa M, Kusakabe K, et al. Left ventricular dysfunction on exercise long-term after total repair of tetralogy of Fallot. Circulation 1995;92:II250–2. [DOI] [PubMed] [Google Scholar]
- 29.Lin SS, Reynertson SI, Louie EK, et al. Right ventricular volume overload results in depression of left ventricular ejection fraction. Circulation 1994;90:II-209–13. [PubMed] [Google Scholar]
- 30.Vogel M, Sponring J, Cullen S, et al. Regional wall motion and abnormalities of electrical depolarization and repolarization in patients after surgical repair of tetralogy of Fallot. Circulation 2001;103:1669–73. [DOI] [PubMed] [Google Scholar]
- 31.Gillette PC. Ventricular arrhythmia after repair of tetralogy of Fallot. J Am Coll Cardiol 1997;30:1384. [DOI] [PubMed] [Google Scholar]