Abstract
Protein C deficiency is a disorder in the coagulation cascade that results in predominantly venous thromboembolism. However, recent studies have implicated this disorder as a possible contributor to arterial thrombosis, especially myocardial infarction. There are six reported cases of myocardial infarction secondary to protein C deficiency in the literature. This is the first report of myocardial infarction and ischaemic stroke in the same patient as a manifestation of protein C deficiency. The investigation of hypercoagulable state is an essential component of the investigation of young patients with myocardial infarction.
Protein C is a vitamin K dependent glycoprotein. Its deficiency is codominantly inherited and is identified when the protein C concentration is below 60–70% of the overall mean concentrations.1–3 Once exposed to thrombomodulin on the endothelial lining of blood vessels, protein C binds to protein S to form the activated protein C complex (APC).1,4 The anticoagulating effect of the APC occurs by inactivation of factors Va and VIIIa, which in turn downregulate the activity of factors Xa and Ixa.1 Protein C deficiency and APC resistance predominantly lead to venous thromboembolism. However, it is important to note that evidence is emerging that protein C deficiency has an important role in arterial thrombosis, which includes myocardial infarction and non-haemorrhagic stroke. We report for the first time a case of myocardial infarction and stroke as the initial presentation of protein C deficiency in a young woman.
CASE PRESENTATION
The patient was a 26 year old white woman who was transferred to our hospital for management of myocardial infarction complicated by ventricular fibrillation. Her medical history was significant for tobacco, alcohol, and marijuana use. Her family history was negative for hypercoagulable states. She had drunk alcohol the evening before and smoked marijuana several hours before her initial presentation but denied any cocaine use. She developed sudden lightheadedness followed rapidly by syncope. Review of rhythm strips obtained by paramedics showed ventricular fibrillation, which required three consecutive defibrillation attempts in conjunction with intubation and cardiopulmonary resuscitation.
At admission, the patient was intubated and had stable haemodynamics. She was admitted directly into the intensive care unit with blood pressure of 126/78 mm Hg, pulse of 72 beats/minute, and oxygen saturation of 92% on 2 l of oxygen. Physical examination showed normal jugular venous return, mild bibasilar crackles on the lung fields, no audible murmur, and no pedal oedema. Neurological examination showed no focal neurological deficit with intermittent episodes of confusion. The initial ECG outside of the hospital had 1–2 mm ST segment elevations in leads II, III, and aVF with reciprocal ST depression in lead V4–6, which were dynamic. Repeat ECG showed 0.5 mm Q waves in leads II, III, and aVF. Her initial troponin T concentration was 1.25 ng/ml (normal 0.0–0.10 ng/ml) and her creatine kinase peaked at 14 375 U/l with creatine kinase MB of more than 500 ng/ml by day 2 of admission. Review of outside records showed that toxin screen was positive for benzodiazepines and tetrahydrocannabinol. Further laboratory investigations found that complete blood count, blood chemistry, lipid profile, homocysteine, βHCG (β human chorionic gonadotrophin), thyroid stimulating hormone, and repeat toxin screen were all within normal limits except for low high density lipoprotein of 32 mg/ml (normal > 55 mg/ml). A hypercoagulable panel examining anticardiolipin antibodies, protein S, antithrombin III, and APC resistance was also normal except for her protein C concentration, which was 53% of the mean value (normal 70–120%).
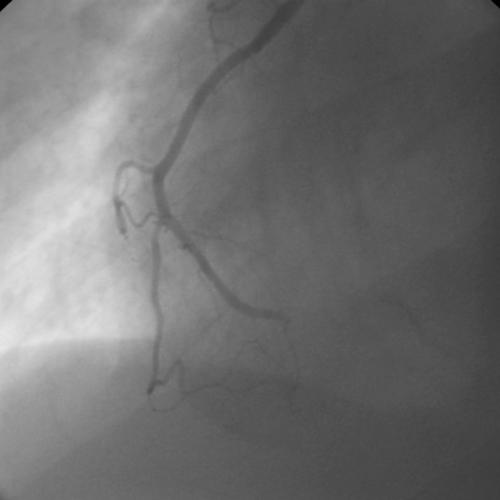
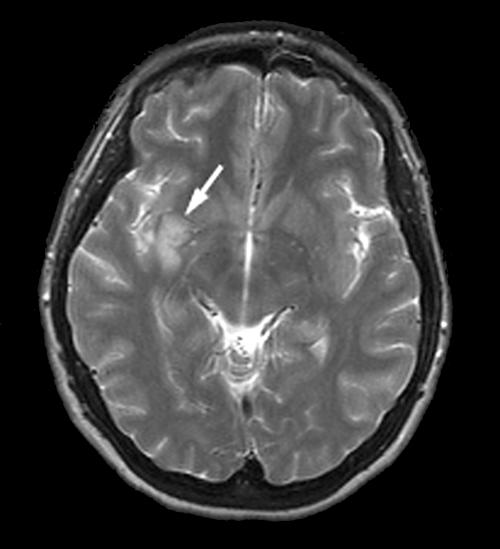
Transthoracic echocardiogram showed a depressed left ventricular ejection fraction of 30% with global hypokinesis with normal valves and without any visible thrombus. Cardiac catheterisation showed acute thrombotic occlusion at the junction of the distal right coronary artery and posterior descending artery with a normal left coronary system (fig 1). By day 3 of admission, her intermittent confusion persisted with now noticeable personality changes without any focal neurological deficit. Magnetic resonance imaging and angiography of the brain showed acute ischaemic stroke in the right subinsular and basal ganglia region—areas supplied by the right middle cerebral artery territory infarction (fig 2). Throughout her hospitalisation, the patient did not have any recurrent tachyarrhythmias and therefore no electrophysiological study was done. She did well and was discharged on warfarin, β blockers, angiotensin converting enzyme inhibitors, and aspirin.
Figure 1.
Right coronary artery in the left anterior oblique projection showing acute thrombotic occlusion at the junction of the distal right coronary artery and posterior descending artery.
Figure 2.
Magnetic resonance imaging of the brain showing acute ischaemic stroke in the right subinsular and basal ganglia region.
DISCUSSION
The prevalence of heterozygous protein C deficiency is 1 in 200–300.1,4 Protein C deficiency or resistance to activated protein C complex results in excess factors Xa and IXa activity and a hypercoagulable state.1,4
Of the six reported cases of arterial thrombosis, the majority involved myocardial infarction followed by strokes and peripheral arterial thrombosis.1–9 In one study only 6% of patients with non-haemorrhagic stroke were found to have protein C deficiency.6 Protein C deficiency by itself does not appear to increase the risk of arterial thrombosis; however, when coupled with smoking, factor VII hyperactivity, cardiac contusion, and high risk family history there is higher incidence of premature myocardial infarction.1–3,5 The age of onset of myocardial event in patients with protein C deficiency remains controversial with some patients presenting before the age of 50 years3,4 and others before the age of 30,5,9 as with our patient.
In conclusion, it is important to recognise protein C deficiency as a significant risk factor in young patients with myocardial infarction who have minimal risk factors. Our patient will be treated with life long warfarin, as well as aggressive lifestyle modification.
Acknowledgments
We thank Dr Benjamin Cheong and Dr Patrick O’Keefe for their contribution in the preparation of this manuscript.
REFERENCES
- 1.Hacker SM, Williamson BD, Lisco S, et al. Protein C deficiency and acute myocardial infarction in the third decade. Am J Cardiol 1991;68:137–8. [DOI] [PubMed] [Google Scholar]
- 2.Miletich, J, Sherman L, Broze G Jr. Absence of thrombosis in subjects with heterozygous protein C deficiency. N Engl J Med 1987;317:991–6. [DOI] [PubMed] [Google Scholar]
- 3.Kario K, Matsuo T, Tai S, et al. Congenital protein C deficiency and myocardial infarction: concomitant factor VII hyperactivity may play a role in the onset of arterial thrombosis. Thromb Res 1992;67:95–103. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi, K, Sone T, Kondoh J, et al. Prevalence of activated protein C resistance in acute myocardial infarction in Japan. Jpn Heart J 1997;38:769–78. [DOI] [PubMed] [Google Scholar]
- 5.Bux-Gewehr I, Nacke A, Feurle GE. Recurring myocardial infarction in a 35 year old woman. Heart 1999;81:316–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Camerlingo M, Finazzi G, Casto L, et al. Inherited protein C deficiency and nonhemorrhagic arterial stroke in young adults. Neurology 1991;41:1371–3. [DOI] [PubMed] [Google Scholar]
- 7.Doggen CJ, Cats VM, Bertina RM, et al. Interaction of coagulation defects and cardiovascular risk factors: increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A. Circulation 1998;97:1037–41. [DOI] [PubMed] [Google Scholar]
- 8.Rosendaal FR, Siscovick DS, Schwartz SM, et al. Factor V Leiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood 1997;89:2817–21. [PubMed] [Google Scholar]
- 9.Sadiq A, Ahmed S, Karim A, et al. Acute myocardial infarction: a rare complication of protein C deficiency. Am J Med 2001;110:414. [DOI] [PubMed] [Google Scholar]