Abstract
Takayasu arteritis is a chronic vasculitis involving the aorta and its main branches, the pulmonary arteries, and the coronary tree, and needs to be considered in a young patient with angina, in particular when pulses are absent. This case illustrates the limitations of exercise testing in diagnosing the extent of coronary artery disease and the risks associated with coronary angiography in patients with inflammatory disease in the left main stem coronary artery. It also highlights the novel use of non-invasive scanning with positron emission tomography using 18-fluorodeoxyglucose in assessing remission from this disease. Revascularisation was performed with percutaneous transluminal coronary angioplasty and stenting as an emergency procedure, but treatment of the restenosis with directional atherectomy was based on a review of the available literature. The lymphocytic alveolitis seen in this patient has not been previously described in Takayasu’s disease.
Keywords: Takayasu’s arteritis, left main stem stenosis
A 27 year old white woman presented in 1994 with dyspnoea, a non-productive cough, and 14 lbs (6.36 kg) weight loss. There was reticulonodular shadowing on her chest radiograph and erythrocyte sedimentation rate was increased to 50 mm. Subsequent atypical pneumonia screen, avian precipitins, tests for acid fast bacilli, and autoantibody screens were negative. An open lung biopsy was performed, with histology showing a lymphocytic alveolitis. She was treated with intermittent oral steroids with some improvement in symptoms but not in the chest radiograph or transfer factor.
She continued clinic follow up and in 1999 appeared well, with mild exertional dyspnoea and a new chest pain occurring only on severe exertion. She also had a pain in her left arm unrelated to walking. She had stopped smoking in 1994, was premenopausal, did not have diabetes mellitus or hypertension, and had a total cholesterol concentration of 3.9 mmol/l. Blood pressure was 130/80 mm Hg supine in the right arm and not recordable in the left arm. Both the left radial and left brachial pulses were impalpable. Exercise testing showed 1 mm of horizontal ST segment depression after 7.5 minutes of exercising in the Bruce protocol in the lateral leads without chest pain. She was started on aspirin and diltiazem and, despite only a mildly positive exercise test, was referred for coronary angiography and arch aortography.
Left and right heart were catheterised through the right femoral approach. The right coronary artery was normal but there was a tight ostial stenosis of the left main stem (LMS) artery without distal disease (fig 1) The left subclavian artery was occluded and there was moderate pulmonary hypertension (pressure 66 mm Hg systolic). At the end of the procedure, while still on the angiogram table, she developed ST elevation anteriorly and had a cardiac arrest. The LMS artery was found to have occluded at repeat angiography but was rapidly reopened with the insertion of a coronary stent (fig 2) Her recovery was uncomplicated, with a creatine kinase peak concentration of 1000 IU/l.
Figure 1.
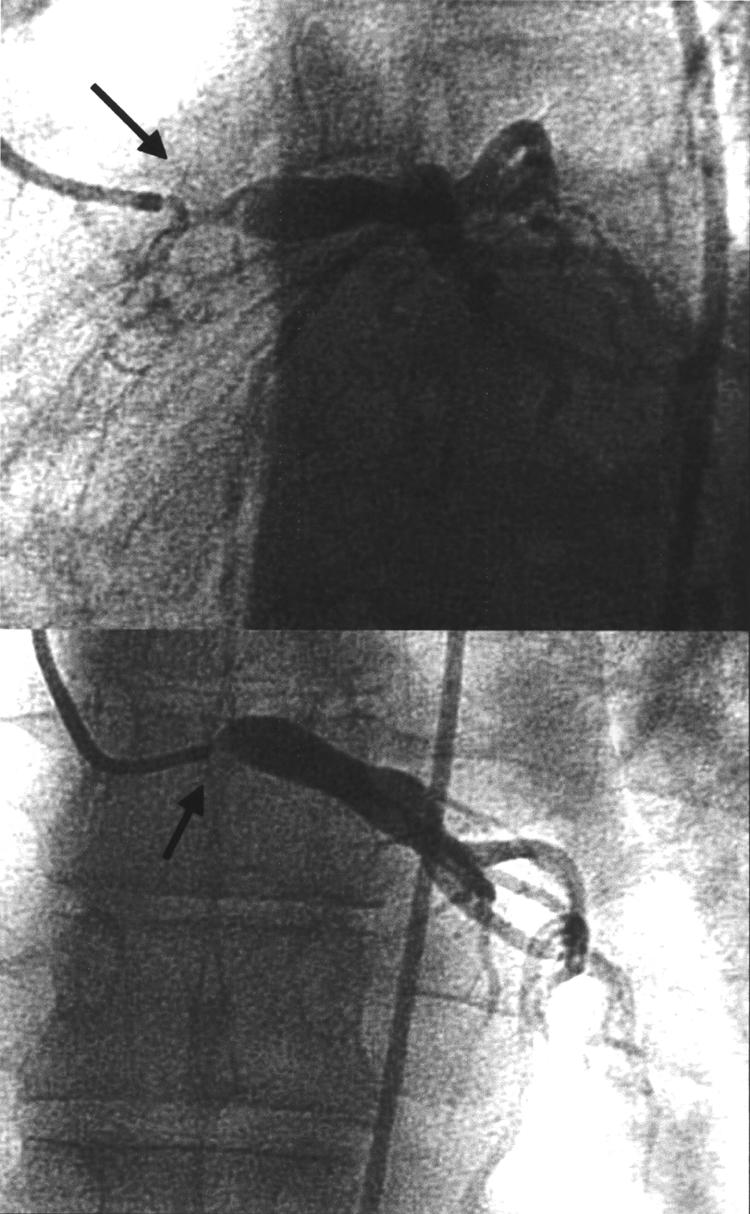
A tight stenosis is seen at the ostium of the left main stem (LMS) coronary artery at diagnostic coronary angiography (black arrows). The right coronary artery had no stenosis.
Figure 2.
The LMS was reopened with percutaneous transcutaneous balloon angioplasty and a 4.0 mm diameter × 9 mm length stent was inserted after acute occlusion following diagnostic angiography. A good angiographic result was achieved.
A clinical diagnosis of Takayasu’s arteritis was made since she had an absent major pulse with arm claudication, an abnormal aortogram, and ostial coronary disease at the age of 34 years.1 Subsequent investigations showed a C reactive protein concentration of 980 mg/l, negative repeat autoantibody screen including antineutrophil cytoplasmic antibody, normal complement concentrations, and negative treponemal serology. An additional stenosis of the origin of the left common carotid artery was shown on magnetic resonance angiography (fig 3).
Figure 3.
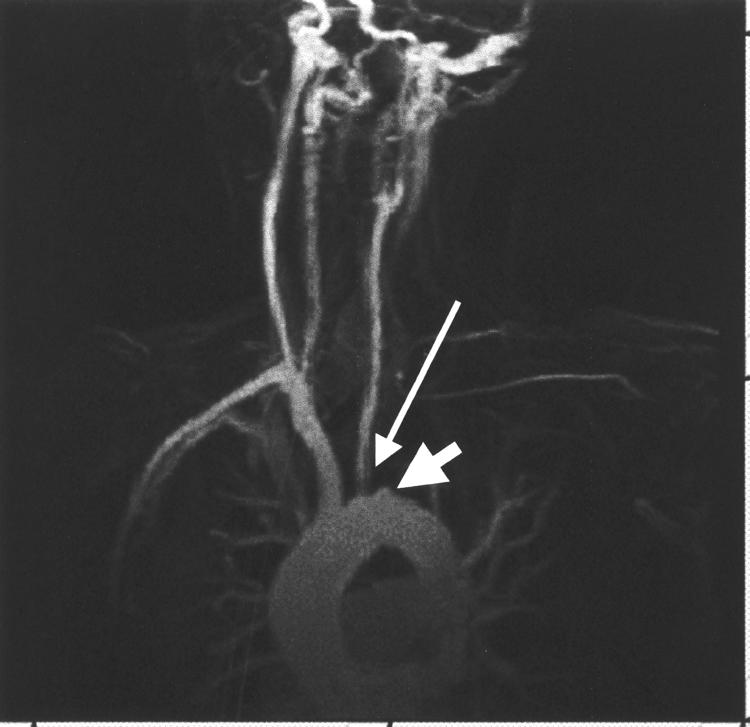
Magnetic resonance angiogram showing total occlusion of the left subclavian artery (wide arrow). In addition, there is a stenosis at the origin of the left common carotid artery (slim arrow).
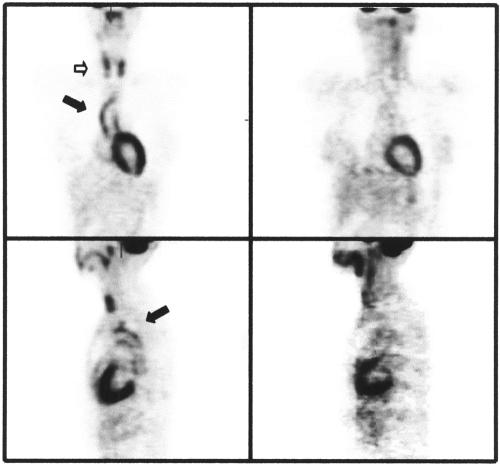
She was treated with oral prednisolone and monthly cyclophosphamide for six months. Her C reactive protein concentration returned to normal. Positron emission tomography using 18-fluorodeoxyglucose (FDG-PET) was used to assess inflammation in the aorta before and after treatment (fig 4). Following immunosuppressive treatment, there was a reduction in FDG uptake in the aortic wall, indicating a reduction in metabolic activity.
Figure 4.
Positron emission tomography using 18-fluorodeoxyglucose (FDG-PET) was used to image the metabolic activity in the aorta (solid arrow) and head and neck vessels (open arrow) both before (left hand panels) and after (right hand panels) treatment with pulsed cyclophosphamide and prednisolone. Uptake in the heart and thyroid glands is similar but the aortic uptake has been dramatically reduced by treatment.
In view of the stent placed in the LMS, she was scheduled for repeat coronary angiography three months after stent placement despite being pain free (fig 5). This showed a tight in-stent stenosis in the LMS artery. It was not immediately treated, as inflammatory markers were still increased. She was kept in hospital to complete her course of immunosuppression. Following reduction in her inflammatory markers and the negative PET scan she underwent percutaneous revascularisation again. A cutting balloon was inserted to produce a tract for the directional atherectomy device. Three passages of the device retrieved a core of white glistening tissue with some stent struts obviously excised (fig 6). Histology showed smooth muscle cells with no significant inflammatory infiltrate. The end result was angiographically good (fig 7).
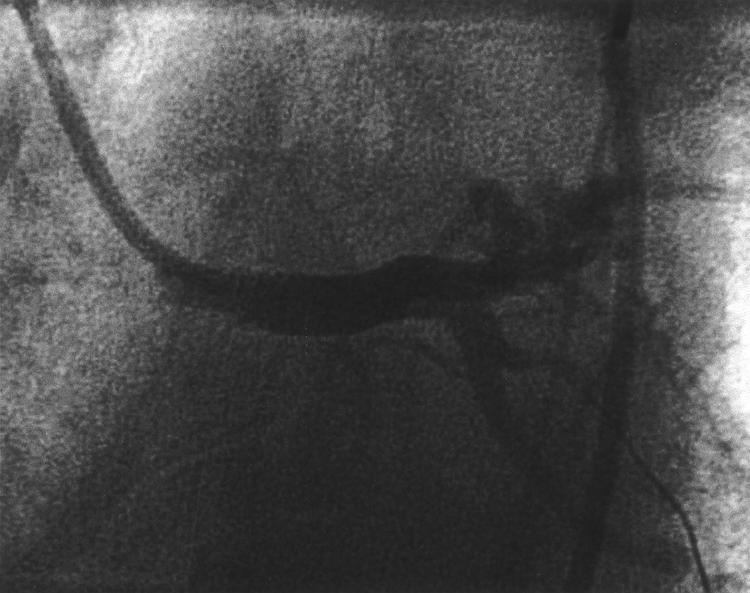
Figure 5.
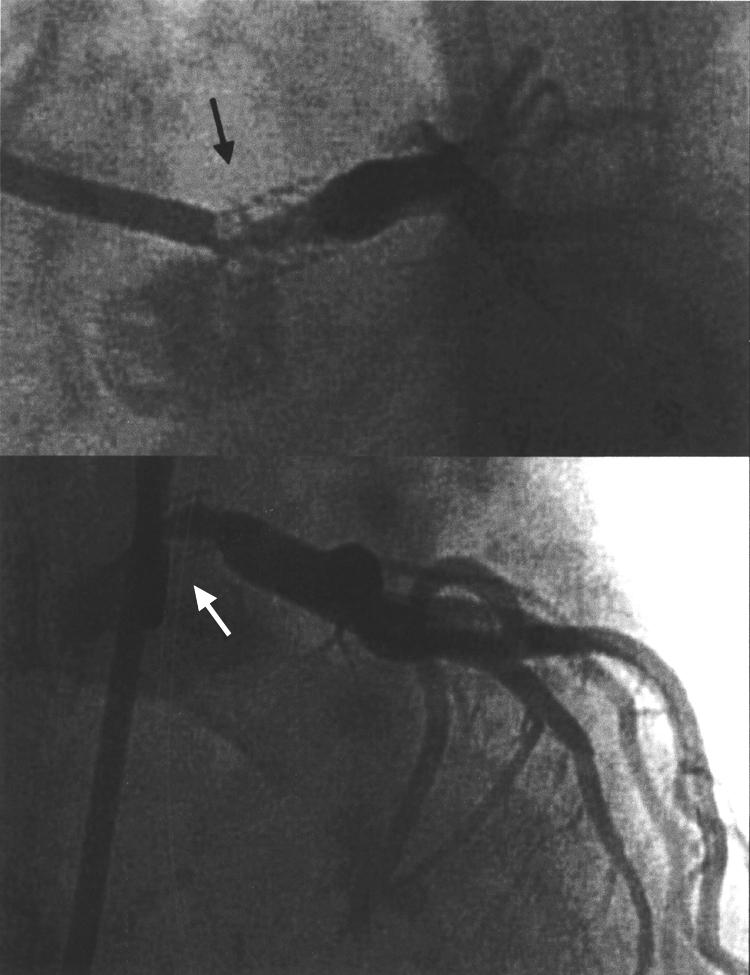
Follow up angiogram of the LMS stent showing severe in-stent stenosis three months after the initial procedure (black arrows).
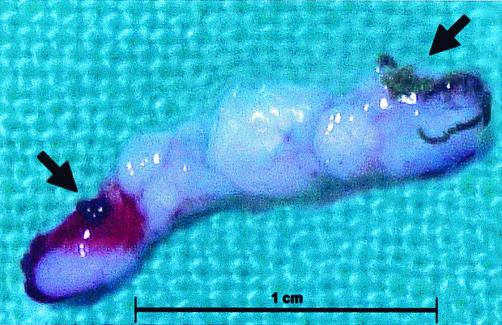
Figure 6.
Core of tissue excised by the directional atherectomy device from the in-stent LMS restenosis. Stent struts are visible at either end of the specimen (black arrows).
Figure 7.
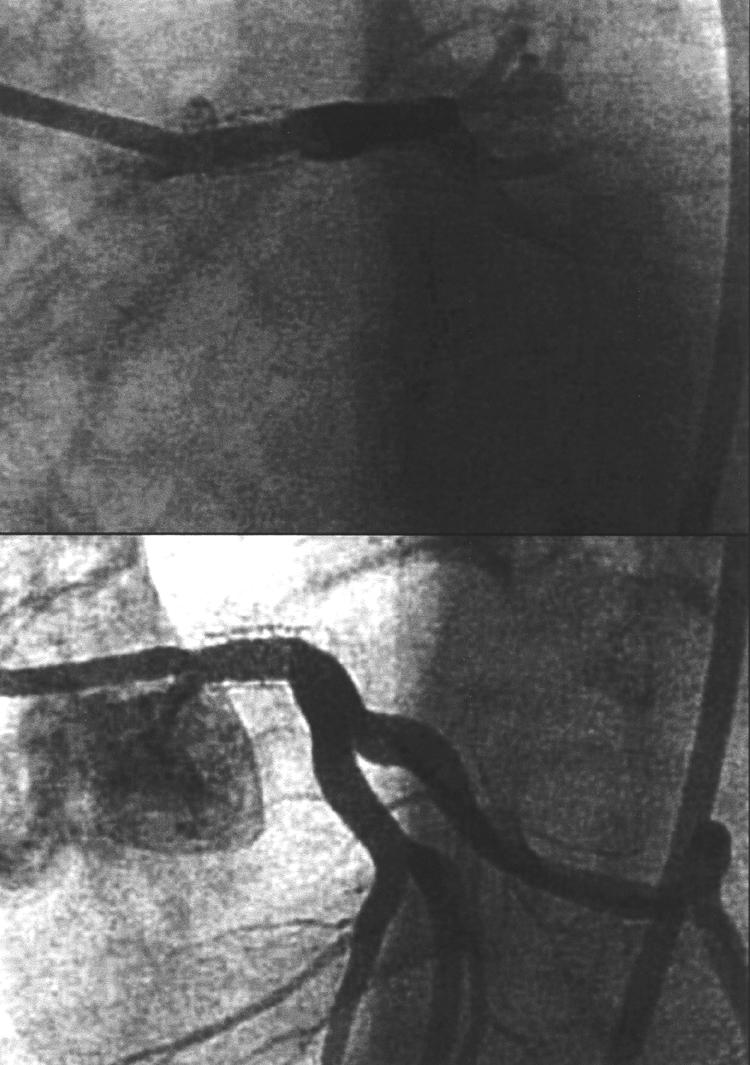
Result of directional atherectomy and balloon angioplasty to the in-stent stenosis of the LMS. There is now a good lumen within the LMS.
She remained free of symptoms and was discharged from hospital. A check angiogram was performed at a three month interval and showed no recurrence of the in-stent stenosis.
DISCUSSION
Takayasu arteritis is a chronic vasculitis involving the aorta and its main branches, the pulmonary arteries, and the coronary tree. It may lead to stenosis with obstruction caused by fibrosis and thrombus formation or aneurysm formation due to weakening of the vessel wall. It was first described in 1908 in a Japanese patient with retinal artery abnormalities but was later characterised as a more generalised arterial disease.2,3 The pathology is of a “panarteritis”, with intimal fibrous thickening and typical atheromatous lesions, medial smooth muscle and elastic lamina destruction, and medial and adventitial cell infiltration and fibrosis. With earlier diagnosis, fatality in the acute phase has been reduced and thus the problem of atherosclerosis has become more prominent.
The estimated worldwide incidence is 2.6 cases per million per year, with women more commonly affected than men. They usually have no risk factors for atherosclerosis and yet have atheromatous aortae, suggesting the importance of inflammation in atherosclerosis.4 The disease has been mainly studied in Japan but western series have also been published suggesting an older population at risk.1,5–10
No serological test is available for diagnosis of Takayasu’s disease.11 Therefore, diagnosis rests on clinical criteria (table 1).1,8,12,13 Cardiac features are present in up to 40% of cases. Aortic regurgitation secondary to aortic root dilatation may occur in 20% of cases. Hypertension may occur in a third of patients, usually related to renal artery stenosis.6,14 Angina pectoris is rarely a presenting feature but has been reported in 6–16% of cases.6,15,16 More rarely, pericarditis, palpitations, and congestive cardiac failure may occur. Pulmonary involvement occurs in 15–17% of cases.17–21 Pulmonary hypertension is well recognised.22 The lymphocytic alveolitis seen in our patient has not been previously described in Takayasu’s disease.
Table 1.
Takayasu’s arteritis: the American College of Rheumatology’s 1990 diagnostic criteria13
| • Patient below 40 years of age at initial diagnosis |
| • Claudication of extremities |
| • Reduced brachial pulse |
| • Difference in blood pressure between right and left arms >10 mm Hg |
| • Bruit above subclavian artery, aorta, or both |
| • Arteriographic appearance |
At least three criteria must be present for a diagnosis. Characteristic angiographic appearances are described.1
First line treatment of Takayasu’s arteritis is immunosuppression, primarily with corticosteroids. With glucocorticoid treatment, remissions occur in 40–60% of all patients. About 40% of all steroid resistant patients respond to the addition of cytotoxic agents. Approximately 20% of all patients are resistant to any kind of treatment.8,15,23 Methotrexate is an alternative immunosuppressive agent to cyclophosphamide and may induce remission if other treatments have failed.24
Angina pectoris is usually caused by premature coronary disease, in particular involving the ostia of the coronary arteries,25 but may be related to extrinsic compression of the coronary tree or a steal phenomenon.26,27 In addition, acute inflammation and the related oedema may be important, as highlighted by two cases where regression of an LMS stenosis was reported with administration of steroids.28,29
Treatment of the coronary disease is problematic, since arterial inflammation is persistent in over 40% of cases even after treatment.8 This would jeopardise the insertion sites of new grafts.30 Surgery may involve coronary bypass with venous rather than arterial conduit (coronary artery bypass graft) or endarterectomy through the aorta. In addition, patches may be used to enlarge diseased segments of the arterial tree.31–33 Studies of surgical series have reported an in-hospital mortality of 7.9–8.7%.25,34 Surgery may have been even more hazardous in our patient in view of her pulmonary hypertension.
Percutaneous transluminal coronary angioplasty (PTCA) has been reported previously in isolated cases of Takayasu’s disease.35–38 The use of intracoronary stents has also been reported.39–43 The largest series had only two cases, and the literature on management of in-stent restenosis in Takayasu’s disease is limited. We chose to use directional atherectomy to reduce the plaque burden before treating the in-stent LMS restenosis. Although some stent struts were excised, the final angiographic result and the clinical outcome three months later were excellent. Although rotational atherectomy (Rotablator rather than directional atherectomy) was described in a previous report on coronary revascularisation in Takayasu’s disease, this was not performed within the LMS artery.40
FDG-PET uptake has been used previously to image other forms of aortitis. Uptake can be reduced within two weeks of initiation of treatment in giant cell arteritis.44 FDG-PET uptake was increased acutely in a previously reported case of Takayasu’s arteritis but follow up data were not provided.45 Residual activity in Takayasu’s disease is difficult to detect based on plasma markers or clinical signs. We have shown that FDG-PET may allow the disease to be monitored non-invasively.
Summary
Takayasu’s arteritis needs to be considered in a young patient with angina, in particular when pulses are absent. This case illustrates the limitations of exercise testing in diagnosing the extent of coronary artery disease and the risks associated with coronary angiography in patients with inflammatory LMS disease. It also highlights the novel use of non-invasive scanning with FDG-PET in assessing remission from this disease. Revascularisation was performed with PTCA and stenting as an emergency procedure, but treatment of the restenosis with directional atherectomy was based on a review of the available literature.
Supplementary Material
Abbreviations
FDG-PET, positron emission tomography using 18-fluorodeoxyglucose
LMS, left main stem
PTCA, percutaneous transluminal coronary angioplasty
REFERENCES
- 1.Ishikawa K. Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu’s arteriopathy. J Am Coll Cardiol 1988;12:964–72. [DOI] [PubMed] [Google Scholar]
- 2.Numano F, Kakuta T. Takayasu arteritis: five doctors in the history of Takayasu arteritis. Int J Cardiol 1996;54(suppl):S1–10. [DOI] [PubMed] [Google Scholar]
- 3.Ohta K. Ein seltener, Fall von beiderseitigem Carotis-Subclavia verschluss: ein Beitrag zur pathologie der Anastomosis peripapillaris des Auges mit fehlendem. Trans Soc Pathol Jpn 1940;30:680–90. [Google Scholar]
- 4.Ross R. Mechanisms of disease: atherosclerosis-an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 5.Koide K. Takayasu arteritis in Japan. Heart Vessels Suppl 1992;7:48–54. [DOI] [PubMed] [Google Scholar]
- 6.Nakao K, Ikeda M, Kimata S, et al. Takayasu’s arteritis: clinical report of eighty-four cases and immunological studies of seven cases. Circulation 1967;35:1141–55. [DOI] [PubMed] [Google Scholar]
- 7.Zheng D, Fan D, Liu L. Takayasu arteritis in China: a report of 530 cases. Heart Vessels Suppl 1992;7:32–6. [DOI] [PubMed] [Google Scholar]
- 8.Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 9.Lande A, Bard R, Rossi P, et al. Takayasu’s arteritis: a worldwide entity. NY State J Med 1976;76:1477–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Numano F. Differences in clinical presentation and outcome in different countries for Takayasu’s arteritis. Curr Opin Rheumatol 1997;9:12–5. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis: a preliminary report from the International Network for the Study of the Systemic Vasculitides (INSSYS). Int J Cardiol 1998;66(suppl 1):S191–4; S195. [DOI] [PubMed] [Google Scholar]
- 12.Sharma BK, Jain S, Suri S, et al. Diagnostic criteria for Takayasu arteritis. Int J Cardiol 1996;54(suppl):S141–7. [DOI] [PubMed] [Google Scholar]
- 13.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 14.Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu’s arteritis: clinical study of 107 cases. Am Heart J 1977;93:94–103. [DOI] [PubMed] [Google Scholar]
- 15.Hall S, Barr W, Lie JT, et al. Takayasu arteritis: a study of 32 North American patients. Medicine (Baltimore) 1985;64:89–99. [PubMed] [Google Scholar]
- 16.Cipriano PR, Silverman JF, Perlroth MG, et al. Coronary arterial narrowing in Takayasu’s aortitis. Am J Cardiol 1977;39:744–50. [DOI] [PubMed] [Google Scholar]
- 17.Yamada I, Shibuya H, Matsubara O, et al. Pulmonary artery disease in Takayasu’s arteritis: angiographic findings. AJR Am J Roentgenol 1992;159:263–9. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Kamalakar T, Rajani M, et al. The incidence and patterns of pulmonary artery involvement in Takayasu’s arteritis. Clin Radiol 1990;42:177–81. [DOI] [PubMed] [Google Scholar]
- 19.He NS, Liu F, Wu EH, et al. Pulmonary artery involvement in aorto-arteritis: an analysis of DSA. Chin Med J (Engl) 1990;103:666–72. [PubMed] [Google Scholar]
- 20.Lupi E, Sanchez G, Horwitz S, et al. Pulmonary artery involvement in Takayasu’s arteritis. Chest 1975;67:69–74. [DOI] [PubMed] [Google Scholar]
- 21.Talwar KK, Kumar K, Chopra P, et al. Cardiac involvement in nonspecific aortoarteritis (Takayasu’s arteritis). Am Heart J 1991;122:1666–70. [DOI] [PubMed] [Google Scholar]
- 22.Lande A, Bard R. Takayasu’s arteritis: an unrecognized cause of pulmonary hypertension. Angiology 1976;27:114–21. [DOI] [PubMed] [Google Scholar]
- 23.Shelhamer JH, Volkman DJ, Parrillo JE, et al. Takayasu’s arteritis and its therapy. Ann Intern Med 1985;103:121–6. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman GS, Leavitt RY, Kerr GS, et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum 1994;37:578–82. [DOI] [PubMed] [Google Scholar]
- 25.Amano J, Suzuki A. Coronary artery involvement in Takayasu’s arteritis: collective review and guideline for surgical treatment. J Thorac Cardiovasc Surg 1991;102:554–60. [PubMed] [Google Scholar]
- 26.Viecili PR, Pamplona D, Cesena FH, et al. Unstable angina due to communication between the coronary artery and the right pulmonary artery in patient with Takayasu’s arteritis. Arq Bras Cardiol 1997;69:129–32. [DOI] [PubMed] [Google Scholar]
- 27.Nemec J, Garratt KN, Schaff HV, et al. Asymptomatic occlusion of the left main coronary artery by an aortic pseudoaneurysm. Mayo Clin Proc 2000;75:1205–8. [DOI] [PubMed] [Google Scholar]
- 28.Iga K, Gohma I, Hori K. Regression of the left main trunk lesion by steroid administration in Takayasu’s aortitis. Chest 1991;99:508–10. [DOI] [PubMed] [Google Scholar]
- 29.Isomatsu Y, Hoshino S, Tsukui H, et al. Regression of left main coronary ostium stenosis after surgical revascularization and steroid therapy. Jpn J Thorac Cardiovasc Surg 2000;48:594–6. [DOI] [PubMed] [Google Scholar]
- 30.Giordano JM, Leavitt RY, Hoffman G, et al. Experience with surgical treatment of Takayasu’s disease. Surgery 1991;109:252–8. [PubMed] [Google Scholar]
- 31.Morgan JM, Honey M, Gray HH, et al. Angina pectoris in a case of Takayasu’s disease: revascularization by coronary ostioplasty and bypass grafting. Eur Heart J 1987;8:1354–8. [DOI] [PubMed] [Google Scholar]
- 32.Nakano S, Shimazaki Y, Kaneko M, et al. Transaortic patch angioplasty for left coronary ostial stenosis in a patient with Takayasu’s aortitis. Ann Thorac Surg 1992;53:694–6. [DOI] [PubMed] [Google Scholar]
- 33.Swahn E, Karlsson JE, Fransson SG, et al. Coronary ostial stenosis operated on by patch technique in a young woman with Takayasu’s arteritis and angina pectoris. Eur Heart J 1993;14:1150–1. [DOI] [PubMed] [Google Scholar]
- 34.Ando M, Sasako Y, Okita Y, et al. Surgical considerations of occlusive lesions associated with Takayasu’s arteritis. Jpn J Thorac Cardiovasc Surg 2000;48:173–9. [DOI] [PubMed] [Google Scholar]
- 35.Sharma BK, Jain S, Bali HK, et al. A follow-up study of balloon angioplasty and de-novo stenting in Takayasu arteritis. Int J Cardiol 2000;75(suppl 1):S147–52. [DOI] [PubMed] [Google Scholar]
- 36.Park JH, Han MC, Kim SH, et al. Takayasu arteritis: angiographic findings and results of angioplasty. AJR Am J Roentgenol 1989;153:1069–74. [DOI] [PubMed] [Google Scholar]
- 37.Antelmi I, Magalhaes L, Caramelli B, et al. Salvage coronary angioplasty in a young patient with Takayasu arteritis and myocardial infarction. Arq Bras Cardiol 1993;60:37–8. [PubMed] [Google Scholar]
- 38.Lee HY, Rao PS. Percutaneous transluminal coronary angioplasty in Takayasu’s arteritis. Am Heart J 1996;132:1084–6. [DOI] [PubMed] [Google Scholar]
- 39.Bali HK, Jain S, Jain A, et al. Stent supported angioplasty in Takayasu arteritis. Int J Cardiol 1998;66(suppl 1):S213–7. [DOI] [PubMed] [Google Scholar]
- 40.Son JW, Koh KK, Dang Q, et al. Recurrent restenosis following stent and rotational atherectomy of coronary artery stenosis in Takayasu’s arteritis. Int J Cardiol 1998;65:295–300. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov NA, Kagan-Ponomarev MI, Zhdanov VS, et al. A case of using Palmaz-Schatz intracoronary stent in a patient with nonspecific aortoarteritis. Vestn Rentgenol Radiol 1997;Jul-Aug:30–3. [PubMed]
- 42.Punamiya K, Bates ER, Shea MJ, et al. Endoluminal stenting for unprotected left main stenosis in Takayasu’s arteritis. Cathet Cardiovasc Diagn 1997;40:272–5. [DOI] [PubMed] [Google Scholar]
- 43.Linnemeier TJ. Left main coronary disease: is stenting an option? Cathet Cardiovasc Diagn 1997;40:276. [DOI] [PubMed] [Google Scholar]
- 44.Turlakow A, Yeung HW, Pui J, et al. Fludeoxyglucose positron emission tomography in the diagnosis of giant cell arteritis. Arch Intern Med 2001;161:1003–7. [DOI] [PubMed] [Google Scholar]
- 45.Hara M, Goodman PC, Leder RA. FDG-PET finding in early-phase Takayasu arteritis. J Comput Assist Tomogr 1999;23:16–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.