Much of medicine is concerned with choosing the right treatment, and cardiologists have done well in recent years to ensure the choice is supported by good evidence. However, the greatest choice often starts where the evidence finishes—namely between drugs of the same class. This article seeks therefore to offer the principles which govern when and how it is appropriate to differentiate drugs within a class, and discuss topical examples among the drugs commonly used in cardiology.
GENERAL OVERVIEW
The parameters which can reasonably be compared between drugs are shown in table 1. If this were all, it might be possible to devise semi-automatic algorithms to calculate which drug(s) have the highest score for any given indication. However, the relatively factual answers that could be filled in each cell of the table for a given drug are only part of the decision making process, and more judgemental are: (1) the strength of evidence for each of the answers; and (2) the second order issues of how to compare, for example, one drug of apparently superior efficacy with another which is better tolerated. Compliance is sometimes cited as a reason for choosing one drug rather than another; compliance is not itself a property of the drug, but a composite phenomenon reflecting the interplay between efficacy, tolerability, frequency or route of administration, and cost. Particularly contentious are questions of cost effectiveness. For example, the quality of the evidence for effectiveness may vary between drugs; or the cost of two drugs may be differentially influenced by factors like laboratory tests or number of visits, where savings can seem more virtual than real.
Table 1.
Parameters influencing rational choice among drugs
| Category | Parameter |
| Pharmacodynamic | Efficacy |
| Surrogate | |
| Morbidity | |
| Mortality | |
| Potency | |
| Pharmacokinetic | Route of administration |
| Frequency of dosing | |
| Drug interactions | |
| Tolerability | Safety |
| Side effects | |
| Cost | Per drug/injection |
| Per treatment (including doctor/nurse/patient/lab time) | |
In this article I shall address some of the controversies regarding choice of drugs from commonly used classes in cardiology: angiotensin converting enzyme (ACE) inhibitors, statins, and β blockers. To illustrate how the parameters in table 1 will be used to resolve controversies, I shall first apply them to non-controversial examples of choices between drugs in a class.
Efficacy
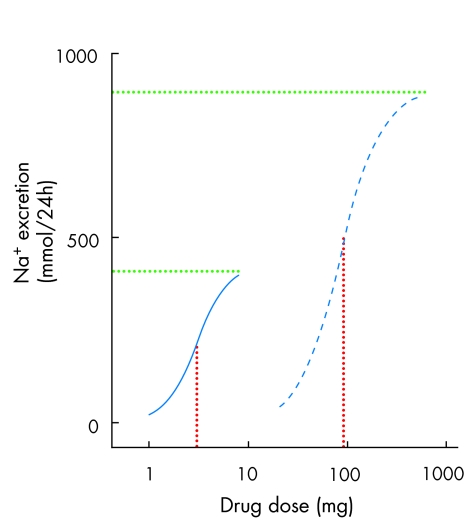
Among diuretics, the loop diuretics cause more sodium (Na+) loss than thiazides because they inhibit a more proximal Na+ channel (the Na+ K+ 2Cl transporter) than the NaCl co-transporter inhibited by thiazides. The term “high ceiling” diuretics refers to the relative shape of the dose–response curve for loop compared to thiazide diuretics (fig 1). Greater efficacy is not an automatic reason for preferring a drug, even when there is no counterbalancing disadvantage in one of the other parameters. When only modest Na+ loss is required, thiazides have the advantage that there is less scope for overdosing the patient.
Figure 1.
Dose response curves comparing furosemide (frusemide) (blue dashed line) and bendrofluazide (blue continuous line). The green dotted lines measure potency, by showing the x axis coordinate for the physical mass of drug at which 50% maximal response is achieved. The red dotted lines measure efficacy, by showing the y axis coordinate for the maximum response achieved. Bendrofluazide has greater potency (50% response at lower dose than furosemide), but furosemide has greater efficacy.
Potency
This parameter can provide the best justification for differentiation within a class, but is also the parameter most often abused in providing spurious justification. In part, the latter occurs because potency and efficacy are often confused. Efficacy refers to the maximum response to a drug; potency refers to the physical mass of drug at which half maximal response occurs. When, loosely, doctors or lay people describe drug A as being “more powerful” than drug B, they are probably referring to efficacy. Under most circumstances the physical mass of drug required to achieve a response is not important. This is illustrated in fig 1, which compares the natriuresis achieved with furosemide (frusemide) or bendrofluazide. The latter is more potent, but the consequentially smaller size of bendrofluazide than furosemide tablets is rarely a reason to prescribe one rather than the other.
Where potency is important is in locally administered drugs. Here the amount of drug in physical contact with a mucosal (or other) surface is critical to determining the pharmacological response. A classic example is the glucocorticoid steroids, where the advent of fluorinated steroids enabled small but effective amounts of drug to be solubilised within an aerosol and used as inhaler treatment for asthma.
Nevertheless, there is a theoretical reason why potency might affect choice of some oral medications. One of the main factors to determine the potency of a drug is its affinity for the target molecule (for example, receptor, ion channel, enzyme). The affinity of a drug for its target is the ratio of its time-for-dissociation to time-for-association. The latter does not vary greatly, being caused mainly by diffusion. So in practice high affinity drugs are those which stick to their target for longer. It is often the case, therefore, that drugs with the lowest dose range within a class are those most likely to have sustained local actions, even when the drug has disappeared from the bloodstream.
Pharmacokinetics
A common reason for choice within a class is frequency of administration. Antibiotics yield a number of examples: once daily azithromycin compared to four times daily use of the older erythromycin; or twice daily doxycycline rather than four times daily tetracycline. Other examples are the development of slow release formulations of drugs (such as nitrates and calcium blockers) which permit less frequent dosing than required for the parent drug. Long lasting is not always better. Short acting nitrates and β blockade (esmolol) have specific roles in treatment. Insulin has been modified to provide short acting formulations for the convenience of covering individual meals.
General overview: key points.
Drugs in the same class are rarely compared with each other
The class paradox, therefore, is that we have no type A evidence to answer the question whether benefits shown for one drug extend to others in the class
There are a small number of parameters by which drugs can be compared. Cost should be the primary parameter for choice, usually after some members have become generic
Cost is overridden as a primary parameter only when an assessment is possible of relative benefit for specific indications, based on non-surrogate efficacy and safety data
Potency is unimportant except for some specific (for example, local) applications, where the physical mass of drug employed is critical
Secondary reasons for choice are either surrogate efficacy (for example, blood pressure, low density lipoprotein), pharmacokinetic (for example, longer dose interval) or tolerability differences
Where data from direct comparisons are lacking, higher drug prices can be justified only by increased probability of benefit or increased convenience of use
Tolerability and safety
Can drugs within the same class vary in their tolerability and safety? Yes, for either pharmacodynamic or pharmacokinetic reasons. For example, metoclopramide and ondansetron are both anti-emetics which block the 5HT3 receptor. But metoclopramide also blocks dopamine receptors and therefore at equi-effective doses to ondansetron causes akathisia. Pharmacokinetic differences are a more common cause of differences in tolerability. Short acting α blocking or vasodilator drugs are more likely to cause hypotension or reflex symptoms of tachycardia and flushing. Long acting sulfonylureas are more likely to cause hypoglycaemia. Drug interactions vary because the drugs are substrates for different isoforms of cytochrome P450; cimetidine but not ranitidine increases drug concentrations of warfarin and phenytoin.
Cost
This is the parameter which will most immediately be perceived by doctor, patient, pharmacist or manager. It is hard to argue against a policy of always prescribing the cheaper of two drugs unless the more expensive has a proven advantage. On the other hand, drug costs are an artificial property of the drug in the sense that they vary with time and place of prescribing.
My example is taken from diabetes to illustrate the pros and cons of letting price influence choice within a class. Among sulfonylureas, generic glibenclamide is now the sulfonylurea of choice, but it not promoted and therefore often replaced by branded drugs like glipizide or gliclazide. However, promotion of branded drugs is one of the main, if not always balanced, modes of education about drugs. When the UK prospective diabetes study showed metformin to be the drug of choice for type 2 diabetes, the take up was more rapid in the USA, where metformin was still a branded drug, than in the UK.
After this illustration of fairly non-contentious choices among drugs within a class, I turn now to recent areas of controversy among cardiovascular drugs.
ACE INHIBITORS AND ANGIOTENSIN BLOCKERS
Under this heading, I shall consider the choices within each of the two groups—ACE inhibitors and angiotensin blockers. But I shall also digress slightly from the main brief of the article to discuss whether there are important differences between them.
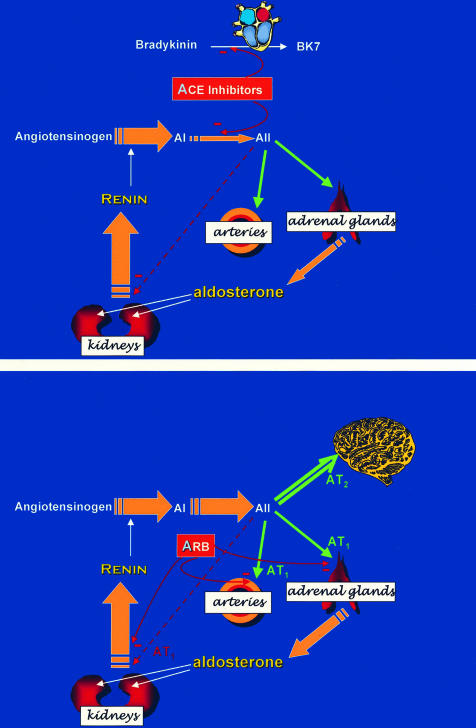
The mechanisms of action of the two groups of agents are illustrated in fig 2.
Figure 2.
Mechanism of action of ACE inhibitors (upper panel) and angiotensin blockers (lower panel). ACE inhibitors achieve their effects both by inhibiting conversion of the inactive decapeptide angiotensin 1 (AI) to the active octapeptide angiotensin II (AII), and by inhibiting breakdown of the vasodilator nonapeptide bradykinin. Angiotensin blockers (ARB) act purely by antagonising actions of AII at the AT1 receptor on arteries and adrenal cortex. Both classes cause increased secretion of renin and AI, by removing the negative feedback of AII; however, AII increases in parallel during ARB treatment, but falls during ACE inhibitor treatment.
ACE inhibitors
The British National Formulary lists 11 ACE inhibitors. Captopril is the only ACE inhibitor which is not a pro-drug, acts immediately, and has much the shortest duration of action. The latter has relegated its use in Europe to that of a diagnostic agent only (including first dose use in heart failure); but worldwide, low cost makes captopril the most widely used drug of its class. Enalapril is also available in generic formulations, and its low cost is a definite advantage that needs to be offset if branded ACE inhibitors are prescribed in its place. Using the principles from the first half of the article, what could these advantages be? The main one is pharmacokinetic, since enalapril at lower doses needs to be given twice daily to provide effective 24 hour ACE inhibition. The duration of action of any ACE inhibitor is increased by increasing the dose, because this prolongs the time for which pharmacologically effective inhibition of ACE (> 95%) is present. However, enalapril at 40 mg daily no longer retains a cost advantage over branded drugs in the class. Although the most popular ACE inhibitors have only slightly longer durations of action than enalapril, the outcome data justifying long term use of enalapril derives from trials employing twice daily administration, whereas all ACE inhibitors other than captopril and enalapril were prescribed once daily in their outcome trials.
In hypertension, there are currently no data to justify the popularity of lisinopril, but this may be rectified by the double blind comparison of lisinopril with chlorthalidone in the ALLHAT study.
Some high affinity inhibitors, such as ramipril and quinapril, may bind to tissue ACE and achieve longer lasting inhibition than the original drugs in the class.1 w1 w2 Angiotensin II (AII) plays an undesirable role in endothelial cells by stimulating NADPH oxidase to produce superoxide that inactivates nitric oxide.2 w3 In the heart, locally produced AII can stimulate hypertrophy, fibrosis, and apoptosis.w4–7 However, there is no evidence of differences between drugs in prevention of these surrogates.
So far, then, there is little to support use of a specific ACE inhibitor for their common indications of hypertension or left ventricular dysfunction/failure. But how about the main area of controversy, concerning novel indications for ramipril and perindopril? The HOPE and PROGRESS trials have shown that when these drugs are added to other treatments in patients with existing cardio- or cerebrovascular disease, they confer a pronounced and significant benefit (compared to addition of placebo) in improving outcome.3,4 The question is whether this benefit is a class effect, or one that can be claimed only by the drugs used in the specific trials—ramipril in HOPE, perindopril in PROGRESS.
The purpose of the general arguments in the first part of this article is to pre-empt special pleading for individual drugs or cases. In other words, any non-class benefits must be due to parameters in table 1. It may be that these relatively high affinity ACE inhibitors cause greater local benefits within the arterial wall than other members of the class, but there is little evidence to support this. Indeed, the publication of a small sub-study from HOPE showing notable variation in blood pressure control over 24 hours argues against the “high potency/long duration” thesis for ramipril.5 I do not therefore consider there to be a strong case on the grounds of efficacy for putting individual drugs before class.
However, when using drugs long term, the parameter of most importance is safety. This does not need randomised controlled trials to be assessed—indeed, even the largest outcome trials are still too small and short to detect the 1/10 000 serious side effects that kill otherwise desirable drugs. When drugs are launched for a new indication, the balance of safety to efficacy cannot be extrapolated from previous use. So when the question is raised whether ramipril may be unique in the benefits conferred on the HOPE population, and perindopril in the post-stroke population, the answer is “no” for efficacy alone, but “unproven” for the critical measure of efficacy/safety. Ironically, neither drug was very effective in lowering blood pressure in these populations: ramipril by only 3 mm Hg more than placebo, and perindopril by 5 mm Hg.4 These small falls are unsurprising in an older, generally low renin population,6 and have raised the question whether the benefit of ACE inhibition outside heart failure (where renin is activated) is caused by an alternate mechanism, like bradykinin potentiation.w8 While this article is not the place to resolve such debate, the point is that drugs often have effects (good and bad) additional to their primary known action. These effects may well have different dose–response relations, and it is likely, for example, that smaller doses of an ACE inhibitor are required for increasing substrate concentrations (angiotensin I or bradykinin), than for reducing product (angiotensin II) concentrations. Bradykinin potentiation may be desirable in increasing endothelial cell production of nitric oxide, but bradykinin and other neuropeptides metabolised by ACE have also been blamed for the cough and angioneurotic oedema.7 w9 The relative importance of these pathways is increased in low renin patients, and it is for this reason that there can be legitimate concern about long term use of untested ACE inhibitors in the normotensive HOPE or PROGRESS type of patient. The argument, however, needs to be set against cost. Where funds are limited, the cheapest ACE inhibitor will be employed in these patients. Where the healthcare system or individual patient wishes to maximise benefit (efficacy v risk), only the trial drug should be used for specific indications. Strictly, the higher cost does not itself purchase increased benefit for the patient, but increases the probability of such benefit.
ACE inhibitors: key points.
For hypertension and heart failure, benefits are likely to be class effects, and there are no primary reasons for preferring individual drugs
For newer indications, in which only one drug has been tested, efficacy is probably a class effect but equal safety cannot be assumed
For these newer indications, the trial drugs, ramipril and perindopril, should be used unless greater cost reduces the number of patients who can be treated by more than the possible increase in safety
Angiotensin blockers (“sartans”)
Pharmacology permits a more precise comparison of drug efficacy among antagonists of cell surface receptors than among drugs affecting ion channels or intracellular pathways. This has permitted more class warfare among the angiotensin blockers than most cardiac drugs. On available evidence, some of the sartans introduced after losartan not only lower blood pressure at maximal dose by more than losartan, but also demonstrate greater reduction in the blood pressure response to exogenously infused angiotensin II.8 w10 While losartan 100 mg was more expensive than other sartans, and had no outcome data to support its use, there was a case for preferring irbesartan or candesartan for antihypertensive treatment. But the confidence intervals around results in the head-to-head comparisons of sartans leave open the clinical significance of differences, and meta-analysis has also been equivocal.9 w11 w12 In hypertensive patients, all three of the sartans mentioned have outcome data to support their value in preventing either stroke or renal impairment, most impressive in diabetic patients.10,11 Even if the impressive results of the LIFE trial, comparing losartan and atenolol, were helped by the problems with atenolol discussed later, the long term safety data with losartan in patients with left ventricular hypertrophy now confers a similar advantage in hypertension as discussed above for selected ACE inhibitors.12 It is now reasonable, if cost differentials are removed, to use the drug with most outcome data for initial treatment, switching to a newer sartan if blood pressure control is unsatisfactory.
If the weight of outcome evidence now favours sartans over ACE inhibitors in preventing nephropathy in type 2 diabetes, and maybe stroke in hypertension, the reverse is true for cardiac end points. Stroke prevention by sartans has been speculatively attributed to a neuroprotective effect of increased angiotensin II concentrations acting upon the unblocked AT2 receptor in the brainw13; myocardial protection by ACE inhibitors is, with greater certainty, caused in part by bradykinin potentiation.w14 w15 The ONTARGET trial is comparing the two subclasses of drug, and the hypothesis that differences between them render a combination more effective than either alone.w16 Pending further trial data, there is little support for using a sartan in heart failure patients unless the patient develops a cough on an ACE inhibitor; addition of one to the other begs the question whether the first was used at maximal dose.w17–19 Since ACE coughers have so far been excluded from the class comparisons, there is no reason to delay prescribing sartans for such patients. Their efficacy should be established during next year by the subgroup of the CHARM study which is comparing sartan with placebo in patients intolerant of ACE inhibition.
STATINS
This is the easiest of the classes discussed here to prepare a scoresheet for each drug, using the parameters listed in the first part of this article.
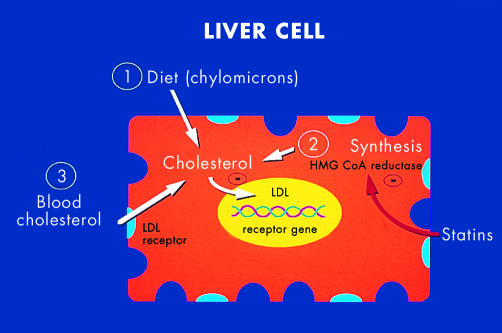
Figure 3 illustrates the mechanism of action of the statins, or HMG-CoA reductase inhibitors.
Figure 3.
Mechanism of action of statins (hydroxymethylglutaryl coenzyme A reductase inhibitors). Circulating low density lipoprotein (LDL) is not regulated but intracellular cholesterol is adapted to requirements. In most cells except hepatocytes, LDL synthesis is switched off. In the liver, cholesterol derives from diet, synthesis, and circulating LDL. Statins inhibit synthesis, so that hepatocytes are stimulated to increase expression of LDL receptors.
In table 2 I have given each parameter a score out of 10 based on my reading of available evidence, including the strength of this evidence. The numbers are therefore all open to debate; the point of the exercise is to illustrate how choices between drugs should be discussed, rather than to provide definitive answers. Cerivastatin is included to illustrate how it should have featured in choices made before the first deaths were announced.
Table 2.
Semi-quantitative method for choosing among statins
| Category | Parameter | Simvastatin | Pravastatin | Atorvastatin | Rosuvastatin | Cerivastatin |
| Pharmacodynamic | Efficacy | |||||
| Reduced mortality | 8 | 8 | 6 | ? | 2 | |
| Reduced morbidity | – | – | – | – | – | |
| Surrogate (LDL reduction) | 8 | 7 | 8 | 8 | 6 | |
| Potency | 4 | 3 | 7 | 8 | 10 | |
| Pharmacokinetic | Route of administration | 10 | 10 | 10 | 10 | 10 |
| Frequency of dosing | 10 | 10 | 10 | 10 | 10 | |
| Drug interactions | 6 | 8 | 7 | 8 | 4 | |
| Tolerability | Safety | 9 | 8 | 8 | 7 | 2 |
| Side effects | 9 | 9 | 9 | 8 | 6 | |
| Cost | Per drug/injection | 5 | 3 | 8 | 8 | 9 |
| Per treatment (including doctor/nurse/patient/lab time) | 4 | 2 | 8 | 9 | 7 |
The table scores five of the statins out of 10 (= most desirable) for each of the parameters by which drugs should be compared. The scores are not themselves definitive, but illustrate how a doctor can summate available evidence to choose the best drug, depending on the weight attached to each of the parameters.
Statins have been arguably the greatest success story in therapeutics for several decades. They save lives, millions of them. This fact should put into perspective the decision processes made using the table. Although each row is scored out of 10, the rows should be weighted for relative importance. Drugs shown to reduce mortality have an almost unanswerable advantage over other drugs, whatever counterbalancing benefits the latter might claim. In this class, the demise of cerivastatin with over 100 deaths shows the overriding importance not only of drug safety, but the size of the evidence base upon which this has been assessed.
Simvastatin was the first statin to demonstrate that effective low density lipoprotein (LDL) reduction leads to the expected reduction in cardiovascular end points, and can be used as a yardstick to compare the other principal statins. The recent Heart Protection Study showed how remarkably safe the drug is, probably paving the way for over-the-counter use13; and by treating virtually allcomers with diabetes or atheroma showed that the correct indication for statin use is risk, not risk factor.
Pravastatin is probably, at the appropriate dose, interchangeable with simvastatin, and in several major outcome studies has also achieved at least the expected reduction in end points.14 w20 It is less potent, meaning that almost twice the dose is required to achieve the same reduction in LDL. This would probably have been of little consequence if appropriately priced, but pravastatin has suffered from appearing expensive relative to simvastatin for a given reduction in LDL. Pravastatin has the advantage of a primary prevention trial, where simvastatin has not been studied.14 In secondary prevention, both these statins have an impressive efficacy and safety record.
There are technical differences between these drugs, based on the number of patient-years and definition of patients studied. The more interesting questions are whether 40 mg of either drug confers maximal benefit, and whether LDL reduction explains the entire benefit of both drugs. Body count (alias outcome) trials are notoriously poor at inferring mechanisms. The similar relative benefit of simvastatin at all baseline concentrations of LDL in the Heart Protection Study might argue either for the importance of reducing even “normal” concentrations of LDL, or for a non-LDL based action. Further outcome trials with both drugs are in progress to address these possibilities: SEARCH, which compares 80 mg with 20 mg of simvastatin; PROVE-IT, which compares pravastatin and atorvastatin at non-equieffective doses.w21 w22 If some or all statins owe part of their clinical benefit to anti-inflammatory effects outside of hepatocytes, pravastatin’s lower efficacy in lowering LDL would be less important.w23–26
Statins: key points.
Available drugs vary in their efficacy and potency in reducing LDL
The rank order of efficacy for LDL reduction is atorvastatin, simvastatin, pravastatin for the equally priced 40 mg of each drug
The rank order for observed reduction in cardiovascular mortality is flatter and appears to be simvastatin, pravastatin, atorvastatin
Further trials in progress should resolve whether greater LDL reduction matters, and whether some or all statins have benefits other than LDL reduction
Safety appears equal for the three main statins
Deaths in patients receiving cerivastatin caused its withdrawal and emphasise the overriding importance of documented safety when choosing between drugs
Atorvastatin was rapidly adopted by cardiologists impressed by its high potency and possibly greater efficacy in reducing LDL. Potency, as discussed above, is irrelevant for an oral drug, but became confused with the issue of price. Not entirely by accident, the starting dose of atorvastatin, 10 mg, is more effective than the similarly priced 10 mg starting dose of its main competitors. But the drug is not, to a pharmacologist, more effective unless the maximal response is greater. After atorvastatin was launched, the previous maximum clinical dose of simvastatin, 40 mg, was belatedly noted to have only submaximal effects in reducing LDL, and simvastatin 80 mg tablets are now available at the same price as 40 mg tablets of simvastatin or atorvastatin. Atorvastatin’s weakness until recently should have been the paucity of long term efficacy and safety data.15 w27 Ironically, the massive uptake of atorvastatin without long term efficacy data means that the absence of reported problems is strongly reassuring about long term safety. Atorvastatin’s main advantage is cost, mainly at lower doses. Maximum efficacy/safety is purchased with simvastatin. More patients can be treated with statins if a fixed sum is spent on atorvastatin—but only at the 10 mg dose which is of unproven long term efficacy.
Cerivastatin was withdrawn following deaths from severe myopathy. Before then, it had the apparent attraction of being cheaper than other statins, and was assumed to have the same long term benefits as seen for other drugs which reduced LDL. Why cerivastatin was less safe remains uncertain, as indeed does the mechanism of the myalgia and myopathy seen in a small proportion of patients on any statin. The unpredictability underlines the importance of having empirical data for individual members of a drug class. Cerivastatin’s high potency was consequent on a high affinity for its target molecule, the enzyme HMG-CoA reductase. As discussed earlier, high affinity is usually a consequence of slow dissociation of a drug from its target and it may be that either the long lasting enzyme inhibition—or cellular compensation for this—led to the toxic consequences.
β BLOCKERS
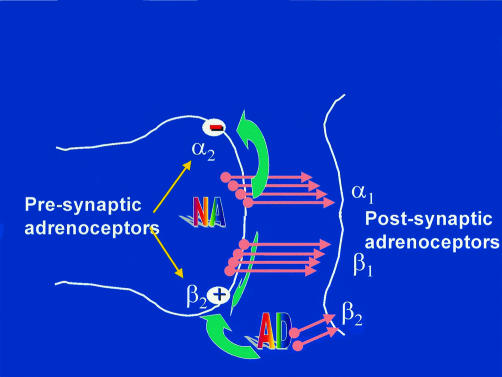
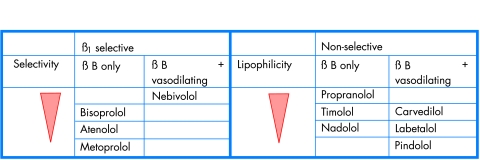
β Blockers, the oldest of the three classes discussed in this article, have the broadest range of indications, embracing hypertension, angina, and (for a few members of this class) heart failure. Figure 4 illustrates their mechanism of action. Although the parameters used to compare drugs are the same as before, there are clear pharmacological differences within the class (fig 5). These differences do not themselves justify choices, but they explain some observed differences in the selection parameters.
Figure 4.
Mechanism of action of β blockers. The principal agonist at β adrenergic receptors is noradrenaline (norepinephrine) released from sympathetic nerve endings. The heart has both β1 and β2 receptors on the postsynaptic side of the cleft; β2 receptors are activated mainly by circulating adrenaline. There are also α and β presynaptic receptors on the nerve ending, which mediate respectively inhibition and facilitation of noradrenaline release. The presynaptic α2 receptors are of a different class from the postsynaptic α1 receptor, which is responsible mainly for vasoconstriction of arteries. Available β blockers vary in how many of the receptors shown in the diagram are blocked, including some mixed α1 and β blockers.
Figure 5.
Selective and non-selective β blockers (βB).
The first β blocker, propranolol, is still used widely worldwide, and—unlike captopril—long acting formulations have helped to preserve a significant if minor share of β blocker use even in developed countries. Since the more popular alternative in this case is an equally cheap old drug, atenolol, the difference in use reflects a real difference in tolerability rather than the legacy of successful marketing. The difference in tolerability is due to two major differences in pharmacology: receptor subtype selectivity and lipid solubility. Indeed, had the manufacturers not been so keen in the late 1970s to promote atenolol as having, unlike propranolol, no dose–response curve, they would have introduced it at 25–50 mg doses rather than the supramaximal doses of 100–200 mg that lose the benefits of β1 selectivity.
Different types of β blocker
There are two β receptor subtypes, β1 and β2. Non-selective β blockers like propranolol and timolol block both receptors to a similar degree (fig 5). β1 Selective blockers, like atenolol, metoprolol and bisoprolol, block the β1 receptor at lower doses than they block the β2 receptor. It is important to recognise that selectivity is a relative property, being measured as the ratio of drug concentration required to block the two receptors, and therefore any benefits of selectivity are progressively lost as the dose is increased. The term “cardioselective” was wrongly introduced as synonomous with β1 selective, on the mistaken assumption that the human heart does not have β2 receptors. Both are present, although the latter are probably of importance only during secretion of high concentrations of adrenaline—in heart failure and during myocardial infarction.16 w28 Therefore the so-called cardioselective blockers may actually be less efficacious than non-selective β blockers in patients with, or at risk from, these conditions.17 Atenolol, the least lipophilic β blocker, is rapidly washed out of the heart after disappearing from the bloodstream.w29 Because of cross talk between the components of the cyclic AMP signalling system coupled to both β receptors, atenolol not only fails to block but actually potentiates cardiac responses to adrenaline.w29 w30
Choice of β blockers in ischaemic heart disease
In the heyday of β blocker trials in the 1980s, intra-class comparisons of drugs were uncommon, so that we do not know how much theory affects practice. But post-hoc comparison of the many secondary prevention trials of β blockers showed most benefit from propranolol and timolol, and the least from the now obsolete practolol.w31 Although atenolol is the most widely used β blocker, it was never tested for secondary prevention, and when used in acute treatment of myocardial infarction achieves only modest protection, mainly from cardiac rupture rather than arrhythmias.w32 On the other hand, except for those with ancillary vasodilator properties (fig 5), non-selective β blockers have a lower tolerability than β1 selective, almost certainly because in patients with normal cardiac output this is reduced more by blockade of both than one receptor subtype. Consequently tiredness and cold extremities are more likely to occur. Use of β blockers after myocardial infarction has tended to suffer from the lack of evidence for their role in the era of thrombolysis and ACE inhibition. The more recent CAPRICORN study showed that carvedilol, which blocks the three major adrenoceptors—α1, β1, and β2—does improve overall survival, although the trial’s impact was diminished by a change in planned end points during the trial.w33
Choice of β blockers in hypertension
In hypertension, where the efficacy of blood pressure reduction is due mainly to blockade of the renal β1 receptor on the renin secreting juxtaglomerular cells, there is no need to contemplate use of non-selective β blockers. In our crossover studies, bisoprolol was as well tolerated as any other class of antihypertensive drug.w34 However, the LIFE trial has recently shown that the prototype β1 blocker, atenolol, is problematic in hypertension, and reminded us how little evidence there is of long term efficacy with atenolol.12 In combination with another older drug, hydrochlorothiazide, atenolol was less effective than the comparator in preventing diabetes; this is probably because both older classes reduce blood flow to skeletal muscle, whereas the newer antihypertensives increase flow. Even more serious was the excess of strokes on atenolol, by almost twofold in patients with stiff arteries (isolated systolic hypertension).w35 This excess may be caused by the unique property of β blockers (unless they have additional vasodilating activity) that central systolic pressure is reduced less effectively than appreciated from measurements in the brachial artery.w36 By slowing heart rate, most β blockers allow reflection of the systolic pulse wave from a stiff aorta to return before the end of systole and thus augment the central aortic pressure.18 w37
Choice of β blockers in heart failure
There have been few such dramatic discoveries in therapeutics than heart failure’s change from absolute contraindication to major indication for use of β blockade. The small choice of licensed drugs for this indication belies the competition, as cardiologists grapple with the clinical significance of pharmacological differences. Three drugs—carvedilol, bisoprolol, and a controlled release formulation of metoprolol—demonstrated large reductions in mortality, with the first two receiving regulatory approval for “adjunct use” in heart failure.19 w38 It is likely, therefore, that benefit is a class effect. However, one other β blocker, bucindolol, was ineffective.w39 This negative result was attributed to a partial agonist effect, although this property of bucindolol has been questioned (see below).w40 Because of the bucindolol result, because of the well known dangers of inappropriate β blocker use in heart failure, and because the mechanism of benefit remains poorly understood, heart failure is the clearest example of where only individual proven drugs should be used.
Between the two licensed drugs there are some interesting differences, as apparent in fig 5. Plasma noradrenaline is one of the major prognostic factors in heart failure, maybe contributing to arrhythmias and cardiomyocyte apoptosis.w41 If noradrenaline is not just a marker of risk, the question is whether its adverse effects are on only some or all adrenoceptors. Figure 4 suggests that stimulation of all might be adverse except for the negative feedback α2 autoreceptor. When vasodilators were introduced for heart failure, α blockade was found to increase ejection fraction and improve early symptoms whereas mortality was unaltered—perhaps because of the baroreflex induced secretion of catecholamines.w42–44 β Blockade also increases catecholamine concentrations, by reducing their clearance. In theory, therefore, a drug with the early benefit of α blockade, and later benefit of protecting all adrenoceptors from catecholamine excess, might maximise potential efficacy and safety. This multiple receptor blockade is achieved by carvedilol, which also blocks the low affinity (previously called β4) site on β1 receptors that seems to have been the site of activation by bucindolol.w45 The rank order of β blocker efficacy in outcome trials correlates with the pharmacological notional rank order for the “net” number of receptors blocked. Carvedilol reduced mortality by 65% in the first outcome trials20 and by 27% in a trial restricted to patients with New York Heart Association (NYHA) functional class III or IV.21 w46; bisoprolol and metoprolol CR both reduced mortality by 34% in trials of NYHA II–IV. However, comparisons of trials cannot prove a hypothesis, and against the case for efficacy should be offset the greater cost and dose frequency of carvedilol. In assessing overall cost effectiveness, this example illustrates the influence of strength of evidence. If COMET,w47 the first outcome trial ever to compare β1 selective (metoprolol) and non-selective (carvedilol) β blockade, confirms the superiority of the latter, it will be possible to calculate the number of lives saved per annum for each extra pound spent on the more expensive agent.
β BLOCKERS: KEY POINTS.
Variation in the number of adrenergic receptors blocked provide genuine differences in pharmacological profile
Lack of direct comparisons mean choices are based on these pharmacological differences when supported by indirect evidence from comparison of different trials
Non-selective β blockers (for example, timolol) are most likely to be beneficial in ischaemic heart disease, by protecting against adrenaline’s activation of cardiac β2 receptors
β1 Selective blockers (for example, bisoprolol) are drugs of choice in young hypertensives in whom blood pressure reduction is caused by renin (β1) blockade, and side effects are caused by reduction in cardiac output (β1 and β2 blockade)
Only carvedilol (β1, β2, and α1 blockade) and bisoprolol are licensed for heart failure. Comparison of outcome trials suggests rank order of efficacy follows the number of receptors which are blocked
SUMMARY
Choices between drugs can be made on a rational basis, by reference to a small number of parameters that characterise clinically relevant properties of a drug. Most drugs within a class are interchangeable. However, there are well substantiated exceptions, which are used in this article to illustrate how more controversial claims for superiority within a class can be resolved.
Supplementary Material
REFERENCES
- 1.Lyons D, Webster J, Benjamin N. Effect of enalapril and quinapril on forearm vascular ACE in man. Eur J Clin Pharmacol 1997;51:373–8. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Cai H, Dikalov S, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002;40:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000;342:145–53. ▸ Landmark study showing that overall risk rather than individual risk factor should be the prime indication for additional treatment in the secondary prevention of vascular disease. [DOI] [PubMed] [Google Scholar]
- 4.Anon. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–41. ▸ Similar message to previous reference, this time for patients with previous stroke. PROGRESS showed more clearly than HOPE that blood pressure reduction from any level is beneficial in high risk patients. [DOI] [PubMed] [Google Scholar]
- 5.Svensson P, de Faire U, Sleight P, et al. Comparative effects of ramipril on ambulatory and office blood pressures: a HOPE substudy. Hypertension 2001;38:28e–32. [DOI] [PubMed] [Google Scholar]
- 6.Brown MJ. Matching the right drug to the right patient in hypertension. Heart 2001;86:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morice AH, Lowry R, Brown MJ, et al. Angiotensin-converting enzyme and the cough reflex. Lancet 1987;2:1116–8. [DOI] [PubMed] [Google Scholar]
- 8.Mazzolai L, Maillard M, Rossat J, et al. Angiotensin II receptor blockade in normotensive subjects: a direct comparison of three AT1 receptor antagonists. Hypertension 1999;33:850–5. [DOI] [PubMed] [Google Scholar]
- 9.Conlin PR, Spence JD, Williams B, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens 2000;13:418–26. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–60. ▸ One of a trio of studies, published together, showing that the nephroprotective effect of angiotensin blockade is additional to the benefit predicted from blood pressure reduction. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9. [DOI] [PubMed] [Google Scholar]
- 12.Dahlof B, Devereux RB, Kjeldsen SE, et al. The losartan intervention for endpoint reduction in hypertension study. Lancet 2002;359:995–1003. ▸ First comparison of antihypertensive drugs to show a difference in primary outcome. The combination of β blockade and diuretic appears undesirable in older patients at risk of stroke or diabetes. [DOI] [PubMed] [Google Scholar]
- 13.Medical Research Council, British Heart Foundation. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22. ▸ Another landmark study showing the importance of treating risk, rather than risk factor. LDL reduction from any level achieved large reductions in coronary disease and stroke.12114036 [Google Scholar]
- 14.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301–7. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–8. [DOI] [PubMed] [Google Scholar]
- 16.Hall JA, Petch MC, Brown MJ. Intracoronary injections of salbutamol demonstrate the presence of functional β2-adrenoceptors in the human heart. Circ Res 1989;65:546–53. ▸ This clinical study of patients undergoing cardiac catheterisation confirmed the in vitro prediction that humans have functioning β2 (as well as β1) adrenoreceptors in the heart. The β2 receptors are activated by high concentrations of adrenaline in patients with myocardial infarction or heart failure.2548759 [Google Scholar]
- 17.Brown MJ. To β block or better block? BMJ 1995;311:701–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly R, Daley J, Avolio A, et al. Arterial dilation and reduced wave reflection. Benefit of dilevalol in hypertension. Hypertension 1989;14:14–21. [DOI] [PubMed] [Google Scholar]
- 19.CIBIS II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 20.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US carvedilol heart failure study group. N Engl J Med 1996;334:1349–55. ▸ The first of several double blind, placebo controlled outcome studies to demonstrate substantial reduction in mortality when patients with heart failure are treated with β blockade. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.