Abstract
Objective: To compare survival and outcome in patients receiving a mechanical or bioprosthetic heart valve prosthesis.
Design: Randomised prospective trial.
Setting: Tertiary cardiac centre.
Patients: Between 1975 and 1979, patients were randomised to receive either a Bjork-Shiley or a porcine prostheses. The mitral valve was replaced in 261 patients, the aortic in 211, and both valves in 61 patients. Follow up now averages 20 years.
Main outcome measures: Death, reoperation, bleeding, embolism, and endocarditis.
Results: After 20 years there was no difference in survival (Bjork-Shiley v porcine prosthesis (mean (SEM)): 25.0 (2.7)% v 22.6 (2.7)%, log rank test p = 0.39). Reoperation for valve failure was undertaken in 91 patients with porcine prostheses and in 22 with Bjork-Shiley prostheses. An analysis combining death and reoperation as end points confirmed that Bjork-Shiley patients had improved survival with the original prosthesis intact (23.5 (2.6)% v 6.7 (1.6)%, log rank test p < 0.0001); this difference became apparent after 8–10 years in patients undergoing mitral valve replacement, and after 12–14 years in those undergoing aortic valve replacement. Major bleeding was more common in Bjork-Shiley patients (40.7 (5.4)% v 27.9 (8.4)% after 20 years, p = 0.008), but there was no significant difference in major embolism or endocarditis.
Conclusions: Survival with an intact valve is better among patients with the Bjork-Shiley spherical tilting disc prosthesis than with a porcine prosthesis but there is an attendant increased risk of bleeding.
Keywords: heart valve, prosthesis, survival
We have previously reported the results of a randomised trial begun in 1975 that compared mechanical with porcine prostheses for heart valve replacement. After a median follow up of 12 years we reported significantly better survival with an intact valve among patients with the Bjork-Shiley prosthesis, because of the increased incidence of reoperation for porcine valve failure seven or more years after implantation. There was a trend towards improved patient survival after 12 years with the Bjork-Shiley prosthesis, but this did not reach significance. The use of the Bjork-Shiley valve carried an attendant increased risk of bleeding associated with the absolute requirement for anticoagulant treatment.1,2 We now report on survival, valve failure, and valve related complications after extending the follow up period to 20 years.
METHODS
The patients and methods have been described in detail previously.1,2 In brief, 541 patients undergoing valve replacement in the period from 1975 to 1979 and who were considered eligible to receive warfarin were randomly assigned at the time of operation to receive either a mechanical Bjork-Shiley 60° spherical tilting disc valve or a porcine bioprosthetic valve. The Bjork-Shiley spherical disc valve preceded the convexo-concave model introduced in 1979, which was subsequently shown to have an unacceptably high rate of strut fracture. In all, 261 patients underwent mitral valve replacement, 211 underwent aortic valve replacement, and 61 had both valves replaced. Eight patients who underwent additional tricuspid valve replacement were excluded from further analysis. Of the 533 patients studied, 267 received the Bjork-Shiley prosthesis. Initially the patients assigned to a porcine bioprosthesis received a Hancock prosthesis (107 patients), but after January 1977 such patients received a Carpentier-Edwards prosthesis (159 patients) because of a substantial cost advantage.
The clinical characteristics of the patients have been described previously.1,2 The mean (SD) age of the patients at the time of valve replacement was 53.9 (10.6) years; 54.4 (10.4) years in the Bjork-Shiley group and 53.4 (10.7) years in the porcine group. The two treatment groups were also comparable with regard to a large number of other preoperative variables. Overall 296 (56%) of the patients in the study were female, 40 (7.5%) had had a previous valve replacement, and fewer than 9% had documented evidence of ischaemic heart disease. Half of those undergoing single mitral valve replacement had had a previous mitral valvotomy. All patients with the Bjork-Shiley prosthesis received warfarin, and those with a porcine bioprosthesis received warfarin at the discretion of their cardiologist. Surviving patients have been followed up as previously described.1,2
Follow up of surviving patients
Since our last report, the 254 surviving patients have been followed up a further 545 times. This follow up was at regular intervals at clinic visits or through mailed questionnaires, with subsequent clarification of events from the patient, hospital records, or family doctor if necessary. Data regarding reoperation were obtained from the cardiac surgery database. All patients enrolled in the study were flagged with the General Register Office for Scotland and a death certificate obtained following the death, in Scotland, of any patient.
There were six patients lost to follow up during the study and the remaining survivors were followed up until at least 1 February 1998; the mean duration of follow up was 20.4 years.
Statistical analysis
Statistical analysis was based on the standard methods for assessing survival data, as previously described.1,2 Analyses comparing the two types of porcine valves showed their results to be very similar and therefore, as before, we present the results for patients receiving the Bjork-Shiley prosthesis as compared with those receiving a porcine prosthesis.
RESULTS
Late postoperative deaths
Survival
Of the 533 patients included in our analysis, 46 died within 30 days of their valve replacement operation, and there have been 358 late deaths, including those occurring after reoperation. Of the 404 deaths, 202 occurred in the group receiving the Bjork-Shiley prosthesis and 202 in those receiving a porcine prosthesis. Death was from a non-cardiac cause in 56 patients (28%) with the Bjork-Shiley prosthesis and in 46 patients (23%) with a porcine prosthesis.
Analysis of survival
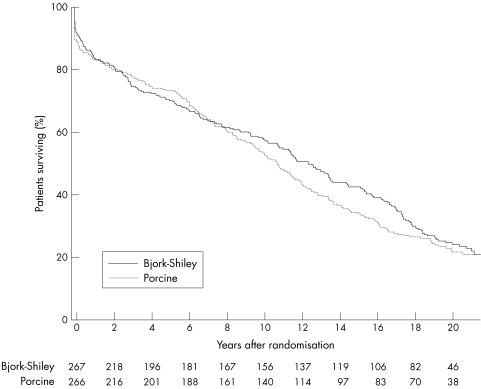
Analysis of the survival of all the patients in the study showed no significant difference between the Bjork-Shiley and the porcine prostheses. The trend towards better survival among patients with a Bjork-Shiley prosthesis seen over 12 years did not continue when the follow up was extended over 20 years (table 1, fig 1). There was no significant difference between the types of prostheses in the subgroups of patients undergoing aortic valve replacement, mitral valve replacement, or combined aortic valve and mitral valve replacement (table 2).
Table 1.
Actuarial survival and occurrence of valve related events after 10 and 20 years in patients receiving a Bjork-Shiley or porcine prosthesis
| All patients | |||
| 10 years | 20 years | p Value* | |
| Survival | |||
| All survivors | |||
| Bjork-Shiley valve | 58.7 (3.0) | 25.0 (2.7) | 0.39 |
| Porcine valve | 53.6 (3.1) | 22.6 (2.7) | |
| Survivors with original prosthesis intact | |||
| Bjork-Shiley valve | 55.7 (3.1) | 23.5 (2.6) | <0.0001 |
| Porcine valve | 42.6 (3.0) | 6.7 (1.6) | |
| Survivors without a major event | |||
| Bjork-Shiley valve | 47.0 (3.1) | 13.8 (2.2) | 0.0007 |
| Porcine valve | 37.3 (3.0) | 4.8 (1.4) | |
| Valve related events | |||
| Reoperation | |||
| Bjork-Shiley valve | 7.1 (1.8) | 12.2 (2.5) | <0.0001 |
| Porcine valve | 27.0 (3.3) | 67.8 (5.0) | |
| Bleeding: all episodes | |||
| Bjork-Shiley valve | 15.3 (2.7) | 55.6 (5.5) | 0.007 |
| Porcine valve | 7.5 (2.2) | 43.6 (8.6) | |
| Bleeding: major episodes | |||
| Bjork-Shiley valve | 11.7 (2.4) | 40.7 (5.4) | 0.008 |
| Porcine valve | 4.9 (1.8) | 27.9 (8.4) | |
| Embolism: all episodes | |||
| Bjork-Shiley valve | 19.4 (2.8) | 36.6 (4.6) | 0.77 |
| Porcine valve | 25.1 (3.3) | 37.9 (6.5) | |
| Embolism: major episodes | |||
| Bjork-Shiley valve | 8.3 (1.9) | 13.9 (3.1) | 0.68 |
| Porcine valve | 9.0 (2.2) | 13.6 (4.9) | |
| Endocarditis | |||
| Bjork-Shiley valve | 3.8 (1.4) | 7.5 (2.6) | 0.60 |
| Porcine valve | 4.6 (1.6) | 10.3 (4.6) | |
Values are % (mean (SEM)).
*p Value from log rank tests, Bjork-Shiley v porcine valves at 20 years.
Figure 1.
Survival among all patients. There was no significant difference between patients receiving a Bjork-Shiley prosthesis and those receiving a porcine prosthesis (log rank test: p = 0.39). The numbers of surviving patients after each year of follow up are shown at the bottom of the figure.
Table 2.
Actuarial survival and occurrence of valve related events after 10–20 years in patients receiving a Bjork-Shiley or porcine prosthesis
| Aortic valve replacement | Mitral valve replacement | Aortic + mitral valve replacement | |||||||
| 10 Years | 20 Years | p Value* | 10 Years | 20 Years | p Value* | 10 Years | 20 Years | p Value* | |
| Survival | |||||||||
| All survivors | |||||||||
| Bjork-Shiley valve | 64.0 (4.6) | 28.4 (4.4) | 0.57 | 52.7 (4.4) | 22.4 (3.8) | 0.41 | 65.5 (8.8) | 24.1 (8.0) | 0.068 |
| Porcine valve | 65.7 (4.7) | 31.3 (4.7) | 46.5 (4.4) | 18.4 (3.6) | 43.8 (8.8) | 11.7 (5.9) | |||
| Survivors with original prosthesis intact | |||||||||
| Bjork-Shiley valve | 63.1 (4.6) | 27.5 (4.3) | 0.025 | 48.8 (4.4) | 20.8 (3.7) | <0.0001 | 58.6 (9.2) | 20.7 (7.5) | 0.002 |
| Porcine valve | 58.8 (4.9) | 13.7 (3.6) | 31.0 (4.1) | 2.5 (1.4) | 37.5 (8.6) | 3.1 (3.1) | |||
| Survivors without a major event | |||||||||
| Bjork-Shiley valve | 53.8 (4.8) | 15.2 (3.5) | 0.34 | 41.1 (4.3) | 11.9 (3.0) | 0.005 | 48.3 (9.3) | 17.2 (7.0) | 0.018 |
| Porcine valve | 52.0 (5.0) | 8.1 (3.0) | 25.6 (3.8) | 2.5 (1.4) | 37.5 (8.6) | 3.1 (3.1) | |||
| Valve related events | |||||||||
| Reoperation | |||||||||
| Bjork-Shiley valve | 4.2 (2.1) | 7.4 (3.0) | <0.0001 | 8.5 (2.9) | 13.4 (3.9) | <0.0001 | 12.8 (6.9) | 24.2 (9.7) | 0.003 |
| Porcine valve | 11.3 (3.6) | 56.2 (8.4) | 38.6 (5.5) | 77.6 (6.7) | 40.7 (10.6) | 70.9 (11.0) | |||
| Bleeding: all episodes | |||||||||
| Bjork-Shiley valve | 16.3 (4.2) | 61.1 (7.6) | 0.001 | 14.3 (3.9) | 53.1 (8.2) | 0.39 | 14.9 (8.1) | 35.0 (14.5) | 0.71 |
| Porcine valve | 5.9 (2.9) | 42.4 (12.1) | 11.1 (4.3) | 37.2 (10.9) | 0.0 (0.0) | 70.4 (24.4) | |||
| Bleeding: major episodes | |||||||||
| Bjork-Shiley valve | 12.2 (3.7) | 37.8 (7.1) | 0.021 | 11.6 (3.5) | 47.3 (8.5) | 0.044 | 9.5 (6.5) | 22.4 (13.2) | 0.24 |
| Porcine valve | 4.2 (2.4) | 32.0 (12.6) | 6.6 (3.1) | 9.5 (4.1) | 0.0 (0.0) | 70.4 (24.4) | |||
| Embolism: all episodes | |||||||||
| Bjork-Shiley valve | 9.8 (3.2) | 24.0 (6.2) | 0.13 | 29.5 (4.8) | 53.4 (7.1) | 0.32 | 13.3 (7.3) | 13.3 (7.3) | 0.68 |
| Porcine valve | 22.6 (4.9) | 39.2 (8.8) | 28.6 (5.1) | 32.0 (5.9) | 18.7 (8.8) | 18.7 (8.8) | |||
| Embolism: major episodes | |||||||||
| Bjork-Shiley valve | 2.0 (1.4) | 10.3 (4.9) | 0.26 | 14.2 (3.6) | 18.3 (4.5) | 0.19 | 7.9 (5.4) | 7.9 (5.4) | 0.54 |
| Porcine valve | 8.9 (3.3) | 15.4 (7.0) | 10.1 (3.4) | 10.1 (3.4) | 4.6 (4.4) | 4.6 (4.4) | |||
| Endocarditis | |||||||||
| Bjork-Shiley valve | 4.8 (2.4) | 8.3 (4.1) | 0.71 | 2.3 (1.7) | 4.5 (2.7) | 0.38 | 5.8 (5.4) | 13.4 (9.0) | 0.40 |
| Porcine valve | 2.2 (1.6) | 8.7 (6.5) | 7.4 (3.4) | 7.4 (3.4) | 4.6 (4.4) | 28.4 (20.9) | |||
Values are % (mean (SEM)).
*p Values from log rank tests: Bjork-Shiley v porcine valves at 20 years.
Late postoperative events
Reoperation
One hundred and thirteen patients (21%) have undergone reoperation for prosthetic valve replacement—22 with a Bjork-Shiley valve and 91 with a porcine bioprosthesis (37 with the Hancock valve and 54 with the Carpentier-Edwards valve). Reoperation was more common in patients with porcine bioprostheses than in those with a Bjork-Shiley prosthesis when all patients were considered together (table 1). Reoperation was also more common in patients with porcine bioprostheses when the subgroups of patients who had undergone single aortic valve, single mitral valve, or combined mitral valve and aortic valve replacement were considered separately (table 2).
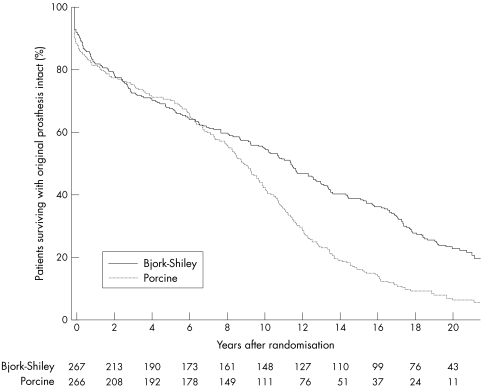
In this study there were only 98 deaths where a necropsy was performed, a necropsy rate of 24%. Although sudden valve failure with the patient dying before a reoperation can be done is not a common event, the valve failure may go unrecognised unless a necropsy is performed. We therefore conducted an analysis in which death or reoperation served as the end point for an assessment of valve survival. This analysis showed significantly better survival with the original prosthesis intact in patients who had undergone a Bjork-Shiley valve replacement when all patients were considered together (table 1 and fig 2).
Figure 2.
Survival among all patients with original prosthesis intact (that is, survival without reoperation). Valve survival was significantly better in the patients with Bjork-Shiley prostheses (log rank test: p < 0.0001). The numbers of surviving patients with the original prosthesis intact are shown at the bottom of the figure.
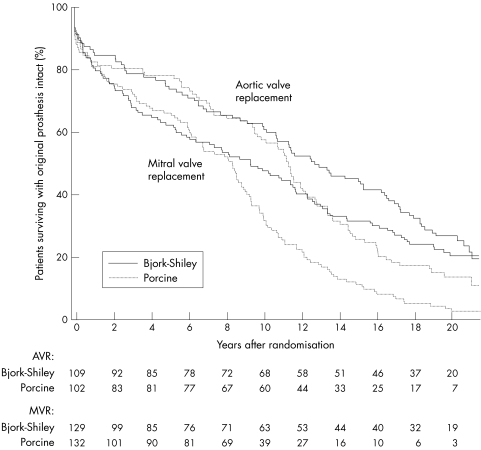
When the subgroups who had undergone single mitral valve replacement, single aortic valve replacement, or combined aortic and mitral valve replacement were considered separately there was significantly better valve survival in patients in whom a Bjork-Shiley valve had been used for single mitral valve replacement; the survival curves had clearly separated after 8–10 years (table 2 and fig 3). For single aortic valve replacement the survival curves had clearly separated after 12–14 years (table 2 and fig 3). For combined aortic and mitral valve replacement the survival curves separated after 8–10 years (table 2).
Figure 3.
Survival among patients with original prosthesis intact, according to site of implantation (aortic or mitral valve). Valve survival was significantly better in those receiving Bjork-Shiley prostheses than in those receiving porcine prostheses, both in the group undergoing aortic valve replacement (log rank test: p = 0.025) and in the group undergoing mitral valve replacement (log rank test: p < 0.0001). The separation of the survival curves occurred earlier in those undergoing mitral valve replacement. The numbers of surviving patients with the original prosthesis intact are shown at the bottom of the figure.
Overall, 16 patients requiring reoperation died within 30 days of operation (a mortality of 14.2%) and 25 patients died within one year (a mortality of 22.2%). For the period from the start of the study to 1987, 30 day mortality after reoperation was 18.3%, and from 1987 the corresponding 30 day mortality after reoperation was 9.4%.
Bleeding
There were 175 episodes of bleeding recorded in 127 patients (24%), including 114 episodes of major bleeding in 87 patients (16%). The incidence of major bleeding was significantly higher in the patients with the Bjork-Shiley prosthesis in all groups with the exception of the group undergoing combined aortic and mitral valve replacement (tables 1 and 2). The incidence of all episodes of bleeding was significantly higher in patients with the Bjork-Shiley prosthesis when all patients were considered together and in the subgroup undergoing single aortic valve replacement; for the subgroups of patients undergoing mitral valve replacement and combined aortic and mitral valve replacement there was no significant difference (table 2).
The 102 patients with a porcine aortic prosthesis received warfarin for 23% of the total patient-years of follow up, while the 132 patients with porcine mitral prostheses received warfarin for 38% of this period. The proportion of patients with a porcine prosthesis receiving warfarin increased with time following their operation. At five years 15% of patients with a porcine aortic prosthesis and 36% of those with a porcine mitral prosthesis were receiving warfarin; by 15 years this proportion had risen to 33% and 57%, respectively.
Embolism
A minor embolism was defined as an episode of transient neurological deficit, amaurosis fugax, limb ischaemia of sudden onset, or other clinical event that could reasonably be attributed to embolism. A major embolism was defined as an embolism resulting in residual neurological deficit, limb ischaemia requiring surgery, or death.
In all, 158 episodes of embolism were recorded in 121 patients (23%), including 53 episodes of major embolism in 47 patients (9%). There was no difference in the incidence of major embolism or any embolism between the Bjork-Shiley valve group and the porcine valve group (tables 1 and 2).
Bacterial endocarditis
Bacterial endocarditis occurred on 32 occasions in 25 patients (5%), and twice in seven patients. There was no difference in the incidence of endocarditis between the prosthesis groups (tables 1 and 2).
All major events
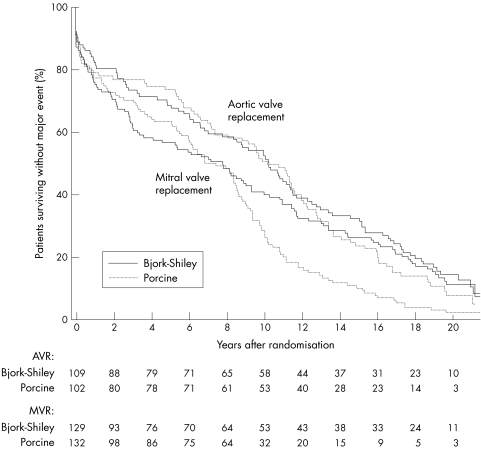
In all, 480 patients have experienced a major event—that is, death, reoperation, major bleeding, major embolism, or endocarditis. These comprised 231 patients with a Bjork-Shiley prosthesis and 249 with a porcine prosthesis. Analysis of survival free from all major events showed a significant difference in favour of the Bjork-Shiley prosthesis when all patients were considered together (table 1). The subgroup undergoing single mitral valve replacement not only had a higher incidence of major events compared with those undergoing single aortic valve replacement, but there was also a significant difference in favour of the Bjork-Shiley prosthesis in the group undergoing single mitral valve replacement (table 2, fig 4). For those undergoing single aortic valve replacement (table 2, fig 4), there was no significant difference in freedom from all major events between prostheses.
Figure 4.
Survival without major event according to site of implantation (aortic or mitral valve). Survival without major event was significantly better in patients undergoing single aortic valve replacement than in those undergoing single mitral valve replacement. For patients undergoing single mitral valve replacement, survival without major event was better with Bjork-Shiley prostheses (log rank test: p = 0.005) but there was no significant difference in the aortic valve replacement group (log rank test: p = 0.34). The numbers of patients surviving without event are shown at the bottom of the figure.
Functional class
Forty one per cent of the survivors in the group originally given a porcine prosthesis were in New York Heart Association functional class III or IV at the end of the study period compared with 36% of the survivors in the Bjork-Shiley group (χ2 test for trend: p = 0.26).
DISCUSSION
After a mean follow up period of 20 years in a large prospective randomised trial we have observed no difference in survival among patients undergoing valve replacement with the Bjork-Shiley tilting disc prosthesis as compared with the Hancock or Carpentier-Edwards porcine prostheses. In our previous report after a mean follow up period of 12 years we had observed a trend towards improved survival with the Bjork-Shiley prosthesis, but this trend did not continue and with further follow up the survival curves have converged. Although there was no difference in overall mortality, there were important differences in morbidity.
The need for reoperation was significantly greater for all patients who had received a porcine prosthesis. As in our previous report we considered that there might be confounding factors to bias the comparison of the failure rates of porcine and Bjork-Shiley valves. Occasionally a valve may fail suddenly, causing death before the patient can be brought to surgery, and as necropsy was rarely done in this study it is possible that some failures of prosthetic valves went unrecognised. We therefore undertook an analysis with death or reoperation as an end point for assessment of valve survival. This analysis showed that valve survival was significantly better in patients with the Bjork-Shiley prosthesis in the whole group and in each of the subgroups undergoing single aortic valve, single mitral valve, and combined mitral valve and aortic valve replacement.
In our previous report, at a mean follow up of 12 years, improved valve survival in patients undergoing single mitral valve replacement had become apparent at 8–10 years2; in the current report after a mean follow up of 20 years, significantly improved valve survival in those undergoing single aortic valve replacement became apparent after 12–14 years. In the smaller subgroup of patients undergoing combined mitral valve and aortic valve replacement, improved valve survival with the Bjork-Shiley prosthesis became apparent after 8–10 years and became significant with the accumulation of more prolonged follow up data.
Non-randomised observational studies with extended periods of follow up have also reported a high incidence of failure of porcine prosthetic valves, with failure occurring more commonly and earlier in patients undergoing mitral valve replacement than aortic valve replacement.3–5 When reoperation for valve failure was required in patients in our series with both aortic and mitral porcine prostheses, the mitral prosthesis was found to have failed more than twice as often as the aortic prosthesis—a finding also reported in other studies.6,7
Reoperation for valve failure was associated with substantial mortality: 14% at 30 days and 22% at one year after reoperation. Even though the 30 day mortality of 9% in the later years of follow up was only half of that before 1987, reoperation carried a high risk of death. Our results are comparable with the UK heart valve registry which reports a 30 day mortality rate for reoperation of 16.6% over the years 1986 to 1997.8 From a single US centre, Akins and colleagues reported a mortality for reoperation of 6.8% for mitral, 7.8% for aortic, and 14.3% for double prosthetic valve replacement over the period 1985 to 1997.9
Bleeding occurred more often in patients receiving the Bjork-Shiley prosthesis, all of whom received oral anticoagulants. Major bleeding episodes were more common with the Bjork-Shiley prosthesis in the single aortic valve and single mitral valve groups, but there was no significant difference in the group undergoing combined mitral valve and aortic valve replacement, which involved a smaller number of patients. When all episodes of bleeding (both major and minor) were considered, there was a higher incidence of bleeding with the Bjork-Shiley prosthesis in patients undergoing single aortic valve replacement but not in those undergoing single mitral valve replacement. This may reflect the higher proportion of patients undergoing single mitral valve replacement with a porcine prosthesis who were also receiving warfarin. With increasing time from operation a greater proportion of porcine valve replacement patients received warfarin. This was mainly because of patient factors such as the development of atrial fibrillation or chamber enlargement rather than factors directly related to the prosthetic valve.
The increased risk of bleeding in patients receiving a Bjork-Shiley prosthesis partly offsets the increased risk of reoperation in those receiving a porcine prosthesis. We found no difference in the occurrence of embolism or endocarditis between the groups. Analysis of survival free from a major event showed a significantly better outcome with the Bjork-Shiley prosthesis in the group undergoing single mitral valve and combined aortic valve and mitral valve replacement, but the difference did not reach significance in the single aortic valve replacement group. The level of anticoagulation required with the current generation of bileaflet mechanical prostheses is lower than for the Bjork-Shiley prosthesis10–12 and the risk of bleeding is reduced, so the relative benefit of mechanical prostheses may now be even more pronounced.
The Department of Veterans Affairs has carried out a similar study to our own, starting in 1977 and comparing the Bjork-Shiley with the Hancock porcine prosthesis in an all male population of patients undergoing single valve replacement.13,14 After a follow up period of 15 years Hammermeister and colleagues reported significantly improved survival in patients who had undergone aortic valve replacement with the Bjork-Shiley prosthesis.15 After 15 years, 34% of those receiving a Bjork-Shiley prosthesis versus 21% of those receiving a Hancock bioprosthesis were still alive. As in our study, Hammermeister and colleagues found no difference in survival in those patients undergoing mitral valve replacement. The difference in survival in patients who had undergone aortic valve replacement with the Hancock bioprosthesis compared with the mechanical valve was, they found, “probably due to more deaths from primary valve failure (8 v 0)”. They also found that “almost all of the excess deaths with bioprosthesis after aortic valve replacement occurred in the 10 to 15 year time period”. Both an increased need for reoperation in patients who had undergone aortic valve replacement with a bioprosthesis and inferior valve survival after about 10 years compared with the mechanical valve were also identified in our study. The Veterans Affairs study enrolled twice as many patients undergoing single aortic valve replacement as in our study, and all were male. Thus, the greater statistical power afforded by a larger cohort and the influence of other patient variables may have operated to produce the difference in survival seen in the Veterans Affairs study. Hammermeister and colleagues also reported an increased risk of bleeding in the Veterans Affairs study in those patients receiving the Bjork-Shiley prosthesis compared with those receiving a bioprosthesis. The absolute risks of bleeding in the Veterans Affairs study were higher than we observed. This may reflect a higher intensity of anticoagulation used in the USA during the major period of the trial compared with that used in the UK, owing to differences in the thromboplastins used to standardise the prothrombin assay.15,16 There was no difference in the incidence of endocarditis or embolism.15,17 Peterseim and colleagues recently reported a large retrospective study comparing outcomes in patients undergoing aortic valve replacement; they found no difference in survival after 10 years of follow up but a significantly increased incidence of reoperation in patients receiving a porcine prosthesis compared with those receiving a St Jude mechanical prosthesis.18
Our study emphasises the need for prolonged follow up of patients undergoing valve replacement in order to show significant differences between treatment groups. Trends towards improved survival seen after 12 years did not develop further with prolonged follow up, probably as a result of the overwhelming effect of patient variables in this aging group of individuals. However, important differences in valve survival did become apparent in all treatment groups during this extended period of follow up.
Recommendations
On the basis of this study, we recommend that patients undergoing single mitral valve or combined aortic valve and mitral valve replacement should receive mechanical prostheses because of their superior durability compared with bioprostheses. Furthermore many such patients will have, or will develop, coexisting conditions such as atrial fibrillation for which anticoagulation is necessary. Patients receiving a single aortic valve replacement who would be expected to live 10 years or more should receive a mechanical valve unless anticoagulation treatment is contraindicated. Our results also have important implications for health care provision, particularly in the UK where there are long waiting lists for cardiac surgery. Patients requiring prosthetic valve replacement use scarce cardiac surgery resources. Mechanical valves have proved to be more durable over several generations of prostheses19 over prolonged periods of follow up, and their use should reduce the need for further cardiac surgery in those in whom they are implanted.
Acknowledgments
Supported by a grant from the Chief Scientist Office, Scottish Executive.
REFERENCES
- 1.Bloomfield P, Kitchin AH, Wheatley DJ, et al. A prospective evaluation of the Bjork-Shiley, Hancock and Carpentier-Edwards heart valve prostheses. Circulation 1986;73:1213–22. [DOI] [PubMed] [Google Scholar]
- 2.Bloomfield P, Wheatley DJ, Prescott RJ, et al. Twelve year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. N Engl J Med 1991;324:573–9. [DOI] [PubMed] [Google Scholar]
- 3.Magilligan DJ, Lewis JW, Tilley B, et al. The porcine bioprosthetic valve: twelve years later. J Thorac Cardiovasc Surg 1985;89:499–507. [PubMed] [Google Scholar]
- 4.Foster AH, Greenberg GJ, Underhill DJ, et al. Intrinsic failure of Hancock mitral bioprosthesis: 10 to 15 year experience. Ann Thorac Surg 1987;44:568–77. [DOI] [PubMed] [Google Scholar]
- 5.Akins CW, Carroll DL, Buckley MJ, et al. Late results with Carpentier-Edwards porcine bioprosthesis. Circulation 1990;82(suppl IV):IV-65–74. [PubMed] [Google Scholar]
- 6.Bolooki H, Mallon S, Kaiser GA, et al. Failure of Hancock xenograft valve: importance of valve position (4- to 9-year follow up). Ann Thorac Surg 1983;36:246–52. [DOI] [PubMed] [Google Scholar]
- 7.Magilligan DJ, Kemp SR, Stein PD, et al. Asynchronous primary valve failure in patients with porcine bioprosthetic aortic and mitral valves. Circulation 1987;76(suppl III):III-141–5. [PubMed] [Google Scholar]
- 8.UK Heart Valve Registry. The United Kingdom Heart Valve Registry report, 1997. London: United Kingdom Heart Valve Registry, Imperial College School of Medicine, Hammersmith Hospital, 1999.
- 9.Akins CW, Buckley MJ, Daggett WM, et al. Risk of reoperative valve replacement for failed mitral and aortic bioprostheses. Ann Thorac Surg 1998;65:1545–52. [DOI] [PubMed] [Google Scholar]
- 10.Poller L. Therapeutic ranges in anticoagulant administration. BMJ 1985;290:1683–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med 1995;333:11–17. [DOI] [PubMed] [Google Scholar]
- 12.Acar J, Iung B, Boissel JP, et al, and the AREVA Group. AREVA: multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation 1996;94:2107–12. [DOI] [PubMed] [Google Scholar]
- 13.Hammermeister KE, Henderson WG, Burchfiel CM, et al. Comparison of outcome after valve replacement with a bioprosthesis versus a mechanical prosthesis: initial 5 year results of a randomised trial. J Am Coll Cardiol 1987;10:719–32. [DOI] [PubMed] [Google Scholar]
- 14.Hammermeister KE, Sethi GK, Henderson WG, et al. A comparison of outcomes in men 11 years after heart valve replacement with a mechanical valve or bioprosthesis. N Engl J Med 1993;328: 1289–96. [DOI] [PubMed] [Google Scholar]
- 15.Hammermeister KE, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomised trial. J Am Coll Cardiol 2000;36:1152–8. [DOI] [PubMed] [Google Scholar]
- 16.Hirsh J. Is the dose of warfarin prescribed by American physicians unnecessarily high? Arch Intern Med 1987;147:769–71. [PubMed] [Google Scholar]
- 17.Grover FL, Cohen DJ, Oprian C, et al. Determinants of the occurrence of and survival from prosthetic valve endocarditis. Experience of the Veterans Affairs Cooperative Study on Valvular Heart Disease. J Thorac Cardiovasc Surg 1994;108:208–14. [PubMed] [Google Scholar]
- 18.Peterseim DS, Cen Ye-Ying, Cheruvu S, et al. Long-term outcome after biologic versus mechanical aortic valve replacement in 841 patients. J Thorac Cardiovasc Surg 1999;117:890–7. [DOI] [PubMed] [Google Scholar]
- 19.Nitter-Hauge S. Mechanical heart valves. Conclusions from long-term follow up. Eur Heart J 1997;18:907–10. [DOI] [PubMed] [Google Scholar]