Abstract
Objective: To determine normal values of plasma B type natriuretic peptide from infancy to adolescence using a commercially available rapid assay.
Setting: Tertiary referral centre.
Design: The study was cross sectional. Plasma BNP concentration was measured in 195 healthy infants, children, and adolescents from birth to 17.6 years using the triage BNP assay (a fluorescence immunoassay).
Results: During the first week of life, the mean (SD) plasma concentration of BNP in newborn infants decreased significantly from 231.6 (197.5) to 48.4 (49.1) pg/ml (p = 0.001). In all subjects older than two weeks plasma BNP concentration was less than 32.7 pg/ml. There was no significant difference in mean plasma BNP measured in boys and girls younger than 10 years (8.3 (6.9) v 8.5 (7.5) pg/ml). In contrast, plasma concentration of BNP in girls aged 10 years or older was significantly higher than in boys of the same age group (12.1 (9.6) v 5.1 (3.5) pg/ml, p < 0.001). Plasma BNP concentrations were higher in pubertal than in prepubertal girls (14.4 (9.7) v 7.1 (6.6) pg/ml, p < 0.001) and were correlated with the Tanner stage (r = 0.41, p = 0.001).
Conclusions: Plasma BNP concentrations in newborn infants are relatively high, vary greatly, and decrease rapidly during the first week of life. In children older than 2 weeks, the mean plasma concentration of BNP is lower than in adults. There is a sex related difference in the second decade of life, with higher BNP concentrations in girls. BNP concentrations in girls are related to pubertal stage.
Keywords: B type natriuretic peptide, children
B type natriuretic peptide (brain natriuretic peptide; BNP) is a cardiac hormone with diuretic, natriuretic, and vasodilator properties.1 This 32 amino acid polypeptide contains a 17 amino acid ring structure common to all natriuretic peptides and is secreted mainly in the ventricles in response to volume expansion and pressure load.2–5 Measurement of plasma B type natriuretic peptide concentrations is increasingly used to aid diagnosis, assess prognosis, and tailor treatment in adults with congestive heart failure.6 Recent studies suggest that BNP may be useful in various other conditions,7 such as hypertrophic cardiomyopathy,8 left ventricular remodelling after myocardial infarction,9 or arrhythmogenic right ventricular dysplasia.10 The few data dealing with B type natriuretic peptide in children suggest that this peptide is also useful in paediatric patients.11 However, investigations to examine the role of B type natriuretic peptide in paediatric cardiology are limited owing to a lack of normative data in children. Our aim in the present study was thus to evaluate normal values of plasma B type natriuretic peptide from infancy to adolescence, using a commercially available rapid assay.
METHODS
Venous samples were collected into tubes containing potassium ethylene diamine tetra-acetic acid (EDTA) and were stored at 4°C. BNP was measured within six hours of blood sampling, using the triage BNP assay (Biosite Diagnostics Inc, San Diego, California, USA), recently approved by the Food and Drug Administration (FDA). The triage BNP assay is a sandwich immunoassay consisting of a disposable device. After addition of 250 μl of whole blood to the sample port of the test device, the cells were separated from the plasma by a filter. The plasma entered a reaction chamber containing murine polyclonal fluorescence tagged BNP antibodies. Plasma and BNP antibodies formed a reaction mixture which was incubated for approximately two minutes. Capillary action caused migration of the reaction mixture through the diagnostic lane to a zone of immobilised murine monoclonal antibody against the ring structure of BNP, binding the BNP fluorescent antibody complex. The unbound fluorescent antibodies were washed away by the excess plasma fluid. The device was then placed in the triage meter, which quantified the fluorescence intensity of the BNP assay zone using an internal calibration curve. The assay was completed in about 15 minutes.
Imprecision, analytical sensitivity, interferences, and stability have been described previously by others.12,13 The total coefficient of variation was between 4.0–12.4%. The measurement range of the BNP assay is < 5.0–1300 ng/l.
Subjects
A total of 195 healthy infants, children, and adolescents (98 female, 97 male) from birth to 17.6 years of age were included in the study. They were admitted for minor operations (hernia repair, tonsillectomy, adenoidectomy, cleft lip or palate repair, orchiopexy), were outpatient attenders, or had a diagnostic work up done (for example, for short stature or headache). The subjects were in the supine position during blood sampling, which was typically done between 8:30 and 11:00 hours after 10 minutes of rest. In newborns, blood samples were taken after mature birth exclusively on the occasion of routine blood analysis for neonatal screening, measurement of serum bilirubin, or exclusion of neonatal infection. All subjects were free of acute illness (fever, infections, water and electrolyte imbalance, neoplastic disease, liver disease, renal disease). They had no history of heart disease, and the parents denied any history of serious disease. They were not on drug treatment, were not receiving intravenous infusions, and their diet was appropriate for their age. Physical examination and laboratory testing (for example, electrolytes, kidney function, blood count) were normal in all subjects.
Written informed consent was obtained from all parents before participation. The study was approved by the local ethics committee.
Statistics
Statistical analyses were done using SPSS 11.0 for Windows. Statistical differences between the age groups, pubertal stages, and the two sexes were evaluated using a rank score test for unpaired data (Mann–Whitney), with a probability value of p < 0.05 considered to be significant. BNP values followed log normal distributions, so correlation and regression analyses were done using natural log transformed BNP values to assess associations with age, body mass index, creatinine, creatinine clearance (univariate regression analyses), pubertal stage, and menarchal stage (Spearman rank correlation).
RESULTS
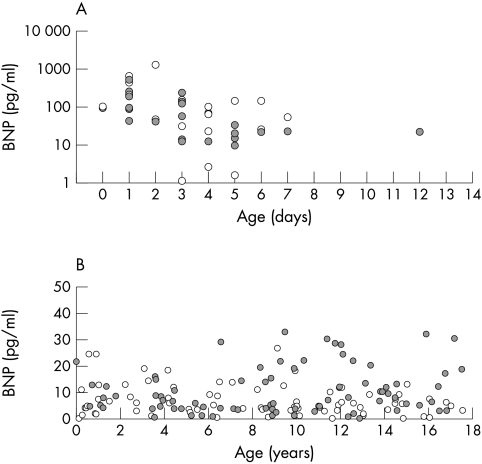
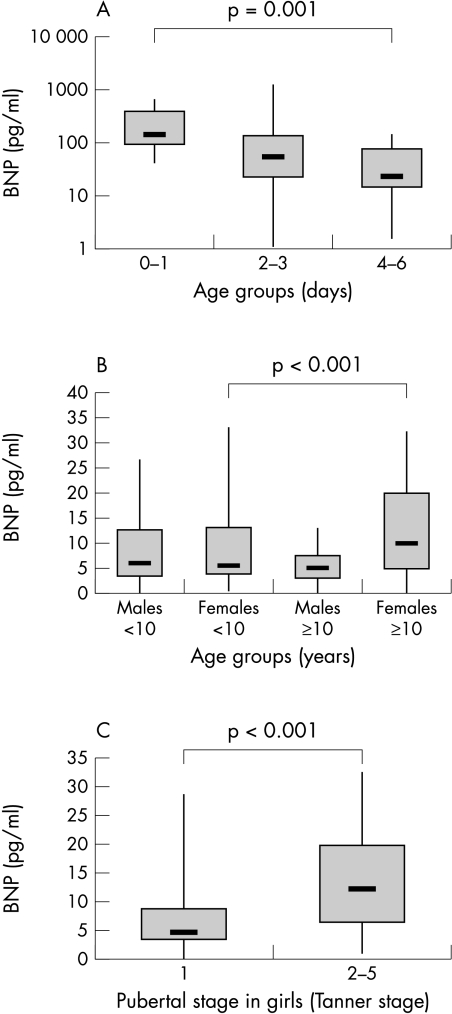
Plasma BNP concentrations according to age of all patients are shown in fig 1. Newborn infants during the first two weeks of life (n = 43) had higher concentrations of BNP than older children, although individual values varied greatly. During the first week, mean (SD) plasma BNP decreased significantly (p = 0.001) from 231.6 (197.5) pg/ml (0 or 1 day of age, n = 12) to 48.4 (49.1) pg/ml (4, 5, or 6 days of age, n = 14) (fig 2). Regression analysis of log BNP on age showed a negative association in the first two weeks (β = −0.45, p = 0.003).
Figure 1.
Plasma concentration of BNP according to age (A) in newborn infants from the first to the 14th day of life, and (B) in infants older than two weeks, children, and adolescents. Open circles, boys; grey circles, girls.
Figure 2.
(A) BNP concentrations of newborn infants aged 0–1 days, 2–3 days, and 4–6 days. (B) Plasma BNP in boys and girls aged < 10 years and > 10 years. (C) Plasma BNP in prepubertal and pubertal girls. Median (bold horizontal line), interquartile range (box), and extreme values (whiskers) are shown.
Clinical data on the subjects older than two weeks are given in table 1. In all subjects older than two weeks (n = 152) BNP values were less than 32.7 pg/ml. There was a significant difference between the mean BNP concentrations in boys and girls (7.0 (5.9) v 10.1 (8.6) pg/ml, p = 0.015). Serum creatinine, creatinine clearance, and body mass index were not correlated with BNP (p = 0.88, 0.37, and 0.86). In boys, there was a trend of decreasing BNP concentrations (β = −0.10, p = 0.41). In contrast, plasma BNP in girls increased non-significantly with age (β = 0.08, p = 0.50). However, neither trend was significant.
Table 1.
Clinical data of subjects older than two weeks
| Boys (n=72) | Girls (n=80) | |
| Age (years), range | 8.19 (5.25), 0.13–17.54 | 9.25 (4.75), 0.61–17.51 |
| Body mass index (kg/m2) | 17.9 (4.7) | 18.2 (4.4) |
| Serum creatinine (mg/dl)* | 0.43 (0.18) | 0.44 (0.15) |
| Creatinine clearance* (ml/min/1.73 m2)† | 156 (35) | 166 (32) |
Values are mean (SD).
*Available in 126 subjects.
†Estimated according to the formula k × body height (cm)/serum creatinine (mg/dl). The value for k is 0.55 in children >1 year and 0.45 in infants <1year.
There was no difference in plasma BNP measured in boys (n = 43, 8.3 (6.9) pg/ml) and girls younger than 10 years (n = 42, 8.5 (7.5) pg/ml). The plasma concentration of BNP in girls aged 10 years or older (n = 36) was significantly higher than in boys of the same age group (n = 31; 12.1 (9.6) v 5.1 (3.5) pg/ml) (p < 0.001) (fig 2). The 95th centile of plasma BNP concentration was 24.5 pg/ml in boys and girls younger than 10 years, and 30.4 pg/ml in girls v 12.1 pg/ml in boys aged ≥ 10 years.
Plasma BNP concentrations measured in prepubertal girls (7.1 (6.6) pg/ml, Tanner stage 1, n = 38) were significantly lower (p < 0.001) than in pubertal or mature girls (14.4 (9.7) pg/ml, Tanner stage 2–5, n = 31) (fig 2). The BNP plasma concentration was correlated with the Tanner stage (r = 0.41, p = 0.001). The difference in BNP values measured in girls before (9.7 (8.6) pg/ml, n = 56) and after menarche (13.3 (9.8) pg/ml, n = 13) was not significant (p = 0.16). There was no correlation of BNP with the Tanner stage in boys (r = −0.18, p = 0.19) and no significant difference between BNP in prepubertal boys (7.4 (6.5) pg/ml, n = 45) and pubertal boys (4.5 (3.7) pg/ml, n = 13) (p = 0.15).
DISCUSSION
The results of our investigations suggest that normal plasma BNP concentrations are lower in children than in adults. Dao and colleagues reported normal values of 38 (4) pg/ml in 139 adults using the triage BNP test system.13 Recently, Wang and colleagues reported considerably lower reference limits derived from a large group of 911 healthy adults using a radioimmunoassay.14 However, even these reference values were 1.1- to 1.4-fold higher than our normal values.14 In addition, the latter study showed that a 10 year increase in age was associated with a 1.4-fold increase in BNP concentrations. Serum creatinine, however, was not associated with higher BNP concentrations.14 Accordingly, the 95th and 97.5th centiles of normal BNP values in adults aged 20–49 years were slightly higher than the corresponding values in our study.
Our data are in line with the very few published reports dealing with BNP values in healthy children. Kawamura and colleagues reported BNP concentrations measured by radioimmunoassay in 26 patients with viral infections aged 2 months to 10 years.15 The mean BNP concentration was 6.8 (7.3) pg/ml, and the maximum value was 32.7 pg/ml. Yoshibayashi and colleagues investigated plasma BNP by radioimmunoassay in a smaller group of 18 neonates and 40 children aged 1 month to 16 years.16 The BNP concentration in nine neonates on the day of birth was significantly higher, at 196 (176) pg/ml, than in the umbilical vein or in the older age groups. The values in six infants aged about four months were at the adult concentration (9.3 (4.8) pg/ml).16 We also observed a striking decrease in mean plasma BNP during the first days of life. Similar significant changes during the first few days have been described in atrial natriuretic peptide (ANP), renin concentration, plasma renin activity, concentrations of aldosterone, and other steroids.17–20 Maturation of the kidney and the haemodynamic changes at birth, with an increase both in systemic vascular resistance because of the removal of the very low resistance placenta, and in pulmonary blood flow as a result of lung expansion may contribute to these findings. The perinatal circulatory changes lead to an increase in left ventricular volume and pressure load. This may stimulate BNP synthesis and secretion in the ventricle and result in the rise in plasma BNP concentrations shortly after birth. In preterm infants, plasma BNP was shown to increase with the size of the shunt through a patent ductus arteriosus.21 In addition, the haemodynamic changes accompanied by a decrease in the ductal shunt were reflected by falling BNP concentrations.21 However, closure of the ductus arteriosus cannot alone be responsible for the fall in BNP, as the concentrations also fell in some infants who had increased shunting.21 The exact mechanisms of these neonatal phenomena are still unclear.
Our data clearly show a sex related difference in the second decade of life. BNP concentrations have been reported recently to be higher in adult women than men, using both radioimmunoassay and fluorescence immunoassay.14,22 Women also had notably higher ANP concentrations.23 There are corresponding striking differences between the sexes in the complementary renin–angiotensin–aldosterone systems, with higher plasma renin concentrations in men.24 The physiological role of these sex related differences is still not resolved.
The finding that sexual dimorphism occurs in the second but not in the first decade of life suggests a close relation with gonadal hormone concentrations. A stimulatory effect of female sex hormones on natriuretic peptide gene expression has been proposed.25 In one study, the BNP concentration was found to be increased in women on hormone replacement therapy.22 Others, however, could not find any effect of menopausal status,14 and no cyclical variation of natriuretic peptides during menstrual cycle has been observed.23 Both findings argue against a direct interaction between oestrogen or progesterone and natriuretic peptide. Another explanation could be an increased metabolic clearance of natriuretic peptides by neutral endopeptidase in men.23 Other endopeptidases such as angiotensin converting enzyme have been shown to be under androgenic control.26 We found a strong positive correlation between plasma BNP concentrations and increasing Tanner stage in girls. Pubertal development in girls is associated with increasing oestrogen concentrations but not progesterone concentrations. On the other hand, there was a decrease in plasma BNP in boys during puberty. However, the number of pubertal boys was small and the difference was not significant. Thus, although a decrease in plasma BNP caused by testosterone is possible, it cannot be confirmed by our data. Our findings suggest that oestrogen accounts for the observed differences between the sexes.
Because it allows rapid and accurate measurements, the triage test system makes possible the widespread clinical use of BNP determinations. Several investigators have reported close correlations between BNP measurements made by radioimmunoassay and by fluorescence immunoassay, but there is a proportional bias, with higher results for the fluorescence assay.12,27 Thus the type of assay used should be taken into account in assessing the results of BNP studies.
Limitations
Some limitations of our study need to be considered. Although this investigation is the largest study in paediatric patients, the groups are too small to determine reference values for all age groups and both sexes. To interpret the great variation and the dramatic decrease in plasma BNP concentrations in the first days of life, haemodynamic data such as data on left ventricular function or patency of the ductus arteriosus would be necessary. However, blood samples were taken exclusively on occasions of routine blood analyses, and no echocardiography was done in the newborn infants. In addition, standardisation of performance characteristics (for example, control of physical activity, 30 minutes of rest in the supine position) is limited in children. On the other hand, it has recently been shown that BNP concentrations remain stable during exercise,28 and from our own experience plasma BNP values in children who have been in the supine position for 30–60 minutes after sedation are in the same range as the samples from the current study (data not shown). However, in previous studies pronounced differences in the performance characteristics of the available BNP assays have caused widely varying reports of normal values and discrimination limits in adults.29 This may also be the case in children. Further studies are needed to clarify these questions.
Conclusions
We have shown age and sex specific trends in normal values of plasma B type natriuretic peptide from infancy to adolescence using a commercially available rapid assay. Plasma BNP concentrations in newborn infants are relatively high, vary greatly, and decrease rapidly during the first week of life. In children older than two weeks, the mean plasma concentration of BNP is lower than in adults. There is a sex related difference in the second decade of life, with higher BNP concentrations in girls. The plasma BNP concentration in girls was correlated with the pubertal stage and was significantly higher in pubertal than in prepubertal girls. Our data indicate the need for age and sex specific normal ranges of BNP in children, as has been shown previously in adults.14,22
Acknowledgments
We gratefully acknowledge VIVA Diagnostika Germany and Biosite Diagnostics Europe for providing the reagents free of cost.
REFERENCES
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321–8. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura M, Yasue H, Okamura, et al. Different secretion pattern of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993;87:464–9. [DOI] [PubMed] [Google Scholar]
- 3.Yandle TG. Biochemistry of natriuretic peptides. J Intern Med 1994;235:561–76. [DOI] [PubMed] [Google Scholar]
- 4.Nagagawa O, Ogawa Y, Itoh H, et al. Rapid transcriptional activation and early mRNA turnover of BNP in cardiocyte hypertrophy: evidence for BNP as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 1995;96:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda K, Takayoshi T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J 1998;135:825–32. [DOI] [PubMed] [Google Scholar]
- 6.Kalra PR, Anker SD, Coats AJS. Water and sodium regulation in chronic heart failure: the role of natriuretic peptides and vasopressin. Cardiovasc Res 2001;51:495–509. [DOI] [PubMed] [Google Scholar]
- 7.Struthers AD. Introducing a new role for BNP: as a general indicator of cardiac structural disease rather than a specific indicator of systolic dysfunction only. Heart 2002;87:97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namakura T, Sakamoto K, Yamano T, et al. Increased plasma brain natriuretic peptide levels as a guide for silent myocardial ischemia in patients with non-obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;39:1657–63. [DOI] [PubMed] [Google Scholar]
- 9.Crilley JG, Farrer M. Left ventricular remodelling and brain natriuretic peptide after the first myocardial infarction. Heart 2001;86:638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo K, Nishikimi T, Yutani C, et al. Diagnostic value of plasma levels of brain natriuretic peptide in arrhythmogenic right ventricular dysplasia. Circulation 1998;98:2433–40. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura T, Wago M. Brain natriuretic peptide can be a useful biochemical marker for myocarditis in patients with Kawasaki disease. Cardiol Young 2002;12:153–8. [DOI] [PubMed] [Google Scholar]
- 12.Fischer Y, Filzmaier K, Stiegler H, et al. Evaluation of a new, rapid bedside test für quantitative determination of B-type natriuretic peptide. Clin Chem 2001;47:591–4. [PubMed] [Google Scholar]
- 13.Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in a urgent-care setting. J Am Coll Cardiol 2001;37:379–85. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–8. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura T, Wago M, Kawaguchi H, et al. Plasma brain natriuretic peptide concentrations in patients with Kawasaki disease. Pediatr Int 2000;42:241–8. [DOI] [PubMed] [Google Scholar]
- 16.Yoshibayashi M, Kamiya T, Saito Y, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: marked and rapid increase after birth. Eur J Endocrinol 1995;133:207–9. [DOI] [PubMed] [Google Scholar]
- 17.Gemelli M, Mami C, DeLuca F, et al. Atrial natriuretic peptide and renin-aldosterone relationship in healthy newborn infants. Acta Paediatr Scand 1991;80:1128–33. [DOI] [PubMed] [Google Scholar]
- 18.Krüger C, Rauh M, Doerr HG. Immunoreactive renin concentrations in healthy children from birth to adolescence. Clin Chim Acta 1998;274:15–17. [DOI] [PubMed] [Google Scholar]
- 19.Dillon MJ, Gillin ME, Ryness JM, et al. Plasma renin activity and aldosterone concentration in the human newborn. Arch Dis Child 1976;51:537–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiselier TJW, Lijnen P, Monnens L, et al. Levels of renin, angiotensin I and II, angiotensin–converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr 1983;141:3–7. [DOI] [PubMed] [Google Scholar]
- 21.Holmström H, Hall C, Thaulow E. Plasma levels of natriuretic peptides and hemodynamic assessment of patent ductus arteriosus in preterm infants. Acta Paediatr 2001;90:184–91. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82. [DOI] [PubMed] [Google Scholar]
- 23.Clark BA, Elahi D, Epstein FH. The influence of gender, age, and the menstrual cycle on plasma atrial natriuretic peptide. J Clin Endocrinol Metab 1990;70:349–52. [DOI] [PubMed] [Google Scholar]
- 24.Danser AH, Derkx FH, Schalekamp MA, et al. Determinants of interindividual variation of renin and prorenin concentrations: evidence of a sexual dimorphism of (pro)renin levels in humans. J Hypertens 1998;16:853–62. [DOI] [PubMed] [Google Scholar]
- 25.Hong M, Yan Q, Tao B, et al. Estradiol, progesterone and testosterone exposures affect the atrial natriuretic peptide gene expression in vivo in rats. Biol Chem Hoppe Seyler 1992;373:213–18. [DOI] [PubMed] [Google Scholar]
- 26.Shih HC, Chao L, Chao J. Age and hormonal dependence of tonin levels in rat submandibular gland as determined by a new direct radioimmunoassay. Biochem J 1986;238:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogeser M, Jacob K. B-type natriuretic peptide (BNP) – validation of an immediate response assay. Clin Lab 2001;47:29–33. [PubMed] [Google Scholar]
- 28.McNairy M, Gardetto N, Clopton P, et al. Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: implications for outpatient monitoring with B-type natriuretic peptide. Am Heart J 2002;143:406–11. [DOI] [PubMed] [Google Scholar]
- 29.Vasan RS, Banjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction. The Framingham Heart Study. JAMA 2002;288:1252–9. [DOI] [PubMed] [Google Scholar]