Abstract
Background: Raised plasma lipoprotein(a) (Lp(a)) is associated with increased risk of cardiovascular disease. It is unknown whether increased Lp(a) is an additional risk factor for coronary artery disease in familial hypercholesterolaemia (FH) or whether statin treatment can reduce Lp(a) concentrations in the long term.
Objective: To investigate Lp(a) concentrations in relation to statin treatment and the progression of atherosclerosis in a large cohort of FH patients.
Design: A two year, randomised, double blind trial (the ASAP trial).
Patients: 325 heterozygous FH patients.
Intervention: Treatment with 80 mg atorvastatin or 40 mg simvastatin.
Main outcome measure: Change in Lp(a) concentrations and intima–media thickness of carotid artery segments at one year and two years.
Results: At baseline, median Lp(a) concentrations were 327 mg/l and 531 mg/l in the atorvastatin and simvastatin arms, respectively (p = 0.03). In the atorvastatin arm, Lp(a) concentrations decreased to 243 mg/l after one year (p < 0.001) and to 263 mg/l after two years (p < 0.001). In the simvastatin arm, Lp(a) concentrations decreased to 437 mg/l after one year (p < 0.001) and to 417 mg/l after two years (p < 0.001). The difference in Lp(a) reduction between the two treatment arms was significant after one year (p = 0.004), but not after two years (p = 0.086). Lp(a) concentrations at baseline were not related to cardiovascular events at baseline. There was no correlation between baseline Lp(a) concentrations and low density lipoprotein cholesterol concentrations or intima–media thickness at baseline. Change in Lp(a) concentrations was not correlated with change in intima–media thickness after one or two years.
Conclusions: Long term statin treatment significantly lowers Lp(a) in FH patients. However, this reduction was unrelated to changes in intima–media thickness and casts doubt on the importance of Lp(a) in the progression of atherosclerotic disease in these patients.
Keywords: familial hypercholesterolaemia; statins; lipoprotein(a); Lp(a); intima, media thickness
Lipoprotein(a) (Lp(a)) is similar to low density lipoprotein (LDL) in both lipid composition and the presence of apolipoprotein (apo)B-100. The difference between these two particles lies in the presence of the apo(a) moiety on Lp(a).1 Although Lp(a) closely resembles LDL, its plasma concentrations and metabolism are quite distinctive. Plasma Lp(a) concentrations can vary over a 1000-fold range (from 1 mg/l to more than 1000 mg/l), and in individuals of African descent, median concentrations are approximately threefold higher than in whites.2
The structural properties of this particle might in part explain the atherogenicity of Lp(a). Various mechanisms have been postulated to explain how Lp(a) may contribute to the development of atherosclerosis. First, it can interact with various tissue matrix components in the intima and it may be susceptible to oxidative modification. Second, it is also reported to inhibit plasmin mediated activation of cytokine transforming growth factor β (TGFβ), thereby promoting smooth muscle cell proliferation, one of the hallmarks of the development of atherosclerotic plaques. Finally, because of the structural similarity between apo(a) and plasminogen, Lp(a) might also interfere with thrombolysis.1,3
Almost all cross sectional studies and most retrospective studies in white populations have shown that Lp(a) concentrations above the 80th centile (250–300 mg/l) are associated with an increased risk of coronary, peripheral, and cerebrovascular disease.2,4 Mean Lp(a) values, as determined in the atherosclerosis risk in community (ARIC) study, were approximately 4 mg/dl for middle aged men and women.3 Although there are still doubts about the causal relation between raised Lp(a) concentrations and coronary artery disease,5 a recent meta-analysis of prospective studies indicated a moderately strong association.6
Patients who are heterozygous for an LDL receptor defect (familial hypercholesterolaemia, FH) not only have a two- to threefold increase in plasma low density lipoprotein cholesterol (LDL-c), but they also have Lp(a) concentrations that are twice as high as normal. Thus, on top of their increased coronary artery disease risk in relation to raised LDL-c concentrations, FH patients are also exposed to potentially atherogenic concentrations of Lp(a).7 The key question is whether increased Lp(a) concentrations constitute an additional risk factor for coronary artery disease in these individuals and whether statin treatment can reduce their Lp(a) concentrations.
We recently reported that aggressive LDL-c reduction over a two year period in patients with heterozygous FH was accompanied by a striking regression of carotid intima–media thickness.8 The ASAP (effects of atorvastatin versus simvastatin on atherosclerosis progression) study protocol also provided us with a unique opportunity to study prospectively the long term effects of statin treatment on Lp(a) concentrations in relation to changes in mean carotid intima–media thickness, our primary outcome variable.
METHODS
Protocol
The design and results of the ASAP trial have been described previously.8,9 In short, ASAP was a randomised, double blind, two year, two centre study that was designed to assess whether aggressive or conventional lipid lowering treatment could retard the progression of atherosclerosis in FH patients. In this study FH patients were treated either with atorvastatin 80 mg/day or simvastatin 40 mg/day. After an eight week placebo run in, baseline measurements of lipoprotein indices, intima–media thickness, and Lp(a) were made. These measurements were repeated after one and two years.
The institutional review boards of both centres approved the study protocol, and written informed consent was obtained.
Laboratory investigations
Lipoprotein indices included total cholesterol, (calculated) LDL-c, high density lipoprotein cholesterol (HDL-c), and triglycerides. These were analysed as described previously.9
At baseline and at one year and two years, EDTA plasma was collected for Lp(a) measurement and stored at −80°C until analysis. All measurements were done in one batch at a central laboratory (department of clinical chemistry, University Hospital Utrecht, Utrecht, Netherlands). Patients were fasting when blood was collected. Lp(a) concentrations were determined in EDTA plasma using the Apo-Tek enzyme linked immunosorbent assay (ELISA) (Sigma Diagnostics Apo-Tek Lp(a) kit, St Louis, USA). The Apo-Tek ELISA assay is known to detect all apo(a) isoform sizes on an equivalent molar basis.
Intima–media thickness
Intima–media thickness measurement procedures have also been reported before.9 In short, ultrasound examinations were done with a Biosound Phase 2 real time scanner (BiosoundEsaote, Indianapolis, Indiana, USA) equipped with a 10 MHz transducer. Three 10 mm segments were scanned bilaterally: the distal portion of the common carotid artery, the carotid bifurcation, and the proximal portion of the internal carotid artery. The mean intima–media thickness represents the average over anterior and posterior walls in the common carotid artery, the carotid bifurcation, and the posterior wall of the internal carotid artery bilaterally.
Intima–media thickness measurements were done on both anterior and posterior walls of the common carotid artery, the carotid bifurcation, and the posterior wall of the internal carotid artery. Images were analysed with a semiautomatic software program (Eurequa; TSA Co, Meudon, France).
Statistical analysis
Because of the skewed distribution of Lp(a) values, median concentrations were used for analyses. Both absolute and relative changes in Lp(a) after one and two years were calculated. The signed rank Wilcoxon test was used to evaluate the significance of Lp(a) changes over time. Changes between treatment groups were analysed with the Wilcoxon two sample test. Spearman correlation coefficients were calculated to assess evidence of an association of Lp(a) concentrations and change in Lp(a) with the following variables: cardiovascular events, intima–media thickness, LDL-c concentrations at baseline, and mean changes in intima–media thickness and LDL-c concentrations. Statistical analyses were done using SAS (version 6.12, SAS Institute Inc).
RESULTS
Patients and primary results
Of the 325 patients in the original intention to treat population, 45 did not complete the study (13.8%). One hundred and sixty patients were allocated to the atorvastatin limb (66 men, 94 women) and 165 to the simvastatin limb (62 men, 103 women). At baseline, no differences between treatment groups were found in either lipid concentrations or mean carotid intima–media thickness. Cardiovascular disease was present in 31% of the patients and was equally distributed between the two treatment groups. Mean intima–media thickness levels were notably increased at baseline.8 These characteristics are summarised in table 1 for patients with at least two baseline Lp(a) values.
Table 1.
Baseline characteristics of patients with heterozygous hypercholesterolaemia
| Atorvastatin (n=139) | Simvastatin (n=148) | |
| Age (years) | 48 (10) | 49 (11) |
| Sex (female/male) | 81/58 | 92/56 |
| Smoking (%) | 32 | 27 |
| CVD (%) | 27 | 25 |
| BMI (kg/m2) | 25.7 (3.3) | 25.7 (3.8) |
| BP (mm Hg) | 130 (16) / 78 (8) | 131 (16) / 79 (8) |
| Lp(a) (mg/l) | 327 (168 to 832) | 531 (184 to 1093) |
Values are means (SD) or n, except Lp(a) which is given as median (interquartile range).
BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; Lp(a), lipoprotein(a).
Table 2 shows concentrations of total cholesterol, LDL-c, HDL-c, triglycerides, and Lp(a) at baseline and after two years. As previously described, while total cholesterol, LDL-c, and triglyceride concentrations were lowered significantly within each treatment arm (p < 0.001 for all variables), atorvastatin reduced total cholesterol, LDL-c, and triglycerides concentrations significantly more than simvastatin.8
Table 2.
Plasma concentrations of lipids, lipoproteins, and Lp(a) at baseline and after two years of treatment with simvastatin (40 mg/day) or atorvastatin (80 mg/day)
| Lipid | Statin | Baseline (n=325 max) | Two years (n=280 max) | Per cent change | p* Value | p† Value |
| Total cholesterol | Simvastatin | 10.27 (2.10) | 6.71 (1.38) | −33.6 | <0.001 | 0.001 |
| Atorvastatin | 9.99 (1.87) | 5.73 (1.31) | −41.8 | <0.001 | ||
| LDL-c | Simvastatin | 8.33 (2.03) | 4.81 (1.38) | −41.2 | <0.001 | 0.001 |
| Atorvastatin | 8.00 (1.83) | 3.88 (1.21) | −50.5 | <0.001 | ||
| HDL-c | Simvastatin | 1.16 (0.28) | 1.30 (0.36) | 13.4 | <0.001 | 0.854 |
| Atorvastatin | 1.18 (0.32) | 1.32 (0.39) | 13.2 | <0.001 | ||
| Triglycerides | Simvastatin | 1.85 (1.34) | 1.41 (0.96) | −17.7 | <0.001 | 0.0023 |
| Atorvastatin | 1.87 (1.09) | 1.23 (0.76) | −29.2 | <0.001 | ||
| Lp(a) | Simvastatin | 531 (184 to 1093) | 417 (137 to 842) | −18.8 | <0.001 | 0.53 |
| Atorvastatin | 327 (168 to 832) | 263 (129 to 707) | −22.1 | <0.001 | ||
Values are means (SD), except for Lp(a) which is in median (interquartile range), in mg/l.
*p Value for relative changes within one treatment group.
†p Value between atorvastatin and simvastatin group.
HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol; Lp(a), lipoprotein(a).
The changes in intima–media thickness have also been described previously. In summary, after two years, intima–media thickness decreased by 0.031 mm in the atorvastatin group (p = 0.0017) and increased by 0.036 mm in the simvastatin group (p = 0.0005). This difference between the two treatments was highly significant (p < 0.001).8
Lipoprotein(a)
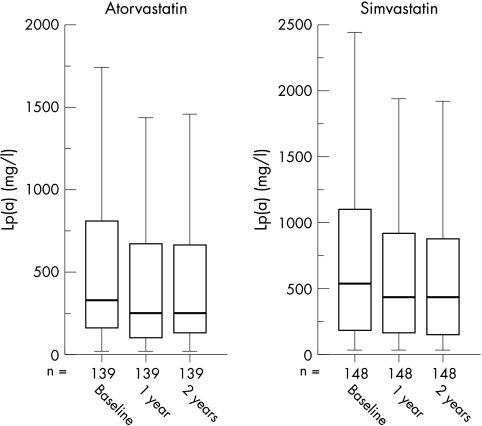
The median Lp(a) concentration at baseline was 327 mg/l in the atorvastatin group and 531 mg/l in the simvastatin group (table 2). This difference at baseline was significant (p = 0.032). There was no difference in Lp(a) concentration between men and women. After one year of treatment the median Lp(a) values were 243 mg/l and 437 mg/l in the atorvastatin and simvastatin groups, respectively. After two years the corresponding values were 263 mg/l and 417 mg/l, respectively (fig 1). The within-group changes were all significant (p < 0.001). The difference between the two treatment arms was significant after one year (p = 0.004), but not after two years (p = 0.086).
Figure 1.
Comparison of effect of simvastatin and atorvastatin treatment after one and two years on lipoprotein(a) (Lp(a)) concentrations.
The median relative decrease in Lp(a) concentrations after one year of treatment was 21.4% (p < 0.001) in the atorvastatin group v 16.3% (p < 0.001) in the simvastatin group. After two years this decrease was 22.1% (p < 0.001) in the atorvastatin group versus 18.8% (p < 0.001) in the simvastatin group. The relative differences between the two treatment groups at one and two years were not significant (p = 0.12 and p = 0.53, respectively). In the atorvastatin group 79% of the patients showed a decrease in Lp(a) concentrations compared with 80% of patients in the simvastatin group.
Baseline Lp(a) concentrations were not correlated with LDL-c concentrations (r = 0.013; p = 0.83). There was no correlation between baseline Lp(a) concentrations and prevalent cardiovascular disease (coronary artery disease or peripheral artery disease) at baseline (p = 0.63). Lp(a) concentrations at baseline were also not correlated with age (r = 0.021, p = 0.728), baseline intima–media thickness (r = 0.045; p = 0.45), or change in intima–media thickness after one year (r = 0.03, p = 0.63) or two years (r = −0.01, p = 0.92). The change in Lp(a) was weakly correlated with the change in LDL-c (r = 0.20; p = 0.001). Change in Lp(a) was not correlated with change in intima–media thickness after one year (r = 0.05, p = 0.39) or two years (r = 0.03, p = 0.65).
DISCUSSION
We have shown here that long term statin treatment in FH patients significantly lowers Lp(a) values. However, there was no correlation between baseline Lp(a) concentrations and the presence of cardiovascular disease. In addition, no correlation was evident between baseline Lp(a) concentrations and baseline intima–media thickness, or between the statin induced change in Lp(a) concentrations and the change in mean carotid intima–media thickness. These findings cast doubt upon the importance of Lp(a) in atherogenesis in FH heterozygotes, at least on the supposed benefit of a reduction in the plasma concentration of this lipoprotein particle.
A recent clinical meta-analysis clearly shows that Lp(a) is a coronary artery disease risk factor,6 notably in the presence of raised plasma LDL-c.10 This has resulted in the search for agents that lower Lp(a) concentrations, and a further exploration of the effects of established lipid lowering drugs in this context.
So far, no specific Lp(a) lowering agents have been discovered. Some drugs (for example, nicotinic acid and oestrogen replacement therapy) have mild to moderate Lp(a) reducing effects, although they hardly ever normalise the concentrations.2 Therefore, more aggressive lowering of LDL-c concentrations is advocated in patients with a raised Lp(a).11 The efficacy of statins in reducing Lp(a) is not well established. Some reports have shown an increase in Lp(a) with statins12,13 while others have shown the opposite,14,15 and it now seems to be assumed that statin treatment does not affect Lp(a) concentrations.16 However, we have shown here for the first time that statins do have a clear Lp(a) lowering effect, albeit not associated with a change in intima–media thickness.
Possible explanations for the observed discrepancies between our study and previous investigations are, first, that most of the studies mentioned only included a small number of patients, whereas a large FH cohort (325 patients) participated in the ASAP study; second, the follow up period in previous trials was generally limited (mostly to less than six months), while in the ASAP it was on average two years; third, the ASAP study only included FH heterozygotes, whereas most of the other studies included mixed hyperlipidaemic subjects; and finally, we cannot exclude the possibility that a reduction in the median Lp(a) concentration of 20% is not large enough to induce measurable changes in intima–media thickness.
It is generally accepted that raised Lp(a) concentrations are a risk factor for coronary artery disease. A recent report which evaluated different risk factors in patients with heterozygous FH is in disagreement with this. The investigators concluded that Lp(a) was only associated with an increased risk in very early coronary cases.17 Furthermore, studies such as the LDL-apheresis atherosclerosis regression study (LAARS) and the familial hypercholesterolaemia regression study investigated the effects of Lp(a) reduction on the changes in the diameter of the coronary arteries. In neither study was any benefit of reducing Lp(a) concentrations seen in patients whose cholesterol had been effectively lowered by drug treatment or apheresis.18,19
Conclusions
In this study, Lp(a) concentrations were not associated with the presence of coronary artery disease or with mean carotid intima–media thickness values at baseline. More importantly, we did not observe a decrease in intima–media thickness after an atorvastatin and simvastatin induced decrease in Lp(a) concentrations. Thus changes in Lp(a) concentrations in this range were not correlated with change in intima–media thickness, indicating either that a beneficial change in intima–media thickness is completely independent of Lp(a) concentrations in FH patients or possibly that much larger reductions in Lp(a) are necessary before a clinical effect becomes obvious.
Acknowledgments
We thank P M Netten, P Bouter, P Lestrade, W Bogers, and B Imholz for assistance with recruitment; A Theloose, M Brok, J Visser, J den Arend, and G van der Biezen for the ultrasound investigations; the department of epidemiology and biostatistics (W Lemmens) for assistance with statistical analyses; and T Terburg and H van Langen for technical assistance. This study was financially supported by Parke Davis BV, Netherlands. JJPK is an established investigator of the Netherlands Heart Foundation.
Abbreviations
ASAP, effects of atorvastatin versus simvastatin on atherosclerosis progression
ARIC, atherosclerosis risk in community
ELISA, enzyme linked immunosorbent assay
FH, familial hypercholesterolaemia
HDL-c, high density lipoprotein cholesterol
LAARS, LDL-apheresis atherosclerosis regression study
LDL-c, low density lipoprotein cholesterol
Lp(a), lipoprotein(a)
TGFβ, transforming growth factor β
REFERENCES
- 1.Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for coronary artery disease. Am J Cardiol 1998;82:57–66U. [DOI] [PubMed] [Google Scholar]
- 2.Hobbs HH, White AL. Lipoprotein(a): intrigues and insights. Curr Opin Lipidol 1999;10:225–36. [DOI] [PubMed] [Google Scholar]
- 3.Superko HR. Did grandma give you heart disease? The new battle against coronary artery disease. Am J Cardiol 1998;82:34–46Q. [DOI] [PubMed] [Google Scholar]
- 4.Berg K, Dahlen G, Christophersen B, et al. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian simvastatin survival study. Clin Genet 1997;52:254–61. [DOI] [PubMed] [Google Scholar]
- 5.Price JF, Lee AJ, Rumley A, et al. Lipoprotein (a) and development of intermittent claudication and major cardiovascular events in men and women: the Edinburgh artery study. Atherosclerosis 2001;157:241–9. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease – meta-analysis of prospective studies. Circulation 2000;102:1082–5. [DOI] [PubMed] [Google Scholar]
- 7.Lingenhel A, Kraft HG, Kotze M, et al. Concentrations of the atherogenic Lp(a) are elevated in FH. Eur J Hum Genet 1998;6:50–60. [DOI] [PubMed] [Google Scholar]
- 8.Smilde TJ, Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001;357:577–81. [DOI] [PubMed] [Google Scholar]
- 9.Smilde TJ, Trip MD, Wollersheim H, et al. Rationale, design and baseline characteristics of a clinical trial comparing the effects of robust vs conventional cholesterol lowering and intima media thickness in patients with familial hypercholesterolaemia. The atorvastatin versus simvastatin on atherosclerosis progression (ASAP) study. Clin Drug Invest 2001;20:67–79. [DOI] [PubMed] [Google Scholar]
- 10.Maher VM, Brown BG. Lipoprotein (a) and coronary heart disease. Curr Opin Lipidol 1995;6:229–35. [DOI] [PubMed] [Google Scholar]
- 11.Maher VM, Brown BG, Marcovina SM, et al. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA 1995;274:1771–4. [PubMed] [Google Scholar]
- 12.Bredie SJ, Westerveld HT, Knipscheer HC, et al. Effects of gemfibrozil or simvastatin on apolipoprotein-B-containing lipoproteins, apolipoprotein-CIII and lipoprotein(a) in familial combined hyperlipidaemia. Neth J Med 1996;49:59–67. [DOI] [PubMed] [Google Scholar]
- 13.Branchi A, Rovellini A, Fiorenza AM, et al. Effects of bezafibrate and of 2 HMG-CoA reductase inhibitors on lipoprotein (a) level in hypercholesterolemic patients. Int J Clin Pharmacol Ther 1995;33:345–50. [PubMed] [Google Scholar]
- 14.Haffner S, Orchard T, Stein E, et al. Effect of simvastatin on Lp(a) concentrations. Clin Cardiol 1995;18:261–7. [DOI] [PubMed] [Google Scholar]
- 15.Goudevenos JA, Bairaktari ET, Chatzidimou KG, et al. The effect of atorvastatin on serum lipids, lipoprotein(a) and plasma fibrinogen levels in primary dyslipidaemia – a pilot study involving serial sampling. Curr Med Res Opin 2001;16:269–75. [DOI] [PubMed] [Google Scholar]
- 16.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation 2000;101:207–13. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins PN, Stephenson S, Wu LL, et al. Evaluation of coronary risk factors in patients with heterozygous familial hypercholesterolemia. Am J Cardiol 2001;87:547–53. [DOI] [PubMed] [Google Scholar]
- 18.Kroon AA, Aengevaeren WR, van der WT, et al. LDL-apheresis atherosclerosis regression study (LAARS). Effect of aggressive versus conventional lipid lowering treatment on coronary atherosclerosis. Circulation 1996;93:1826–35. [DOI] [PubMed] [Google Scholar]
- 19.Thompson GR, Maher VM, Matthews S, et al. Familial hypercholesterolaemia regression study: a randomised trial of low-density-lipoprotein apheresis. Lancet 1995;345:811–16. [DOI] [PubMed] [Google Scholar]