Abstract
Objective: To assess the influence of acute α and β blockade on ventilation and symptoms of breathlessness during exercise in patients with chronic heart failure and in controls.
Methods: 11 patients with chronic heart failure and 11 control subjects underwent repeated exercise testing with metabolic gas exchange after random, double blind administration of either an α blocker and placebo, a β blocker and a placebo, both an α blocker and a β blocker, or double placebo.
Results: Patients had a lower peak oxygen consumption (mean (SD) 20.7 (4.9) v 37.6 (9.6) ml/kg/min, p < 0.0001) and a steeper slope relating ventilation to carbon dioxide production (VE/V̇co2 slope) (26.5 (4.1) v 37.1 (8.2), p = 0.0011), than controls. Blood pressure was lower following α and β blockade (p < 0.05) and the gradients of the slopes relating heart rate to oxygen consumption following the β blocker were reduced (p < 0.05). Exercise time and peak ventilatory variables following β or α blockers were unchanged. Ventilation was reduced during submaximal exercise following the active medications. Combined α and β blockade produced the greatest difference (p < 0.005), but the α and β blockers alone also reduced ventilation (p < 0.05). There was no difference in perceived exertion during exercise with any of the treatments.
Conclusion: Acute sympathetic inhibition can reduce submaximal ventilation during exercise in patients with heart failure and control subjects, suggesting that autonomic nervous system activation has an important role in the abnormal ventilatory response to exercise in chronic heart failure.
Keywords: α receptors, β receptors, ventilatory response, chronic heart failure, exercise testing
Patients with chronic heart failure complain of exercise intolerance, usually because of breathlessness and fatigue.1 This can be objectively assessed as a reduction in peak oxygen consumption (PV̇o2) during incremental exercise testing with metabolic gas exchange analysis.2 Patients also have an increased ventilatory response to exercise as shown by an increase in the slope relating ventilation to carbon dioxide production (VE/V̇co2 slope).3,4 The VE/V̇co2 slope is abnormal throughout exercise5 and correlates inversely with PV̇o2, so that the greater the ventilatory response, the lower the exercise capacity.3,4
The cause of this abnormal ventilatory response is not clearly understood. Lung function is abnormal in patients with chronic heart failure,6,7 and we have previously shown that patients’ exercise capacity is partly limited by a reduced maximal tidal volume, leading to a greater dependence on frequency early during exercise.8 Skeletal muscle is also involved in ventilatory control. Skeletal muscle contains receptors, termed ergoreceptors, that stimulate ventilation in response to work.9 These receptors are overactive in heart failure and are linked to the increased sympathetic activity.10
A further influence on ventilation during exercise may be through central chemoreceptors.11 Arterial blood gas tensions are normal in patients with chronic heart failure during exercise,12,13 but the reflexes associated with these chemoreceptors are overactive in patients.14
Chronic heart failure is a state of sympathetic overactivity,15 and it may be through the abnormal sympathetic nervous system that these factors exert an influence upon ventilatory control during exercise. β Blockade in chronic heart failure improves echocardiographic variables of cardiac function, patients’ symptoms, and their prognosis,16,17 but no consistent improvement in maximal18 or submaximal19,20 exercise capacity has been seen.
Exogenously infused catecholamines increase ventilation in control subjects.21–23 Yohimbine, which increases catecholamine release, increases ventilation during exercise in normal subjects.24 The aim of the present study was to assess the influence that short term α and β blockade has on the ventilatory response to exercise and symptoms of breathlessness in patients with chronic heart failure compared with a group of control subjects.
METHODS
We enrolled 11 patients with chronic heart failure caused by ischaemic heart disease and 11 control subjects into the study. Each subject gave informed, written consent before involvement in the study, which was approved by the local ethics committee. Chronic heart failure was defined as the presence of symptoms of fatigue or breathlessness on exertion and a left ventricular ejection fraction on echocardiography of < 35%. The condition had to be of at least three months’ duration with no recent exacerbation. We excluded patients with neurological conditions or inducible ischaemia upon exercise and those already taking either α or β antagonists. The controls were individuals of a similar age chosen at random from the patient lists of local general practitioners. None was taking regular medication and all underwent echocardiography to exclude left ventricular dysfunction. People in both groups were excluded if there was any history of pulmonary disease or if their forced expiratory volume in one second (FEV1) was < 80% of predicted.
Each subject underwent a familiarisation symptom limited exercise test. We used the Bruce treadmill protocol modified by the addition of a stage 0 at onset consisting of three minutes of exercise at 1.61 km/h (1 mile/h) with a 5% gradient. During the tests patients wore a tightly fitting facemask to which was connected a capnograph and a sample tube enabling online measurement of ventilation and metabolic gas exchange (Oxycon Delta system, Jaeger, Hoechberg, Germany). A respiratory exchange ratio (RER) (V̇co2/V̇o2) > 1 was taken to suggest a maximal effort. The subjects were asked to score their symptoms of breathlessness or fatigue between 0–10 (0 being no symptoms and 10 being maximal) on a standard scale of perceived exertion25 at the end of each stage during the test.
At four subsequent visits, the exercise tests were repeated 30 minutes after the administration of either an α blocker (doxazocin 2 mg) and a placebo, a β blocker (metoprolol 25 mg) and a placebo, both (metoprolol 25 mg and doxazocin 2 mg), or double placebo in a randomised double blind fashion.
Results are reported as mean (SD). The anaerobic threshold was calculated by the V̇co2/V̇o2 slope method.26 We plotted the relation of Borg score against expired minute ventilation (VE) (Borg/VE slope) and compared the slopes acquired by simple regression. To compare submaximal responses, we used data from the last stage completed by all subjects (stage 0). We used unpaired Student’s t test for between group comparisons of the baseline data and analysis of variance with Fisher’s PLSD correction for comparisons between treatments. A probability value of p < 0.05 was taken to be significant.
RESULTS
Table 1 shows the baseline variables for the patients and control subjects. Patients had a shorter exercise time and a lower PV̇o2. The relation between VE and V̇co2 was steeper in patients than in controls. There was a significantly steeper slope relating expired volume (VE) to symptoms (Borg score) in patients, so that for a given VE, patients were more breathless.
Table 1.
Subject characteristics
| Patients (n=11) |
Controls (n=11) |
p Value | |
| Age (years) | 68 (6.1) | 67 (9.0) | 0.67 |
| Height (cm) | 174 (5.5) | 177 (8.5) | 0.22 |
| Weight (kg) | 80.6 (15.9) | 83.4 (8.8) | 0.62 |
| LVEDD (cm) | 6.9 (0.7) | 5.0 (0.7) | <0.0001 |
| LVEF (%) | 31.8 (2.9) | 56.7 (10.4) | <0.0001 |
| Drugs | |||
| Furosemide* | 83.4 (53.4) | ||
| ACEi/AIIA (n) | 23 | ||
| Spironolactone (n) | 5 | ||
| New York Heart Association functional class (n) | |||
| I | 0 | ||
| II | 4 | ||
| III | 7 | ||
| IV | 0 | ||
| PV̇o2 (ml/kg/min) | 20.7 (4.9) | 37.6 (9.6) | <0.0001 |
| Exercise time (s) | 471 (226) | 855 (225) | 0.0007 |
| RER | 1.06 (0.06) | 1.08 (0.08) | 0.50 |
| HR/O2 slope | 4.25 (1.4) | 2.92 (0.7) | 0.01 |
| AT | 15.5 (4.8) | 27.0 (7.4) | 0.0003 |
| VE/V̇co2 slope | 37.1 (8.2) | 26.5 (4.1) | 0.0011 |
| VE peak (l/min) | 64.5 (13.5) | 97.4 (21.3) | 0.0003 |
| Borg/VE slope | 0.28 (0.39) | 0.12 (0.05) | 0.017 |
Values are means (SD).
*Mean (SD) daily dose of furosemide (frusemide).
ACEi, angiotensin converting enzyme inhibitor; AIIA, angiotensin II inhibitor; AT, anaerobic threshold;
HR, heart rate; LVEDD, left ventricular end diastolic dimension form M mode echocardiography; LVEF, left ventricular ejection fraction; PV̇o2, peak oxygen consumption; RER, respiratory exchange ratio (V̇co2/V̇o2); VE, minute ventilation.
There was no difference in metabolic gas analysis variables between the familiarisation test and the test conducted with double placebo.
Haemodynamic variables
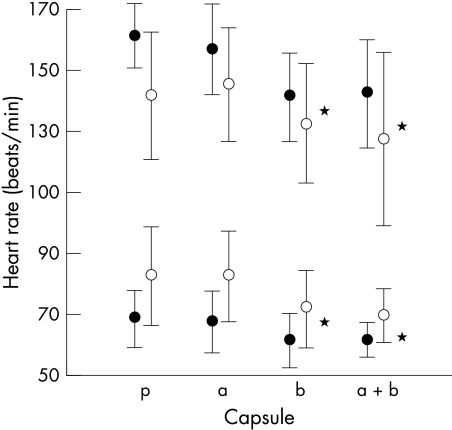
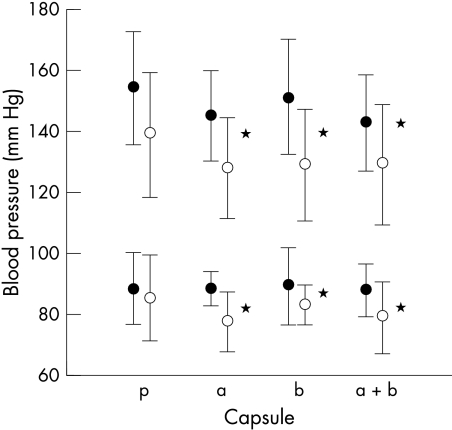
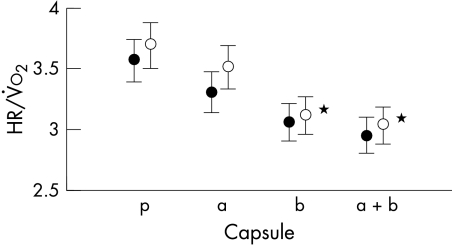
Figures 1 and 2 show the rest and peak heart rates and blood pressures for patients and controls. The differences in heart rate between the tests performed following β blockade and those following placebo and α blockade are significant (p < 0.05). The systolic blood pressure at rest and at peak exercise was significantly lower in both patients and controls for the tests following α and β blockade (p < 0.05). The effect of the β blocker can also be seen in fig 3 with significantly lower gradients of the slopes relating heart rate to oxygen consumption during the tests following β blocker ingestion (p < 0.05).
Figure 1.
The effect of treatments on heart rate at rest (open circles) and at peak exercise (solid circles). *p < 0.05. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
Figure 2.
Effect of treatments on systolic and diastolic blood pressure at rest (open circles) and peak exercise (solid circles). *p < 0.05. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
Figure 3.
Effect of treatments on the ratio of heart rate to oxygen consumption during the tests in patients (open circles) and controls (solid circles). *p < 0.05 for reductions from placebo for both patients and controls. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
Ventilatory variables
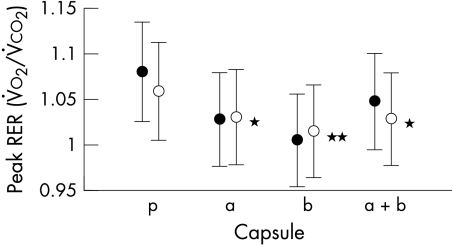
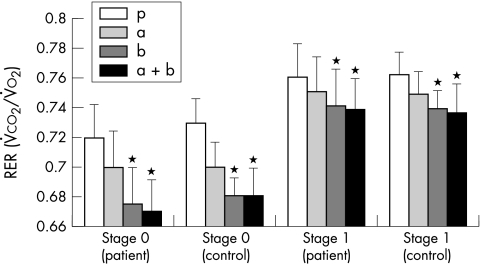
There were no significant differences in exercise time or peak ventilatory variables following β or α blocker for either patients or controls. The PV̇o2, VE/V̇co2 slope, and tidal volume were also not different. Table 2 displays the results for the patients and controls combined. Despite this lack of differences, patients and controls reached a significantly lower RER after the β blocker (p < 0.02) than after either the α blocker or placebo (fig 4). At matched submaximal workload, the RER was significantly lower after the β blocker and the combination treatments (p < 0.05) (fig 5).
Table 2.
Peak exercise variables after drug and placebo administration
| Treatment | |||||
| Variable | a | b | a+b | p | p Value |
| Exercise time (s) | 730 (335) | 692 (294) | 700 (313) | 712 (300) | 0.97 |
| PV̇o2 (ml/kg/min) | 29.5 (11.1) | 28.9 (10.8) | 29.0 (11.6) | 29.2 (11.4) | 1.0 |
| VE/<V̇co2 slope | 31.8 (7.7) | 31.8 (7.6) | 31.8 (8.1) | 31.8 (8.4) | 1.0 |
| VE (l/min) | 82.0 (23.1) | 76.7 (20.9) | 81.9 (27.7) | 81.0 (24.2) | 0.94 |
| Vt (l) | 2.2 (0.5) | 2.2 (0.5) | 2.2 (0.6) | 2.2 (0.5) | 0.99 |
| Frequency (breaths/min) | 37.4 (6.9) | 36.0 (6.3) | 36.5 (6.6) | 36.5 (7.8) | 0.97 |
| RER | 1.03 (0.03) | 1.01 (0.08) | 1.04 (0.11) | 1.07 (0.07) | <0.02 |
| HR (beats/min) | 151 (19) | 137 (19) | 136 (26) | 151 (21) | <0.05 |
| SBP (mm Hg) | 190 (35) | 186 (31) | 181 (37) | 197 (33) | <0.05 |
| DBP (mm Hg) | 97 (26) | 106 (24) | 98 (26) | 107 (27) | <0.05 |
| Borg/VE slope | 0.13 (0.05) | 0.15 (0.05) | 0.17 (0.15) | 0.20 (0.28) | 0.57 |
| HR/O2 | 3.4 (0.9) | 3.1 (0.9) | 3.0 (0.9) | 3.5 (1.0) | <0.05 |
p Values are for analysis of variance differences between the active treatments and placebo.
a, α blocker; b, β blocker; a+b, α and β blocker; DBP, diastolic blood pressure; p, double placebo;
SBP, systolic blood pressure; Vt, tidal volume.
Figure 4.
Effect of treatments on peak respiratory exchange ratio (RER) in patients (solid circles) and controls (solid circles). **p < 0.02; *p < 0.05 for patients and controls. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
Figure 5.
Effect of treatments on RER at the end of stage 0 and stage 1 in patients and controls. *p < 0.05. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
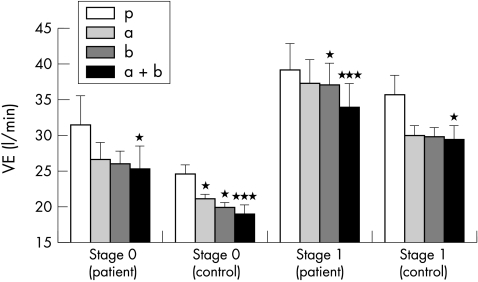
There was also a significant reduction in VE following administration of the active medication at submaximal exercise (fig 6). The combination treatment produced the greatest difference in the patients (p < 0.005), but the α and β blockers alone also reduced ventilation (p < 0.05). None of the active treatments reduced ventilation significantly more than any other. Reductions in ventilation were also seen at higher levels of exercise, but only the effect of the combined active treatments reached significance at the second stage (stage 1) (p < 0.05) and this was no longer significant by the third (stage 2) (p < 0.07). During the early stages of exercise ventilation decreased more with all active treatments than with placebo (p < 0.01) in the control group compared with patients, with the greatest effect following the combination treatment (p < 0.005). The effect was significant to the end of the second stage (p < 0.05) but was not significant by the end of the third (p = 0.28).
Figure 6.
Effect of treatments on ventilation at the end of stage 0 and stage 1 in patients and controls. * p < 0.05; ***p < 0.005. a, α blocker; b, β blocker; a+b, α and β blocker; p, double placebo.
Symptom scores
Overall there was no difference in the slopes relating symptoms to VE (Borg/VE). The Borg score at each stage was also not influenced by the adrenergic blockers (p = 0.44 for the difference between Borg score with placebo and the combination treatment tests at the end of the first stage).
DISCUSSION
The origin of the increased ventilatory response to exercise in patients with chronic heart failure remains elusive. However, there is good evidence for a role of the sympathetic nervous system. Heart failure is a state of chronic sympathetic overactivity that may lead to increased sensitivity of the central chemoreceptors and hence an increased ventilatory response to hypercapnia.11 Further receptors that can stimulate ventilation and are sensitive to sympathetic activation exist in the peripheral muscles.9,10
Each of these mechanisms may contribute to the pathophysiological response to exercise in patients with chronic heart failure. The common factor in the heightened sensitivity of the receptors and the increased ventilatory response is sympathetic nervous system activation.21–23
The potential influence of increased plasma catecholamine concentrations was shown in a group of normal subjects with the use of yohimbine. This agent prevents the negative feedback of catecholamines at the presynaptic membrane and leads to increased noradrenaline and adrenaline release, increases the ventilatory response of normal subjects, and heightens symptoms of breathlessness during steady state exercise.24
In the present study heart rate and blood pressure were decreased with the β and α blockers. There was also a reduction in the slope relating heart rate to oxygen consumption. The effect of the α and β receptor blockers on these haemodynamic variables implies that the delay between administration of the treatment and the tests was sufficient for the agents to be taking effect. Given that blood pressure and heart rate changes are unrelated to exercise intolerance in patients with chronic heart failure, it is unlikely that the changes in the haemodynamic variables caused the altered ventilatory response.27,28
Submaximal ventilation was reduced, however, with the greatest effect seen following the combination of α and β blockade. The influence of the β and α blockers on submaximal ventilation was most pronounced in the control group, and one may speculate that this reflects the activity of the agents in the presence of lower baseline catecholamine concentrations. This result supports previous findings that the sympathetic nervous system influences ventilation, both in patients with chronic heart failure and in normal subjects.21–23 It also suggests that the increased response is not fixed and may therefore be modifiable. There was no influence on basic exercise variables such as PV̇o2 and VE/V̇co2 slope or on the ventilatory equivalent for carbon dioxide (V̇eqco2) by either β or α blockade.
There was no effect on the perception of breathlessness, unlike the changes noted with yohimbine. However, that study looked at normal people at submaximal exercise. It is possible that the degree of exertion required to perform a peak exercise test masked a subtle reduction in symptoms in the present study.
The influence of β and α blockade on the RER has not been described previously. The well recognised fatiguing effects of β blockade may have led subjects to perform less well in the presence of these agents at peak. However, the exercise time, PV̇o2, and other peak ventilatory variables were not different. The lower RER at the end of the early stages may suggest altered substrate utilisation. It is possible that after adrenergic blockade fatty acids are metabolised in preference to glucose, thereby lowering the RER.
Despite plausible explanations for the abnormalities of ventilation in heart failure, large multicentre randomised controlled trials have failed to show any benefit on exercise capacity and symptom scores from long term β blockade.18–20 The studies suggesting improved exercise capacity in patients on long term β blockade used small study populations.29,30 There is also no benefit on exercise capacity of adding α blockers to standard β blockers.31
Conclusion
The major symptom of chronic heart failure is exercise intolerance caused by breathlessness or fatigue. Despite the well documented beneficial effects of β blockade on prognosis and left ventricular ejection fraction there is no evidence of an improvement in exercise capacity. The present study, examining the effects of acute sympathetic blockade in a group of patients and control subjects, has documented reduced ventilation during the early stages of exercise in both patients and controls. This supports the concept that sympathetic activation contributes to the excessive ventilation in chronic heart failure.
Limitations
We feel that randomising the order of treatment administration and keeping the time between tests to at least one week eliminated the effects of training. The influence of performing five peak tests over a period of five weeks remains a potential but unlikely confounding variable. A possible further limitation is that of reproducibility. However, we found no difference between the familiarisation tests and those performed after double placebo suggesting good reproducibility and a lack of training effect.
We chose the doses of the α and β blockers to avoid possible side effects of hypotension and bradycardia, but it is possible that in the chronic state of sympathetic overactivity associated with heart failure, greater doses would have led to more pronounced effects. As mentioned above, the effects of yohimbine were tested at submaximal exercise in normal subjects. It is possible that the influence of the sympathetic nervous system is greatest at low to medium levels of exertion, before the respiratory and cardiovascular systems are stressed to peak, and that beneficial effects in the present study were masked by the nature of the peak tests. Indeed, any benefits at submaximal workloads would be most appropriate for patients with chronic heart failure, who rarely exercise to the levels achieved during a peak test.
Acknowledgments
This study was made possible by a grant from Pfizer Inc.
Abbreviations
PV̇o2, peak oxygen consumption
RER, respiratory exchange ratio
V̇co2, carbon dioxide production
VE, minute ventilation
REFERENCES
- 1.Clark AL, Sparrow JL, Coats AJS. Muscle fatigue and dyspnoea in chronic heart failure: two sides of the same coin? Eur Heart J 1995;16:49–52. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Poole-Wilson PA, Coats AJS. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 1996;28:1092–102. [DOI] [PubMed] [Google Scholar]
- 3.Buller NP, Poole-Wilson PA. Mechanism of the increased ventilatory response to exercise in patients with chronic heart failure. Br Heart J 1990;63:281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies SW, Emery TM, Watling MIL, et al. A critical threshold of exercise capacity in the ventilatory response to exercise in chronic heart failure. Br Heart J 1991;65:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witte KK, Clark AL. Is the elevated slope relating ventilation to carbon dioxide production in chronic heart failure a consequence of slow metabolic gas kinetics? Eur J Heart Fail 2002;4:469–72. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite haemodynamic and pulmonary abnormalities. Circulation 1988;77:552–9. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman K, Zhang Y-Y, Gitt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997;96:2221–7. [DOI] [PubMed] [Google Scholar]
- 8.Witte KKA, Thackray SDR, Nikitin NP, et al. Pattern of ventilation during exercise in chronic heart failure. Heart 2003;89:610–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepoli M, Clark AL, Coats AJS. Muscle metaboreceptors in the hemodynamic, autonomic and ventilatory responses to exercise in man. Am J Physiol 1995;269:H1428–36. [DOI] [PubMed] [Google Scholar]
- 10.Piepoli M, Clark A, Volterrani M, et al. Contribution of muscle afferents to the hemodynamic, autonomic and ventilatory responses to exercise in patients with chronic heart failure. Circulation 1996;93:940–52. [DOI] [PubMed] [Google Scholar]
- 11.Narkiewicz K, Pesek CA, van de Borne PJH, et al. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation 1999;100:262–7. [DOI] [PubMed] [Google Scholar]
- 12.Clark AL, Coats AJS. Usefulness of arterial blood gas estimations during exercise in patients with chronic heart failure. Br Heart J 1994;71:528–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark AL, Volterrani M, Swan JW, et al. Increased ventilatory response to exercise in chronic heart failure: relation to pulmonary pathology. Heart 1997;77:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua TP, Clark AL, Amadi A, et al. Relationship between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1996;27:650–7. [DOI] [PubMed] [Google Scholar]
- 15.Francis GS, Cohn JN, Johnson G, et al. Plasma norephinephrine, plasma rennin activity and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT II follow-up. Circulation 1993;87(suppl VI):40–8. [PubMed] [Google Scholar]
- 16.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349–55. [DOI] [PubMed] [Google Scholar]
- 17.CIBIS II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS II). Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 18.Anon. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Australia/New Zealand Heart Failure Research Collaborative Group. Lancet 1997;349:375–80. [PubMed] [Google Scholar]
- 19.Anon. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US carvedilol heart failure study group. Circulation 1996;94:2800–6. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JN, Fowler MB, Bristow MR, et al, for the US Carvedilol Heart Failure Study Group. Safety and efficacy of carvedilol in severe heart failure. J Card Fail 1997;3:173–9. [DOI] [PubMed] [Google Scholar]
- 21.Wheelan RF, Young IM. The effect of adrenaline and noradrenaline infusions on respiration in man. Br J Pharmacol 1953;8:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heistad DD, Wheeler RC, Mark AL, et al. Effects of adrenergic stimulation on ventilation in man. J Clin Invest 1972;51:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butland RJA, Pang JA, Geddes DM. The selectivity of the β adrenoceptor for ventilation in man. Br J Clin Pharmacol 1982;14:707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark AL, Galloway S, MacFarlane N, et al. Catecholamines contribute to exertional dyspnoea and to the ventilatory response to exercise in normal humans. Eur Heart J 1997;18:1829–33. [DOI] [PubMed] [Google Scholar]
- 25.Borg G. Subjective effort and physical activities. Scand J Rehabil 1978;6:108–13. [PubMed] [Google Scholar]
- 26.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol 1986;60:2020–7. [DOI] [PubMed] [Google Scholar]
- 27.Faggiano P, D’Aloia A, Gualeni A, et al. Relative contribution of resting haemodynamic profile and lung function to exercise tolerance in male patients with chronic heart failure. Heart 2001;85:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraemer MD, Kubo SH, Rector TS, et al. Pulmonary and peripheral vascular factors are important determinants of peak exercise oxygen uptake in patients with heart failure. J Am Coll Cardiol 1993;21:641–8. [DOI] [PubMed] [Google Scholar]
- 29.Metra M, Nardi M, Giubbini R, et al. Effects of short and long term carvedilol administration on rest and exercise haemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1994;24:1678–87. [DOI] [PubMed] [Google Scholar]
- 30.Krum H, Schwartz B, Sackner-Bernstein J, et al. Double-blind pacebo-controlled study of the long-term efficacy of carvedilol in patients with severe chronic heart failure. Circulation 1995;92:1499–506. [DOI] [PubMed] [Google Scholar]
- 31.Kukin ML, Kalman J, Mannino M, et al. Combined alpha-beta blockade (doxasocin plus metoprolol) compared with beta-blockade alone in chronic congestive heart failure. Am J Cardiol 1996;77:486–91. [DOI] [PubMed] [Google Scholar]