Each year in the UK since 1988, an average of 165 patients have undergone surgical repair of interventricular septal rupture complicating myocardial infarction (cardiac surgical registry of the Society of Cardiothoracic Surgeons of Great Britain and Ireland). From each surgeon’s viewpoint this operation is a rare event. Given that there are now some 200 or so consultant cardiac surgeons in the UK, the current workload averages out at less than one case per surgeon per year.
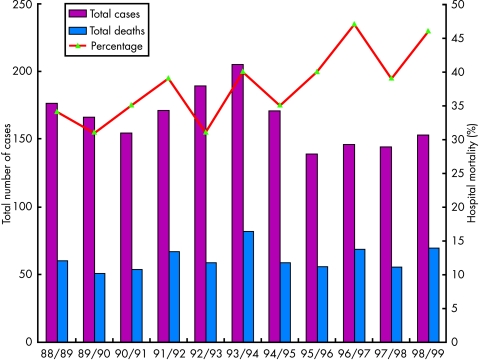
The overall hospital mortality of patients undergoing surgical repair in the UK in the same time period was 38%. So that it is not only a rare operation but it is also difficult to end up with a survivor. The national figures suggest that hospital mortality has increased with time (fig 1). In addition, in the last five years for which data are available, the number of patients undergoing repair has decreased. These two statistics are almost certainly associated with the increasing use of thrombolytic agents in the management of patients with acute myocardial infarction.
Figure 1.
Incidence and mortality of surgical closure of ventricular septal rupture in the UK (1988–1999).
So ventricular septal rupture is a rare surgical intervention with a high complication rate. On the other hand, it is a condition that when untreated has such a high mortality1 that each survivor is something to treasure. This article will attempt to describe best practice and the evidence on which such practice is based.
PATHOLOGY
Acute ventricular septal rupture only rarely comes to the attention of each surgeon, but it is a relatively common condition. Before the introduction of thrombolysis it complicated 1–2% of all myocardial infarctions. The incidence has declined to about 0.2% in the thrombolysis era. At this rate, of the 270 000 myocardial infarctions suffered in the UK in 2002, approximately 550 will be complicated by a ventricular septal rupture. Considerably less than 50% of these come to surgery.
Ventricular septal rupture results from full thickness infarction of the interventricular septum followed by sufficient necrosis to result in the septal rupture. It is one of the three mechanical complications that can occur following myocardial infarction. The others are free wall rupture, which is usually rapidly fatal, and papillary muscle rupture, which results in sudden onset of mitral regurgitation. The respective frequencies of these complications are in approximate proportion to the respective volumes of muscle that are available to be involved, so that free wall rupture is most common, ventricular septal rupture next, and papillary muscle rupture least.
The two pathological types of ventricular rupture are simple and complex. In a simple rupture there is a through and through opening connecting the two ventricles, without gross haemorrhage or laceration and with the right and left ventricular openings at about the same horizontal level of the ventricular septum. A complex rupture is an interventricular communication with a convoluted course, with a tract that might extend into regions remote from the primary acute myocardial infarction site, and with haemorrhage and disruption of myocardial tissue. Complex morphology is more common in ruptures complicating inferior myocardial infarct while simple morphology is more common after anterior myocardial infarction.2
A post mortem study by Mann and Roberts compared hearts from victims of acute myocardial infarction with and without ventricular septal rupture.3 They found that more epicardial coronary arteries were narrowed in those without rupture than those with, indicating that patients with diffuse disease are less likely to develop septal rupture. In a clinical study the coronary angiographic findings in 91 patients with ventricular septal rupture were compared with angiography in 123 infarct survivors without septal rupture. Rupture was associated with a higher incidence of single vessel disease and less evidence of collateral circulation.4 This was confirmed in a more recent but smaller Swiss study.5 It has also been demonstrated that multi-vessel disease is more common when rupture complicates an inferior infarct rather than an anterior infarct.6
The advent of widespread use of thrombolysis has had a dramatic effect upon the nature of ventricular septal rupture. In the early days of thrombolysis it was thought that the incidence may be increased, but it has subsequently been demonstrated repeatedly that the incidence is significantly reduced. In the GUSTO-I (global utilization of streptokinase and t-PA for occluded coronary arteries) trial there was an incidence of 0.2% of ventricular septal rupture in over 41 000 patients,7 a 5–10 fold reduction compared with the pre-thrombolytic era. However, the nature of presentation has changed. Whereas the average time interval between infarction and rupture used to be 5–6 days, it is now closer to one day.8 As we have already seen, the surgical mortality has increased at the same time.
It is likely that the nature of the patients coming to surgery has changed. The thrombolytic treatment may increase the proportion of ruptures that are complex rather than simple, and therefore more difficult to repair. Furthermore, patients in the first 24–48 hours after infarction are probably less well able to sustain the insult of surgery than they would be a week or so later.
PATHOPHYSIOLOGY
Interventricular septal rupture results in shunting of blood from the left to the right ventricle. The right ventricle, which is usually involved in the infarct, suffers the added burden of an increased volume load. Clearly the size of the rupture will have an influence on the size of the shunt, but also physiological factors contribute to the overall haemodynamic consequences. In particular, any increase in systemic vascular resistance will increase the size of the shunt. If pulmonary vascular resistance increases then the size of the shunt will be reduced. If the left ventricle fails to the extent that systolic pressure falls then the size of the shunt will again be reduced. The pathophysiology will be dependent upon the balances between these interactive factors.
MAKING THE DIAGNOSIS
The typical presentation is of a patient whose condition deteriorates after a myocardial infarction, with increasing dyspnoea, sometimes chest pain, often cardiogenic shock, and a harsh pansystolic murmur on auscultation loudest at the left sternal edge, together with a parasternal thrill in a proportion of cases. Not all patients present with a typical history. In one series of 109 consecutive cases a substantial proportion (22%) presented with signs of interventricular rupture having had a silent myocardial infarction.9
The most important differential diagnosis is mitral valve regurgitation resulting from papillary muscle rupture. The murmur of acute papillary muscle rupture is loudest at the apex, often has a diastolic component, and rarely has a palpable thrill.1 Unfortunately the progressive loss of clinical skills may put such differentiation beyond many of us.
The ECG will show an anterior or inferior infarction. Although the site of the rupture may be predicted from the site of the infarct, on some occasions the rupture is apical or central even when the infarct is inferior.6
Chest x ray is likely to show a combination of cardiomegaly, increased pulmonary interstitial fluid, and pleural effusions, although these findings are certainly not specific for ventricular sepal rupture and are present in a substantial proportion of patients with other complications after myocardial infarction.
Right heart catheterisation was the mainstay of diagnosis until the late 1980s and is still diagnostic if there is inadequate echocardiographic expertise available.10 A Swann Gantz catheter can be inserted by the bedside. Sampling for oxygen saturation demonstrates a step up between the right atrium and the pulmonary artery whenever there is a left to right shunt at ventricular level. The size of the shunt is indicated by the ratio of flows between the pulmonary and systemic circulations. The excess pulmonary blood flow is equal to the amount of blood coming through the defect. Since the shunted blood has just come through the pulmonary circulation it is oxygenated to the same level as aortic blood.
The pulmonary to systemic flow ratio is estimated from the oxygen saturations in blood samples taken simultaneously from the right atrium (mixed venous saturation), pulmonary artery, and any artery which represents an aortic sample. The calculation of the shunt is very simple and is represented in the following formula:
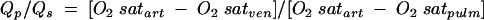 |
where Qp is pulmonary blood flow and Qs is systemic blood flow.
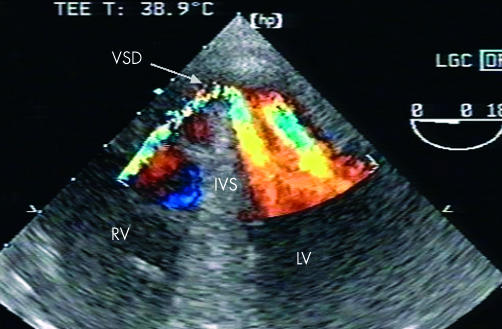
The principle means of diagnosis is now two dimensional echocardiographic imaging with colour flow Doppler (fig 2). As well as distinguishing septal rupture from papillary muscle rupture, echocardiography characterises the rupture with respect to size and site, and degree of right and left ventricular impairment, and gives an estimate of the shunt size.11
Figure 2.
Transoesophageal two dimensional echocardiography of ventricular septal rupture after anterior ventricular septal rupture.
Although left ventriculography demonstrates septal rupture, the contrast load is likely to be detrimental to both ventricular function and to renal function, and so is best avoided.
WHO IS TOO ILL FOR SURGERY?
There are some circumstances when attempts at repair of ventricular septal rupture is a futile if heroic endeavour. Nobody would wish to spend one of their last few hours on earth stuck in the back of an ambulance. What guidelines should we develop to avoid unnecessary referral?
As a first step, what factors have been shown to have a relationship with poor outcome? Norell and colleagues12 in a univariate analysis found hypotension, oliguria, elevated creatinine, and cardiogenic shock to be associated with non-survivors. Moore and colleagues showed that survival was associated with better right ventricular function and with anterior as opposed to inferior myocardial infarction.13 A multivariate analysis of pre-existing clinical variables demonstrated increasing age, anterior infarction, and female sex as predictors of ventricular septal rupture.7
A consistent finding is that rupture following inferior infarction carries a considerably higher operative mortality than following anterior infarction. For example, in a relatively early series of 31 patients from Manchester,14 there were four out of 13 (31%) survivors with inferior infarction compared with 12 out of 18 (67%) with anterior infarction. The authors went on to demonstrate that the degree of right ventricular involvement in the infarction had an even greater association with poor outcome, whereby 12 out of 15 (80%) patients with good right ventricular free wall contraction survived compared with only four out of 17 (24%) with poor right ventricular free wall contraction.
In a report of 109 consecutive patients from Nieuwegein9 the overall mortality at 30 days was 27.5% (better than the UK national outcome data). In a multivariate logistic regression analysis, the investigators were able to show a significant survival advantage for those patients without preoperative shock, and those patients with a right atrial oxygen saturation of more than 60%.
Pathology of ventricular septal rupture: key points.
Ventricular septal ruptures can be classified as either simple or complex
Although associated with single vessel disease, multiple vessel disease is not uncommon necessitating coronary angiography
Inferior septal rupture is associated with right ventricular infarction
In a series of 150 operated patients from Southampton,15 logistic regression analysis showed that the only preoperative factors that were associated with improved survival were better preoperative New York Heart Association (NYHA) functional class, and anterior rather than inferior myocardial infarction. Age was not a significant factor, and the Southampton group recommend that age by itself should not be a bar to surgical intervention.
We are left with the sad truth that the only patient who is too ill for surgery is a patient who has been sent too late and in whom multisystem failure has already developed. Our systems of care should be geared to avoid organisational failure. Early diagnosis and immediate referral to the nearest surgical centre should be the first thought as soon as signs of ventricular septal rupture appear.
WHAT IS THE PLACE FOR NON-SURGICAL TREATMENT?
There existed a vogue some time ago for managing patients with ventricular septal rupture non-surgically in the first instant. After a period of perhaps six weeks, often with intra-aortic balloon counterpulsation, the patient then underwent surgery. The advantages for the surgeon of this strategy were several. The procedure itself was a good deal more straightforward than the same operation in the acute phase, because the remaining septum was no longer mushy necrotic muscle, but had begun to fibrose and was thus more receptive to sutures. Furthermore the least well patients often failed to survive the weeks of non-surgical management, so that only the least ill survived to undergo surgery. This process of “unnatural selection” was first expressed in the literature nearly 40 years ago by Honey and his colleagues from the London Chest Hospital.16
Some time later, from the same institution, Norell and colleagues reported a series of 55 consecutive patients who had presented with ventricular septal rupture and which they divided into two temporal groups.12 Of the first group of 26 patients up to 1982, who were managed with delayed surgery, two had only minimal haemodynamic abnormality and did not go forward to operation. The remaining 24 patients included six who died without surgery and a further three who died after surgery. Thus the operative mortality was only 17%, and the overall mortality for the whole group was 38%. In the second era the philosophy was for early surgery. Even then there was an average delay of 10 days between clinical recognition and surgical repair. Of the 29 patients in this group, two were too well to require intervention, but a further five patients were considered to be too unwell for surgery and all of these died. Of the remaining 22 patients who underwent repair seven died, so that the operative mortality was 32%, and the overall mortality was 44%.
The literature in the late 1970s and early ’80s established that there was no place for procrastination. It was established that the great majority of patients died while waiting for the surgical procedure and the mood shifted to early surgical correction. That not withstanding, there are some patients who are so well that surgery is not necessary in the acute phase, and some for whom surgery is not necessary at all. Such a patient may be characterised as having no need for haemodynamic support, being in a completely stable condition and with no deterioration over time.
PRE-SURGICAL MANAGEMENT
Once a decision to operate has been made, then the sooner the patient is on the operating table the better. I know of no evidence that delaying surgery to allow for a period of optimisation provides any survival benefit. While preparations are being made and when necessary, transportation to a surgical centre is being undertaken, oxygenation is maintained, and measures to support the circulation are provided. A substantial proportion of these patients will require inotropic support at this early stage.
There is often some delay involved in setting up the operating theatre, and this time should be used to good effect. The patient should have an intra-aortic balloon pump inserted, as this decreases left ventricular afterload and therefore reduces the volume of the shunt. Although no study has demonstrated a survival advantage, the short time taken to insert an intra-aortic balloon and the mechanical advantage to the cardiac haemodynamics, together with increased coronary blood flow, are likely to be of benefit to all patients and stabilise the patient for induction of anaesthesia.
In addition, if possible—and it should always be possible—the patient should undergo coronary angiography. The pathophysiology of interventricular rupture, which depends on full thickness infarction, which in turn results when there is a relative lack of collateralisation, is such that many will have only a single vessel occlusion without other vessel involvement with significant stenoses. However, it is likely that long term outcome is improved when significantly stenosed vessels are grafted at the time of rupture repair. Obtain an angiogram whenever you can then.
OPERATIVE SURGERY
Denton Cooley and colleagues described the first surgical repair of a ventricular septal rupture in 1957.17 The patient had survived acute septal rupture but developed worsening right and left sided cardiac failure and came to surgery some three months after the original event. The ventricular septum was approached through the anterior wall of the right ventricle and the repair was accomplished with a pad of polyvinyl sponge sutured to the septum with interrupted silk sutures. The patient suffered the eventful postoperative course that is all too familiar more than 50 years later. He required a tracheostomy, developed a degree of renal failure, and required sternal resuture for a dehiscence. Despite all this he survived for six weeks after the operation, only to die from cardiac failure.
There followed a number of reports with progressive improvement in technique and outcome. By 1977 Daggett and colleagues at Massachusetts General Hospital had accumulated an experience amounting to 36 patients undergoing surgical intervention.18 At the start of this experience the approach to the septum was through the anterior wall of the left ventricle as described by Cooley,17 but the Boston group soon changed to making a ventriculotomy through the infarct. This helped to minimise myocardial dysfunction after surgical intervention. If after exposure the rupture was found to be small and apical, then the apex of the heart incorporating the rupture was amputated and the defect repaired with interrupted horizontal mattress sutures strengthened with Teflon strips. This in turn resulted in distortion of ventricular geometry and also placed considerable tension on friable myocardial tissue. To overcome these problems Teflon or Dacron patches were employed. They also described in their findings that the approach to rupture complicating an inferior myocardial infarction was best through the infarct in the inferior wall of the right ventricle. By the end of the series there was routine use of intra-aortic balloon counterpulsation to provide some haemodynamic stability during assessment, and concomitant coronary bypass graft and, when necessary, mitral valve replacement.
Komeda and colleagues19 have described a surgical modification whereby the geometry of the ventricular cavities is better preserved by the application of a pericardial patch to the ruptured septum without infarctectomy and without interfering with the right ventricle at all. These and other authors advocate suturing the patch to the non-infarcted portion of the septum.20
Although there are reports of various methods of minimising ventricular impairment21 the standard form of myocardial protection for this operation is moderate hypothermia on cardiopulmonary bypass and cold blood cardioplegia. It is possible to repair septal ruptures complicating anterior infarction by employing normothermic bypass with the heart beating and perfused, providing there is no aortic regurgitation, thus avoiding any myocardial ischaemia.20
OUTCOME OF SURGERY
The hospital survival after ventricular septal rupture repair in the UK for each year between 1988 and 1999 varied between 31–47%. The four highest annual mortalities have occurred in the last six years for which data is available. In the GUSTO-I study only 34 patients underwent surgical repair, with a 30-day mortality of 47%. In the SHOCK (should we emergently revascularise occluded coronaries for cardiogeuic shock?) trial the hospital mortality for surgical repair of ventricular septal rupture (all of whom were in cardiogenic shock) was 81%.22 These high mortalities have to be set against the mortality without surgery. In GUSTO-I the 30 day mortality for those patients who did not undergo surgery was 94%. The largest published series of surgically treated ventricular septal rupture in the UK is from Southampton.15 Their hospital mortality of 31% for acute rupture closure is similar to that achieved nationally.
We have no means of knowing what the longer term survival is for the UK wide patients. In the Southampton series five and 10 year survivals were 60% and 31%, respectively. A proportion of patients who survive surgical repair end up with a residual defect. Parry and colleagues6 reported an incidence of 17% in operative survivors. Another series incorporating careful echocardiographic follow up had a higher incidence of 43%.23 Both of these series found that 83% of survivors were in NYHA functional classes I and II.
Management of acute ventricular septal rupture: key points.
- Ventricular septal rupture (VSR) in the thrombolysis era
- thrombolysis has reduced the incidence of VSR 10–20 fold
- with thrombolysis VSR presents earlier after myocardial infarction and is more often complex than simple
- surgical mortality for surgical repair is higher in the thrombolysis era
- Echocardiography provides the following information:
- differentiation from papillary muscle rupture
- site and size of interventricular rupture
- eight and left ventricular function
- size of shunt
- Surgical principles of VSR closure:
- hypothermic cardiopulmonary bypass with myocardial protection
- trans-infarction approach to the VSR
- trimming of infarcted muscle around the VSR
- closure of the VSR with a patch to avoid tension
- closure of the ventricle without tension with buttressed sutures
FUTURE TREATMENT OPTIONS
It must surely only be a matter of time before there is a device that permits routine transcatheter closure of ventricular septal rupture in the acute setting. Up to the present time there have only been single case reports and small series. Lee and colleagues reported successful closure, using an Amplatzer septal occluder, of a residual defect following surgical patch closure.24 Others have suggested that device closure may provide temporary haemodynamic relief and therefore allow surgical closure after the infarcted myocardium around the rupture has had time to fibrose.25 With the current devices closure is most difficult while the margins of the rupture are soft.26
Another adjunct for those patients who are severely haemodynamically compromised are ventricular assist devices. As the technology of devices advances they should become increasingly available and increasingly adaptable; one can imagine the situation where a temporary device will maintain a patient’s haemodynamics while the edges of the septal rupture fibrose sufficiently to support a percutaneously implantable occlusion device.
CONCLUSION
For each individual surgeon closure of ventricular septal rupture following myocardial infarction is an infrequent operation with a very high operative risk. Nonetheless, early surgical intervention offers the only realistic chance of survival and this opportunity should not be denied patients. Thrombolysis for myocardial infarction has altered the pattern of the condition, reducing the incidence but providing the surgeon with an even greater challenge. Immediate intra-aortic balloon counterpulsation provides some haemodynamic optimisation while preparations are made for surgery. The good long term outcome for survivors makes the high early mortality worthwhile.
REFERENCES
- 1.Sanders RJ, Kern WH, Blount SG. Perforation of the interventricular septum complicating myocardial infarction. Am Heart J 1956;51:736–48. ▸ Provides outcome data before surgical intervention was possible. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BS, Edwards WD, Edwards JE. Ventricular septal rupture complicating acute myocardial infarction: identification of simple and complex types in 53 autopsied hearts. Am J Cardiol 1984;54:1201–4. [DOI] [PubMed] [Google Scholar]
- 3.Mann JM, Roberts WC. Acquired ventricular septal defect during acute myocardial infarction: analysis of 38 unoperated necropsy patients and comparison with 50 unoperated necropsy patients without rupture. Am J Cardiol 1988;62:8–19. ▸ These two papers provide a thorough analysis of the morphology of postinfarction ventricular septal rupture. [DOI] [PubMed] [Google Scholar]
- 4.Skehan JD, Carey C, Norrell MS, et al. Patterns of coronary artery disease in post-infarction ventricular septal rupture. Br Heart J 1989;62:268–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pretre R, Rickli H, Qing Y, et al. Frequency of collateral blood flow in the infarct-related coronary artery in rupture of the ventricular septum after acute myocardial infarction. Am J Cardiol 2000;85:497–9. ▸ These two papers provide analysis of the coronary angiographic findings in patients with postinfarction ventricular septal rupture. [DOI] [PubMed] [Google Scholar]
- 6.Parry G, Goudevenos J, Adams PC, et al. Septal rupture after myocardial infarction: is very early surgery really worthwhile? Eur Heart J 1992;13:373–82. [DOI] [PubMed] [Google Scholar]
- 7.Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000;100:27. [DOI] [PubMed] [Google Scholar]
- 8.Rhydwen GR, Charman S, Schofield PM. Influence of thrombolytic therapy on the patterns of ventricular septal rupture after acute myocardial infarction. Postgrad Med J 2002;78:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox FF, Morshuis WJ, Plokker T, et al. Early mortality after surgical repair of postinfarction ventricular septal rupture: importance of rupture location. Ann Thorac Surg 1996;61:1752–8. [DOI] [PubMed] [Google Scholar]
- 10.Heitmiller R, Jacobs ML, Daggett WM. Surgical management of postinfarction ventricular septal rupture. Ann Thorac Surg 1986;41:683–91. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinides S, Geibel A, Kasper W, et al. Noninvasive estimation of right ventricular systolic pressure in postinfarction septal rupture: an assessment of two Doppler echocardiographic methods. Crit Care Med 1997;25:1167–74. [DOI] [PubMed] [Google Scholar]
- 12.Norell MS, Gershlick AH, Pillai R, et al. Ventricular septal rupture complicating myocardial infarction: is earlier surgery justified? Eur Heart J 1987;8:1281–6. ▸ Discusses the merits of early intervention versus the earlier philosophy of prolonged support followed by delayed surgery. [DOI] [PubMed] [Google Scholar]
- 13.Moore CA, Nygaard TW, Kaiser DL, et al. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation 1986;74:45–55. [DOI] [PubMed] [Google Scholar]
- 14.Fananpazir L, Bray CL, Dark JF, et al. Right ventricular dysfunction and surgical outcome in postinfarction ventricular septal defect. Eur Heart J 1983;4:155–67. [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple-Hay MJR, Monro JL, Livesey SA, et al. Postinfarction ventricular septal rupture: the Wessex experience. Seminars in Thoracic and Cardiovascular Surgery 1998;10:111–16. ▸ The largest reported single centre experience of the condition and its surgical treatment. [DOI] [PubMed] [Google Scholar]
- 16.Honey M, Belcher JR, Hasan M, et al. Successful early repair of acquired ventricular septal defect after myocardial infarction. Br Heart J 1967;29:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooley DA, Belmonte BA, Zeis LB, et al. Surgical repair of ruptured interventricular septum following acute myocardial infarction. Surgery 1957;41:930–7. ▸ The first report of surgical repair for postinfarction ventricular septal rupture. [PubMed] [Google Scholar]
- 18.Daggett WM, Guyton RA, Mundth ED, et al. Surgery for post-myocardial infarct ventricular septal defect. Ann Surg 1977;186:260–71. ▸ This paper sets out the principles of surgical repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komeda M, Fremes SE, David TE. Surgical repair of postinfarction ventricular septal defect. Circulation 1990;82 (suppl IV):243–7. ▸ Sets out surgical modifications which aim to reduce ventricular cavity distortion. [PubMed] [Google Scholar]
- 20.Pretre R, Stadler N, Ye Q, et al. Surgical repair of postinfarction structural failure of the posterobasal part of the heart. Ann Thorac Surg 1999;68:2152–7. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Kadoba K, Taniguchi K, et al. Repair of postinfarction ventricular septal defect on a beating heart. Ann Thorac Surg 1996;61:1817–19. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Webb JG, Hilis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK trial registry. J Am Coll Cardiol 2000;36:1110–16. ▸ Describes the changes in the nature of postinfarction ventricular septal defect in the thrombolysis era. [DOI] [PubMed] [Google Scholar]
- 23.Deja MA, Szostek J, Widenka K, et al. Post infarction ventricular septal defect- canwe do better? Eur J Cardiothoracic Surg 2000;18:194–201. ▸ Provides long term follow up data of patients following surgical intervention [DOI] [PubMed] [Google Scholar]
- 24.Lee EM, Roberts DH, Walsh KP. Transcatheter closure of a residual postmyocardial infarction ventricular septal defect with the Amplatzer septal occluder. Heart 1998;80:522–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benton JP, Barker KS. Transcatheter closure of ventricular septal defect: a nonsurgical approach to the care of the patient with acute ventricular septal rupture. Heart and Lung 1992;21:356–64. [PubMed] [Google Scholar]
- 26.Szkutnik M, Bialkowski J, Kusa J, et al. Postinfarction ventricular septal defect closure with Amplatzer occluders. Eur J Cardiothoracic Surg 2003;23:323–7. [DOI] [PubMed] [Google Scholar]