The foramen ovale, while vital to our formative development, assumes mischievous potential if it persists post-utero. Similar to other vestigial structures, presence of a patent foramen ovale (PFO) appears to carry no physiologic or survival benefit for normal individuals. In states of abnormal right sided cardiac or pulmonary vascular capacitance or resistance, PFO has been implicated in worsening hypoxaemia caused by right to left intracardiac passage of deoxygenated blood (see box). More recently, a causative role of PFO in much more commonly occurring syndromes, including embolic ischaemic stroke, migraine with aura, and cerebral and cutaneous decompression disease, has been suggested. Highlighting cryptogenic embolic stroke associated with PFO, we will systematically review the evidence for these relations, discuss therapeutic potentials, and propose guidelines for therapeutic decisions as we await the completion of randomised controlled interventional trials.
CRYPTOGENIC STROKE AND PFO
Incidence
Stroke databases suggest that despite intensive evaluation, approximately 40% of all patients suffering ischaemic strokes (80% of all stroke victims) remain without clearly identifiable precipitant or cause (2002 heart and stroke statistical update, American Heart Association). In 1989, Webster and Lechat separately reported small case–control series with increased prevalence of PFO in patients with cryptogenic stroke (CS).1,2 To date, these descriptive series have been followed only by additional case–control studies, without prospective collection of primary occurrence of CS + PFO in a well defined population. While these case–control series have significant limitations, meta-analysis by Overell and colleagues suggested a strong correlation between PFO and primary occurrence of CS.3 In this analysis, prevalence of CS + PFO in persons of all ages was three times greater (95% confidence interval (CI) 2.0 to 4.3) than in non-stroke controls; this relation was even more compelling in persons with CS aged < 55 years of age, where PFO prevalence was five times greater (95% CI 3.2 to 8.3) than in healthy controls.
PFO and patient related risks
Attempts to risk stratify primary CS occurrence by anatomic features of PFO have had even greater limitation, due to smaller numbers of case–control studies including fewer patients. Most evaluation has focused on hypermobility (atrial septal aneurysm) of the septum primum. Most rigorous definition of atrial septal aneurysm requires ⩾ 10 mm tissue sway in either direction from the septal plane (or ⩾ 15 mm total sway) with a base of moving tissue that extends ⩾ 10 mm. Anatomically, atrial septal aneurysm typically, if not invariably, is associated with either septal fenestrations or PFO. Case series meta-analysis points to a strong association between the presence of atrial septal aneurysm + PFO and primary occurrence of CS, with all ages, and those aged < 55 years with atrial septal aneurysm + PFO having five times greater (95% CI 2.4 to 10.4) and 16 times greater (95% CI 3.0 to 86.1) associative risk.3 This association was confirmed in a recent large case–control analysis.4 “High risk” PFO features of (1) atrial septal aneurysm, and (2) spontaneous intracardiac passage of bubble contrast without provocative manoeuvres have been applied to existing stroke databases and have been shown to carry several fold higher risk of stroke/transient ischaemic attack (TIA) recurrence when compared to CS + PFO patients without these features.5
Causative mechanisms of association between CS + PFO remain speculative. Anecdotes of thrombi viewed passing from systemic venous circulation through PFO to the systemic arterial circulation have led to the suggestion of embolisation of systemic venous thrombus via PFO as a primary mechanism of disease. Such thrombi have generally appeared quite large, and would typically account for large vessel cerebro-occlusive disease and symptomatology. However, CS patients in primary occurrence trials and CS + PFO patients in secondary prevention trials have tended to present with smaller territory, or milder, neurologic events.6–9 This has led to speculation that smaller embolic material forms in situ within certain PFO. The two dimensional “tunnel”, or three dimensional “wind sock” nature of certain PFO suggests a plausibility of a pro-coagulant milieu with stagnation as well as potential for embolisation in people harbouring such PFO. However, direct evidence implicating such is lacking to date.
TREATMENT: MEDICAL, SURGICAL AND PERCUTANEOUS
Currently there are no consensus guidelines on treating patients with CS + PFO using available treatments including medical therapies (antiplatelet, anticoagulant), surgical PFO closure, and percutaneous closure.
Medical
Case series have suggested a significant risk of stroke/TIA recurrence (4–20%/year depending upon relative “high risk” features) for patients with CS + PFO using medical treatment.5,10–13 Use of warfarin, despite increased risk of associated haemorrhage, has been clinically favoured over aspirin, though confirmatory data are lacking. A prospective registry of CS patients aged 15–55 years treated with aspirin analysed recurrence over four years of stroke/TIA by presence or absence of PFO with/without atrial septal aneurysm.14 The authors suggested that there was notable attributable risk for future stroke/TIA of PFO with atrial septal aneurysm, with four year stroke/TIA recurrence of 15.2%/19.2% in patients with PFO + atrial septal aneurysm (hazard ratio 4.17). Of note, in this trial patients with PFO were significantly younger and had decreased additional stroke risks of systemic hypertension, diabetes, and higher body mass index.
Typical conditions when PFO may cause or worsen hypoxaemia.
Valvar pulmonary stenosis
Ebstein’s disease of right ventricle and tricuspid valve
Right ventricular infarction
Orthodeoxia-platypnoea syndrome
Chronic lung disease (obstructive or restrictive)
Pulmonary embolism (acute or chronic)
Pulmonary arteriolar hypertension (primary and secondary)
The WARSS trial randomised patients aged 30–85 years with recent ischaemic stroke to use of either daily aspirin (325 mg) or warfarin (target international normalised ratio (INR) 1.4–2.8), assessing recurrence of ischaemic neurologic events or death.15 No statistical difference in occurrence of the primary end point at two years was noted between groups (16% v 17.6%, respectively). Subgroup analysis of a much smaller number of patients with PFO and ischaemic stroke (only some with CS) found similar risk of high stroke/TIA recurrence regardless of aspirin or warfarin treatment.16 In this trial as well, patients with PFO had decreased additional stroke risks of systemic hypertension, diabetes, and sedentary life style.
Surgical
Surgical PFO closure appears a safe and effective means of eradicating PFO as a potential risk for stroke/TIA occurrence/recurrence, with limited supportive data. Ischaemic neurologic event recurrence rates of 4–17%/year, seen in surgical series of PFO closure for patients with index stroke, are likely to be distorted by limited and selected enrolment, as well as the single institutional nature of these case series.17–19
Percutaneous
Percutaneous PFO closure, first performed in 1989, is now possible with any of 5–7 different devices depending upon availability during various phases of investigational development (fig 1).20 In the USA, PFO may be closed percutaneously under Food and Drug Administration mandated humanitarian device exemption (HDE) guidelines in limited specific circumstances, both with CardioSEAL (HDE granted 2000) and the Amplatzer PFO Occluder (HDE granted 2002). PFO closure with all other devices remains limited to investigational trials: currently no device has FDA pre-market approval for this indication.
Figure 1.

Available percutaneous PFO occlusion devices.
Assessing efficacy of percutaneous PFO closure for each device has been troublesome given the case series nature of existing studies, lack of randomised controlled trials, as well as a lack of defined and clinically meaningful end points for comparison. The oldest, continuous database examining percutaneous PFO closure safety and efficacy outcomes has suggested that, for progressive generations of double umbrella devices culminating in CardioSEAL and its modifications, annual recurrent combined stroke/TIA event rate following percutaneous closure has been consistently less than 4%.6,7,9,20,21 Complete PFO closure at follow up can be expected in 90–95% of patients utilising CardioSEAL (and its current STARFlex self adjusting modification) or the Amplatzer PFO Occluder.6–9,21 For all devices, choice, duration, and benefit and risks of peri-implant antiplatelet or anticoagulant strategies remain unclear and undefined, with current clinical practice mirroring post-coronary stent implantation pharmacologics (1–6 months clopidogrel, 75 mg daily, plus six months aspirin, 325 mg daily). Device related adversity has been documented with every occluder, with most notable occurrences including device embolisation, tissue erosion, pericardial inflammation, device related thrombosis, infection, device fracture or dislodgement, and stroke. Important other complications have included device related arrhythmia, transfusion requirement, and precipitation of migraine and chest pain. While exact incidences of such adversity are difficult to determine from limited available published series, clinically meaningful adversity most recently appears to occur in less than 1–3% of all patients undergoing percutaneous PFO closure.6–9,21 The incidence and clinical relevance of device related thrombosis and early and late post-implant atrial arrhythmias has yet to be determined and compared to other therapeutic modalities.
SYSTEMATIC REVIEW AND POOLED ANALYSIS
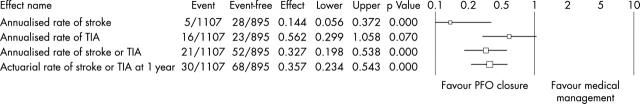
In a recent systematic review we chose to estimate the relative benefit of percutaneous PFO closure compared to medical therapy (tables 1 and 2, fig 2).22 Adjusting for attributable risk due to the higher prevalence of diabetes mellitus and smoking in medically treated patients with PFO, percutaneous PFO closure was shown to have a protective effect on stroke or TIA recurrence compared to medical treatment (annualised incidence 1.9% v 5.4%, relative risk 0.346, 95% CI 0.209 to 0.573; p < 0.0001). At one year follow up, PFO closure was associated with a relative risk of 0.385 (95% CI 0.252 to 0.589) and absolute risk difference of 4.4%. Otherwise expressed, after the first year of follow up, for every 23 patients who had their PFO closed percutaneously, one stroke or TIA was prevented compared to use of medical treatment.
Table 1.
Systematic review: patient characteristics—transcatheter closure versus medical management of PFO
| Characteristic | Transcatheter closure (n = 1107) | Medical treatment (n = 895) | p Value |
| Baseline characteristics | |||
| Mean (SD) age (years) | 45.8 (13.4) | 47.6 (12.8) | 0.0024 |
| Male sex (%) | 48.3 | 57.0 | 0.0004 |
| Atrial septal aneurysm (%) | 22.4 | 22.1 | 0.8771 |
| Thromboembolic events at presentation | |||
| Stroke (%) | 67.0 | 89.3 | <0.0001 |
| TIA (%) | 53.1 | 24.5 | <0.0001 |
| Other systemic embolic event (%) | 3.9 | – | − |
| Multiple embolic events (%) | 45.2 | 12.2 | <0.0001 |
| Risk factors | |||
| Hypertension (%) | 28.3 | 26.3 | 0.3465 |
| Diabetes (%) | 4.5 | 25.0 | <0.0001 |
| Smoking history (%) | 32.6 | 49.5 | <0.0001 |
| Hypercholesterolaemia (%) | 15.5 | 18.4 | 0.2372 |
| Means (SD) follow up (months) | 18.2 (15.1) | 30.2 (16.2) | <0.0001 |
TIA, transient ischaemic attack.
Table 3.
Systematic review: recurrent neurologic thromboembolic events with transcatheter PFO closure compared to medical treatment
| Recurrent events | PFO closure | Medical treatment | RD | NNT | RR (95% CI) | P Value |
| Crude annualised rate | ||||||
| Stroke | 0.42% | 3.09% | 2.67% | 37 | 0.144 (0.056 to 0.372) | <0.0001 |
| TIA | 1.43% | 2.58% | 1.15% | 87 | 0.562 (0.299 to 1.058) | 0.0703 |
| Stroke or TIA | 1.86% | 5.80% | 3.94% | 25 | 0.327 (0.198 to 0.538) | <0.0001 |
| Crude rate of stroke/TIA at 1 year | 2.71% | 7.56% | 4.85% | 21 | 0.357 (0.234 to 0.543) | <0.0001 |
| Adjusted annual rate of stroke/TIA* | 1.86% | 5.42% | 3.56% | 28 | 0.346 (0.209 to 0.573) | <0.0001 |
| Adjusted rate stroke/TIA at 1 year* | 2.71% | 7.07% | 4.36% | 23 | 0.385 (0.252 to 0.589) | <0.0001 |
*Adjusted by indirect standardisation for imbalances in diabetes and smoking.
NNT, number needed to treat; RD, risk difference; RR, relative risk; TIA, transient ischaemic attack.
Figure 2.
Systematic review: stroke and TIA following transcatheter PFO closure compared to medically treated patients
RANDOMISED CONTROLLED TRIALS: THE TIME HAS COME
A number of attempts at randomised controlled trials (RCTs), including the percutaneous closure (PC) trial and the Paradoxical Embolism Prevention Study in Ischemic Stroke (PEPSIS) trial, occurred throughout the past decade, but failed largely due to a lack of: (1) neurologist–cardiologist–primary physician teamwork and coordination of goal and effort; (2) modern precise definition of ischaemic neurologic outcome; (3) data to generate realistic hypotheses and sample size requirements; (4) referring physician and investigator motivation to enrol all candidate patients into randomised expert care; (5) industry based sponsorship of a sufficiently sized trial to adequately address power concerns; and (6) a “tipping point” mentality that CS + PFO is a true and highly morbid disease, requiring study and relief. This milieu has radically shifted, setting the stage for current RCTs of percutaneous PFO closure and other treatments for persons affected by CS. The largest such trial, CLOSURE-1, is a > 1600 patient trial (neurologist principal investigatorships), testing superiority of CardioSEAL-STARFlex versus best medical treatment in persons with imaging confirmed index stroke, and evaluating similar hard neurologic end points as primary outcome. A second trial, RESPECT, is a 300 patient trial (primarily cardiologist principal investigatorship) evaluating equivalency of Amplatzer PFO Occluder PFO occlusion with clinician-determined “best medical therapy” in persons with “clinically symptomatic” index stroke, evaluating similar symptomatology as primary outcome. Both trials are projected to complete enrolment within 12–18 months.
In light of the above trials, we strongly advocate the following:
Rapid investigation of patients with CS, including prompt assessment for and anatomic definition of PFO. When transthoracic echocardiography with Mueller manoeuvre (sniff forcing right atrial pressure to bow the atrial septum leftward) does not sufficiently define the presence of shunting or PFO anatomy, transoesophageal echocardiography is employed to provide greatest anatomic detail of intracardiac shunting, though likely with lesser sensitivity in diagnosis. While individual institutions may offer various “first line” testing for presence of intravascular shunting, including contrast echocardiography23 and transcranial Doppler sonography,24 we recommend standard transoesophageal echocardiography25 for all patients with CS and suspected PFO.
Recognition of “high risk” patient (age < 55–60 years, (+) known circulating pro-coagulant) and PFO anatomic risk (“spontaneous echocardiographic shunting”, hypermobile septum primum, tunnel-like PFO) features potentially raising risk of recurrent neurologic ischaemic events.
Patient education regarding association and particular risk features of CS and PFO, and removal or reduction of all potential procoagulant risks (trauma, obesity, inactivity, oral contraception, cigarette use, etc).
In patients with new onset CS, enrolment of all eligible candidates into RCTs evaluating safety and efficacy of treatment arms. While we strongly favour data acquisition to answer questions regarding superiority of therapeutic choice in trials based upon most rigorous data analysis, both CLOSURE-1 and RESPECT remain reasonable enrolment options.
- Choice of primary PFO closure in patients:
- – with recurrent CS, not eligible for trial enrolment, despite compliant medical treatment or with inability to comply safely with medical treatment; while we favour percutaneous PFO closure, surgical closure is likely a reasonable, though rarely chosen, alternative, with fewer supportive data
- – with known circulating hypercoagulable states with recognised increased risks of thrombosis/embolism despite recommended warfarin treatment (lupus anticoagulant/antiphospholipid antibody syndrome)
- – with persistent procoagulant risk despite best medical treatment.
RIGHT SIDED CARDIAC DISEASE AND PFO
Persons affected by either compliance or capacitance abnormalities of right sided filling may have sufficient elevation of right atrial pressure so as to promote right-to-left shunting at the level of the foramen. Understanding the aetiology of the underlying muscle abnormality and targeting treatment to that, as is possible, is the mainstay of therapy for these patients. On occasion, control of cyanosis by means of PFO closure may be a reasonable acute and intermediate term palliation, with recognition that longer term worsening of muscle function caused by increased right sided volume may occur. In the adult patient with such disease, temporary PFO occlusion may offer some degree of mimicry of acute haemodynamic effects of closure, though there are no recognised manoeuvres that predict successful longer term outcomes with PFO closure. We advocate prolonged discussion of unknown intermediate and long term outcomes with such patients, and device implantation for PFO closure when indicated, at centres participating in closure registries.
DECOMPRESSION SICKNESS IN DIVERS AND PFO
Decompression sickness (DCS I—musculocutaneous; DCI II—neurologic) arises from nitrogen and oxygen gas formation in various body tissues at increased ambient pressure. Gas passage from the systemic venous to arterial circulation can occur due to either pulmonary barotrauma or intravascular shunting.
Several studies have suggested increased incidence of PFO in divers with DCS. From initial recognition of association of PFO with DCS in 1986, to current reports of PFO closure for divers affected by DCS, differences in outcomes of case series highlight the differences in techniques used to: (1) select and enrol studied patients and controls; (2) clinically diagnose DCS; (3) diagnose PFO; and (4) image “neurologic events”.26–32 In light of these limitations, general reviews suggests that: (1) regardless of presence of PFO, increasing numbers of dives may be associated with increasing incidence of DCS or asymptomatic neurologic events (ANEs); (2) occurrence of ANEs is likely to be common (25–50% of screened patients) in high volume divers; (3) multiple recurrences of DCS II, ANEs, and migraine headaches with aura may cluster in persons with “large shunt volume” PFO (atrial septal aneurysm or spontaneous shunting).
Standardisation of diagnosis and outcome remains a major obstacle for assessment of treatment for DCS prevention. Typically, more than 50% of high volume divers with ANEs remain asymptomatic, and there are no recognised risks predictive of future symptomatic events. In this context, nonetheless, previously symptomatic or high volume divers with ANEs or with “high risk” anatomic PFO features, who wish to continue diving may warrant closure in centres maintaining closure registries or participation in trials. Intervention for low volume sports divers with PFO without ANEs or symptoms of DCS, regardless of anatomic concerns, remains unfounded at the present.
Abbreviations.
ANE: asymptomatic neurologic event
CS: cryptogenic stroke
DCS: decompression sickness
HDE: humanitarian device exemption
M+A: migraine syndrome with aura
PEPSIS: Paradoxical Embolism Prevention Study in Ischemic Stroke
PFO: patent foramen oval
RCT: randomised controlled trial
RESPECT: Randomized Evaluation of recurrent Stroke comparing PFO closure to Established Current standard of care Treatment
TIA: transient ischaemic attack
WARSS: Warfarin-Aspirin Recurrent Stroke Study
Of interest, DCS, regardless of presence of PFO, has been noted in compressed air tunnel workers, high altitude aviators, and astronauts. Treatments offering potential for limiting the occurrence of DCS may carry particular import for such persons whose employment in high risk situations may place themselves and others at particular risk.
MIGRAINE SYNDROME AND PFO
The recognition of an association between migraine syndrome with aura (M+A) and PFO appears to have come “full circle” over the past two decades. Initial concerns from cardiologists focused on post-percutaneous PFO closure precipitation of migrainous events that mimicked original neurologic presentation. Effects from general anaesthesia, raised ambient catecholamines, and embolisation of metal or procoagulant microaggregates were all theorised as being related to occurrence.
Indications for closure of PFO: key points.
A 3 to 5-fold higher prevalence of PFO is noted in patients with cryptogenic stroke
Proposed “high risk” PFO features include: atrial septal aneurysm, spontaneous right-to-left shunting, and “tunnel-like” appearance
Although warfarin is often preferred over aspirin for secondary prevention of cryptogenic stroke, confirmatory data are lacking
Percutaneous transcatheter PFO device closure may be performed safely with a low incidence of recurrent neurologic events
Clinical trials comparing medical treatment versus transcatheter closure are underway
If ineligible for randomised trials, PFO closure may be considered in patients with recurrent events unable to safely comply with or despite medical treatment, “high risk” PFO feature, and/or “high risk” medical features (for example, hypercoagulable state, age < 55–60 years)
High volume divers with decompression sickness or asymptomatic neurologic events who wish to continue diving may warrant PFO closure in appropriate centres
There is insufficient evidence to recommend PFO closure for the indication of migraine with aura
Small epidemiologic studies have suggested a notably increased PFO prevalence in persons suffering M+A.33,34 The relation between this association and the recognition of M+A as a risk factor for ischaemic stroke in the young is unclear, though right to left passage of circulating factors has been postulated in both syndromes.35
Despite case series documenting PFO closure effects in persons with M+A, the competing concerns of both precipitation and reduction of M+A in persons with PFO lead to our recommendation not to pursue PFO closure at the present for persons with M+A without CS.36,37 We support improved basic aetiologic and epidemiologic study of M+A, as well as study of the effects of various antiplatelet and anticoagulant agents as they are employed after percutaneous PFO closure.
CONCLUSIONS
Patency of the foramen ovale, occurring in 20–30% of persons, may be a commonly occurring intracardiac anatomic variation, yet our knowledge of even basic epidemiologic principles leading to association with disease remains lacking. This vestige of embryologic physiology has little if any relation to health and, on increasingly recognised occasion, harbours potential for association with catastrophic disease. Relative risks and benefits of treatments aimed at controlling or eliminating syndromes associated with PFO hinge upon study of the mechanisms of these diseases, the effects of specific planned treatments, and randomised controlled comparison trials to guide individual and population treatment recommendations. Current therapeutic options for control of PFO associated disease, including percutaneous PFO closure for recurrent stroke risk reduction as a model, have low adverse potential. While systematic review suggests a strong favour for percutaneous PFO closure when compared to medical treatments, these data are gleaned from case series. RCTs, including a large “superiority trial”, CLOSURE-1, and a smaller, “equivalency trial”, RESPECT, are active and ongoing, and will likely contribute to answering the necessary scientific and clinical questions to allow for improved patient care. We have been called “to arms” to recognise patients with PFO who are at risk of associated devastating disease, and to forcefully attack such by enrolling such patients, whenever possible, into RCTs testing risk limiting strategies. Until such study is completed, percutaneous PFO closure remains an effective and acceptable treatment for specific patients with high risk features falling outside protocol entry criteria.
REFERENCES
- 1.Webster MW, Chancellor AM, Smith HJ, et al. Patent foramen ovale in young stroke patients. Lancet 1988;ii:11–12. [DOI] [PubMed] [Google Scholar]
- 2.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988;318:1148–52. ▸ Among the first reports showing a higher prevalence of PFO in patients with strokes compared to controls (40% v 10%; p < 0.001). [DOI] [PubMed] [Google Scholar]
- 3.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000;55:1172–9. [DOI] [PubMed] [Google Scholar]
- 4.Agmon Y, Khandheria BK, Meissner I, et al. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation 1999;99:1942–4. ▸ Determined and compared the prevalence of atrial septal aneurysms in the general population (2.2%) and in patients with crypotogenic stroke (7.9%). [DOI] [PubMed] [Google Scholar]
- 5.De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000;31:2407–13. [DOI] [PubMed] [Google Scholar]
- 6.Windecker S, Wahl A, Chatterjee T, et al. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation 2000;101:893–8. [DOI] [PubMed] [Google Scholar]
- 7.Hung J, Landzberg MJ, Jenkins KJ, et al. Closure of patent foramen ovale for paradoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol 2000;35:1311–6. [DOI] [PubMed] [Google Scholar]
- 8.Braun MU, Fassbender D, Schoen SP, et al. Transcatheter closure of patent foramen ovale in patients with cerebral ischemia. J Am Coll Cardiol 2002;39:2019–25. [DOI] [PubMed] [Google Scholar]
- 9.Martin F, Sanchez PL, Doherty E, et al. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation 2002;106:1121–6. [DOI] [PubMed] [Google Scholar]
- 10.Bogousslavsky J, Garazi S, Jeanrenaud X, et al. Stroke recurrence in patients with patent foramen ovale: the Lausanne study. Lausanne stroke with paradoxical embolism study group. Neurology 1996;46:1301–5. [DOI] [PubMed] [Google Scholar]
- 11.Comess KA, DeRook FA, Beach KW, et al. Transesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: prevalence of findings and recurrent stroke risk. J Am Coll Cardiol 1994;23:1598–603. [DOI] [PubMed] [Google Scholar]
- 12.Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French study group on patent foramen ovale and atrial septal aneurysm. Am Heart J 1995;130:1083–8. [DOI] [PubMed] [Google Scholar]
- 13.Hanna JP, Sun JP, Furlan AJ, et al. Patent foramen ovale and brain infarct. Echocardiographic predictors, recurrence, and prevention. Stroke 1994;25:782–6. [DOI] [PubMed] [Google Scholar]
- 14.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740–6. ▸ Reported that patients with both PFO and atrial septal aneurysms with cryptogenic stroke constitute a high risk group for recurrent events. [DOI] [PubMed] [Google Scholar]
- 15.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001;345:1444–51. ▸ Randomised trial comparing warfarin (target INR 1.4–2.8) to aspirin (325 mg/day) for the prevention of recurrent ischaemic stroke in 2206 patients with ischaemic strokes. No differences in the primary outcome or major haemorrhage were found. [DOI] [PubMed] [Google Scholar]
- 16.Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation 2002;105:2625–31. [DOI] [PubMed] [Google Scholar]
- 17.Homma S, Di Tullio MR, Sacco RL, et al. Surgical closure of patent foramen ovale in cryptogenic stroke patients. Stroke 1997;28:2376–81. [DOI] [PubMed] [Google Scholar]
- 18.Ruchat P, Bogousslavsky J, Hurni M, et al. Systematic surgical closure of patent foramen ovale in selected patients with cerebrovascular events due to paradoxical embolism. Early results of a preliminary study. Eur J Cardiothorac Surg 1997;11:824–7. [DOI] [PubMed] [Google Scholar]
- 19.Dearani JA, Ugurlu BS, Danielson GK, et al. Surgical patent foramen ovale closure for prevention of paradoxical embolism-related cerebrovascular ischemic events. Circulation 1999;100:II171–5. [DOI] [PubMed] [Google Scholar]
- 20.Bridges ND, Hellenbrand W, Latson L, et al. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992;86:1902–8. ▸ First case series reporting transcatheter PFO closure in 36 patients with implantation of a double-umbrella device. [DOI] [PubMed] [Google Scholar]
- 21.Butera G, Bini MR, Chessa M, et al. Transcatheter closure of patent foramen ovale in patients with cryptogenic stroke. Ital Heart J 2001;2:115–8. [PubMed] [Google Scholar]
- 22.Khairy P, Landzberg MJ. Systematic review and analysis of transcatheter closure versus medical therapy for patent foramen ovale [abstract]. Circulation 2003;107:e175. [DOI] [PubMed] [Google Scholar]
- 23.Ha JW, Shin MS, Kang S, et al. Enhanced detection of right-to-left shunt through patent foramen ovale by transthoracic contrast echocardiography using harmonic imaging. Am J Cardiol 2001;87:669–71, A11. [DOI] [PubMed] [Google Scholar]
- 24.Zanette EM, Mancini G, De Castro S, et al. Patent foramen ovale and transcranial Doppler. Comparison of different procedures. Stroke 1996;27:2251–5. [DOI] [PubMed] [Google Scholar]
- 25.Schuchlenz HW, Weihs W, Beitzke A, et al. Transesophageal echocardiography for quantifying size of patent foramen ovale in patients with cryptogenic cerebrovascular events. Stroke 2002;33:293–6. [DOI] [PubMed] [Google Scholar]
- 26.Kerut EK, Truax WD, Borreson TE, et al. Detection of right to left shunts in decompression sickness in divers. Am J Cardiol 1997;79:377–8. [DOI] [PubMed] [Google Scholar]
- 27.Kerut EK, Norfleet WT, Plotnick GD, et al. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001;38:613–23. [DOI] [PubMed] [Google Scholar]
- 28.Knauth M, Ries S, Pohimann S, et al. Cohort study of multiple brain lesions in sport divers: role of a patent foramen ovale. BMJ 1997;314:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germonpre P, Dendale P, Unger P, et al. Patent foramen ovale and decompression sickness in sports divers. J Appl Physiol 1998;84:1622–6. [DOI] [PubMed] [Google Scholar]
- 30.Schwerzmann M, Seiler C, Lipp E, et al. Relation between directly detected patent foramen ovale and ischemic brain lesions in sport divers. Ann Intern Med 2001;134:21–4. [DOI] [PubMed] [Google Scholar]
- 31.Wilmshurst PT, Pearson MJ, Walsh KP, et al. Relationship between right-to-left shunts and cutaneous decompression illness. Clinical Science (Lond) 2001;100:539–42. [PubMed] [Google Scholar]
- 32.Walsh KP, Wilmshurst PT, Morrison WL. Transcatheter closure of patent foramen ovale using the Amplatzer septal occluder to prevent recurrence of neurological decompression illness in divers. Heart 1999;81:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sztajzel R, Genoud D, Roth S, et al. Patent foramen ovale, a possible cause of symptomatic migraine: a study of 74 patients with acute ischemic stroke. Cerebrovasc Dis 2002;13:102–6. [DOI] [PubMed] [Google Scholar]
- 34.Anzola GP, Magoni M, Guindani M, et al. Potential source of cerebral embolism in migraine with aura: a transcranial Doppler study. Neurology 1999;52:1622–5. [DOI] [PubMed] [Google Scholar]
- 35.Milhaud D, Bogousslavsky J, Van Melle G, et al. Ischemic stroke and active migraine. Neurology 2001;57:1805–11. [DOI] [PubMed] [Google Scholar]
- 36.Wilmshurst P, Nightingale S. Relationship between migraine and cardiac and pulmonary right-to-left shunts. Clinical Science (Lond) 2001;100:215–20. [PubMed] [Google Scholar]
- 37.Wilmshurst PT, Nightingale S, Walsh KP, et al. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet 2000;356:1648–51. [DOI] [PubMed] [Google Scholar]