Cardiac rhythm during sleep is influenced by the autonomic nervous system and various pathologic states. Most arrhythmias that occur during sleep are detected incidentally on Holter recordings, and are in fact benign. Although such findings may not necessitate further investigation, they may be an important clue to an underlying disorder in need of investigation and treatment. It is important to recognise predisposing factors to sleep related arrhythmia in order to prevent and treat potentially dangerous cardiac rhythms. It is also important to establish a threshold for referral to a cardiologist or electrophysiologist once such arrhythmias are detected.
NORMAL CARDIAC RHYTHM DURING SLEEP
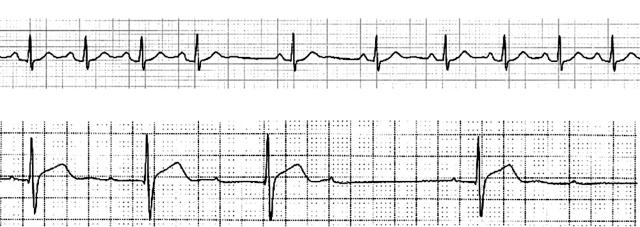
Sleep is a dynamic and complex process. The stages of sleep, conventionally divided into rapid eye movement (REM) and non-rapid eye movement (non-REM), are characterised by unique autonomic influences over cardiac rhythm and haemodynamics. Non-REM sleep is graded 1–4 according to electroencephalogram (EEG) characteristics and diminished arousability. REM sleep occurs at approximately 90 minute intervals, encompasses most dream activity, and is characterised by rapid eye movements and reduced voluntary muscle activity. Studies of individuals free of cardiac disease show that sinus bradycardia, sinus pauses, and type 1 second degree atrioventricular (AV) block are common during sleep (table 1).1 Sinus pauses up to two seconds in duration occur commonly in young people in association with sinus arrhythmia (fig 1).1 This is seen more frequently in athletes,w1 and less frequently in those over 80 years of age.w2 These arrhythmias are, for the most part, both asymptomatic and benign. They are a reflection of changes in autonomic tone that occur during sleep and require no intervention unless accompanied by symptoms.2
Table 1.
| ECG finding | Prevalence |
| Sinus pause (>2 s) | 4–10% |
| Sinus bradycardia (<40 bpm) | 24% |
| First degree AV block | 8–12% |
| Wenckebach second degree AV block | 6–11% |
AV, atrioventricular; bpm, beats per minute
Figure 1.
Typical arrhythmias demonstrated in a healthy male during sleep. Upper panel: pronounced sinus arrhythmia. Lower panel: type 1 second degree atrioventricular block (Wenckebach).
AUTONOMIC INFLUENCE ON CARDIAC RHYTHM DURING SLEEP
Non-REM sleep is characterised by an overall increase in parasympathetic tone, and a decrease in sympathetic tone (table 2). Parasympathetic tone is controlled by circadian rhythms, and sympathetic tone by sleep stage and the changes in posture and activity accompanying sleep.w3 In a study that compared results from individuals after a night of sleep and then a subsequent night deprived of sleep, sympathetic activity, as measured by the systolic pre-ejection period, differed significantly between the two nights.w4 There was no difference in parasympathetic activity which was measured by the degree of sinus arrhythmia. This implies that parasympathetic mediated arrhythmias may persist in the awake patient overnight. This may give rise to arrhythmias detected at night on Holter monitoring or inpatient cardiac monitoring even in the awake patient—a finding that does not necessarily imply the need for therapeutic intervention. Further monitoring may be appropriate to ensure that such arrhythmias are within normal limits, as described above.
Table 2.
Autonomic changes during sleep
| Sleep stage | Parasympathetic tone | Sympathetic tone |
| Non-REM | ↑ | ↓ |
| REM | ↓ | (due to surges) ↑↓ |
REM, rapid eye movement.
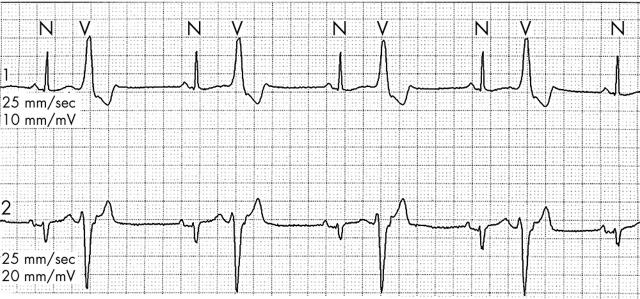
Night time reduction in sympathetic tone is reflected in the diminished incidence of premature ventricular contractions (PVCs) during sleep2w5 despite a relatively slow heart rate. Evidence in support of this demonstrates that suppression of PVCs by sleep predicts success of β blockade in PVC suppression.w6 Sleep suppression of PVCs is attenuated in patients with coronary artery disease or congestive heart failure,w5 likely due to increased baseline sympathetic tone. The natural suppression of hypercapnia induced ventilation during sleep and resulting hypoxia may also be a factor. Thus persistent, frequent PVCs may well be detected in patients with coronary artery disease when monitored (fig 2). Although such a finding may be unsettling, an abundance of evidence has shown that aggressive treatment of asymptomatic PVCs with antiarrhythmic medications is inappropriate in most situations. Non-sustained ventricular tachycardia (VT) may also be detected on monitoring, the management of which depends on assessment of symptoms, haemodynamic compromise, and ejection fraction. For patients with non-sustained VT in the presence of a poor ejection fraction, recent primary prevention trials have shown survival benefit with implantable cardioverter-defibrillators (ICDs).3,4
Figure 2.
Ventricular bigeminy seen in overnight monitoring of patient with coronary artery disease.
Further evidence for the role of sympathetic withdrawal in stabilising the cardiac rhythm overnight is provided by the observation that electrical recovery time of ventricular myocardium is longer during sleep than while awake.w7 Factors suggesting instability, such as shortest refractory period, as well as the greatest rate of shortening, occur during the transition from sleep to wakefulness. This may play an important role in the observation that the rates of sudden cardiac death, ventricular arrhythmias, and shocks delivered by ICDs are highest upon awakening in the morning hours.5w8 This phenomenon is likely related to an increase in sympathetic tone involved in assuming an upright posture, increasing activity level, and mental stress. Higher neural mechanisms have also been shown to play a role in sudden cardiac death,w9 as has the change in haemodynamics and its effect on coronary perfusion after waking.6 A circadian pattern of rhythm disturbance has also been demonstrated in patients with Brugada syndrome. This syndrome is characterised by right bundle branch block, ST segment elevation in the right precordial leads, and an elevated risk of sudden death.7,8w10 Matsuo and colleagues9 have determined that patients with Brugada syndrome experience ventricular fibrillation more frequently during sleep between midnight and 6 am than throughout the rest of the day. Sodium channel defects underlie the Brugada syndrome and type 3 long QT syndrome, a disorder of cardiac repolarisation in which most clinical events also occur during sleep. Autonomic effects likely play an indirect role in both these disorders, although the mechanism has not yet been established.
REM sleep occurs approximately 4–6 times per night for 90 minute periods, characterised by surges of sympathetic activity and decreased baroreceptor regulation and control.10 The highest incidence of non-fatal myocardial infarction, implanted defibrillator discharges, and sudden cardiac death occurs in a non-uniform manner throughout the night.10 A surge in each of these events occurs between 5 and 6 am, coinciding with an increased incidence of REM sleep. Several mechanisms are likely involved in this phenomenon, including diminished myocardial perfusion and increased electrical instability caused by high sympathetic tone, as well as a diminished ventilatory response to hypercapnia resulting in relative hypoxia and acidosis.
SLEEP RELATED ARRHYTHMIAS OF PATHOLOGIC SIGNIFICANCE
Arrhythmias during sleep may represent a direct risk to the patient, such as sudden death caused by ventricular arrhythmias in congestive heart failure or sudden infant death syndrome. In contrast, arrhythmias observed during overnight monitoring may represent a marker of other potentially harmful pathology such as obstructive sleep apnoea (OSA). Such arrhythmias may be characterised by symptoms such as palpitations or secondary angina, or may be detected incidentally on Holter or inpatient monitoring. They may also be recorded during a sleep study in a patient with no cardiac complaints but symptoms suggestive of OSA such as excessive daytime sleepiness, loud snoring, and intermittent apnoea during sleep which is noted by others.
Obstructive sleep apnoea
OSA is a disorder characterised by periodic episodes of apnoea during sleep. These apnoeic episodes are a result of laxity in the pharynx and the resulting obstruction to inspiration. OSA patients are usually males who sleep supine, with high body mass index, and a large neck and waist circumference.w11 w12 Main complaints include morning fatigue and headache, and loud snoring, frequent choking or absent respirations as reported by bed partners. The recognition and treatment of OSA is important because of the symptoms and complications that may arise, including hypertension, ischaemic heart disease, and stroke.11 Cardiac arrhythmias, although usually benign, commonly occur in patients with OSA and may assist with the diagnosis by appearing incidentally on overnight monitoring. It is important to recognise that these arrhythmias are caused by autonomic influences over initiation of impulses, and not intrinsic disease of the conduction system.
The gold standard for diagnosis of OSA is the overnight sleep study. This involves continuous overnight monitoring of the EEG, electro-oculogram, electromyogram, ECG, thoraco-abdominal motion, oronasal airflow (expired carbon dioxide), and arterial oxygen saturation via pulse oximetry. These measures allow for assessment of sleep onset, sleep stage, respiratory effort, airflow, and the calculation of an apnoea–hypopnoea index (AHI). The AHI indicates the average number of significant apnoeic or hypopnoeic episodes occurring per hour overnight. The minimum AHI used to diagnose OSA varies among studies, but generally lies in the range of 5–15. Several aids to the diagnosis of OSA have been developed including surveys,w11 models based on morphological measurements,w12 and assessment of clinical symptoms and signs. The role of rhythm monitoring as a diagnostic aid for OSA has yet to be determined, and continues to undergo investigation.
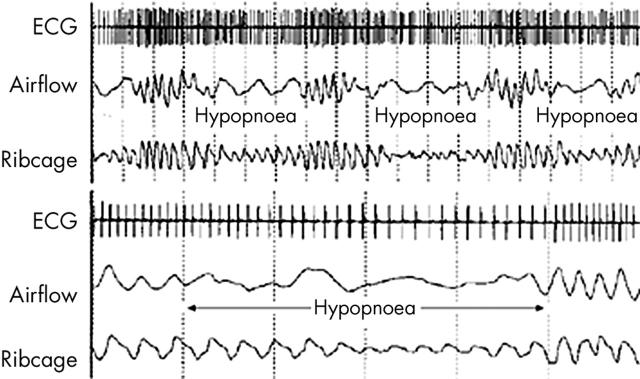
Most arrhythmias caused by obstructive apnoea occur with OSA that is at least moderate in severity. The prevalence of apnoeic bradycardia, in particular, increases in proportion to worsening OSA indices.w13 In fact, arrhythmias caused by mild or moderate sleep disordered breathing are rare, even among patients with other potentially arrhythmogenic factors such as disabling angina. The arrhythmia most commonly observed in association with OSA is cyclic variation of heart rate (CVHR).11,12 CVHR is characterised by progressive bradycardia during the apnoeic period with subsequent tachycardia on resumption of respiration (fig 3). Bradycardia generally begins with the onset of apnoea, with a decrease in heart rate that is proportional to the degree of hypoxaemia. The mechanism of CVHR involves both hypoxaemia and changes in autonomic tone, evidenced by elimination of CVHR by tracheostomy12 or administration of atropine.13 Furthermore, CVHR is absent in OSA patients with autonomic dysfunction caused by autonomic neuropathy, Shy Drager syndrome, and heart transplantation.13 The mechanism of the post-apnoeic tachycardia is likely due to a combination of arousal from sleep and vagal withdrawal caused by the pulmonary inflation reflex which results in increased heart rate, decreased systemic vascular resistance, and bronchodilation. The tachycardia is not sustained, probably because of the return of parasympathetic influence soon after breathing resumes.
Figure 3.
Illustration of cyclic variation of heart rate in relation to arterial oxygen saturation in obstructive sleep apnoea.
Ventricular ectopy has also been noted to occur more frequently among patients with OSA than normals,w14 although the incidence of non-sustained VT is similar to that of the normal population.12 Among patients with ICDs for life threatening VT, the number of shocks delivered at two years was found to be no different among patients with OSA, central sleep apnoea (CSA), and those without sleep disordered breathing.w15 Ventricular late potentials, a risk factor for life threatening arrhythmias, are infrequent among patients with OSA (6%), and have not predicted life threatening events among these patients during 45 months of follow up.w16 Other arrhythmias that have been noted to occur in OSA patients include prolonged sinus arrest (up to 13 seconds in duration), and Mobitz type II second degree AV nodal block. Sinus bradycardia and pauses noted on Holter monitoring may represent cyclic variation of heart rate. Evidence has shown that over 80% of apnoea associated bradycardia occurs during REM sleep,w17 further illustrating the vulnerability of the heart to autonomic influences during this sleep stage.
Abbreviations.
AHI: apnoea–hypopnoea index
AV: atrioventricular
CHF: congestive heart failure
CSA: central sleep apnoea
CPAP: continuous positive airway pressure
CVHR: cyclic variation of heart rate
EEG: electroencephalogram
ICD: implantable cardioverter-defibrillator
OSA: obstructive sleep apnoea
Paco2: arterial carbon dioxide partial pressure
PVC: premature ventricular contraction
REM: rapid eye movement
SIDS: sudden infant death syndrome
VT: ventricular tachycardia
The most effective treatment for OSA is nasal continuous positive airway pressure (CPAP). Worn overnight, CPAP maintains airway patency and notably reduces or eliminates obstructive episodes. Furthermore, arrhythmias characteristic of severe OSA, including CVHR, are not observed in patients using CPAP.14 Harbison and colleagues14 studied 45 OSA patients with an AHI greater than 50. Thirty five patients demonstrated rhythm disturbances, eight of which were severe. These were eliminated in seven of the eight patients after introduction of CPAP. This finding illustrates that overnight arrhythmias in many patients with OSA is directly related to obstructive events and their autonomic effects, and are not likely caused by structural heart disease or other causes unless suspected on other clinical grounds. Patients with significant bradycardia caused by obstructive apnoea usually have a normal conduction system but impaired initiation of impulses by the sinus node. When treated with CPAP, these patients have an excellent prognosis with regard to five year risk of syncope or cardiac arrest.15
An emerging area of research interest involves the relation between cardiac pacing and OSA. Small observational studies initially suggested that patients with sleep disordered breathing may have a reduction of AHI with cardiac pacing.w18 A recent study by Garrigue examined the effect of atrial overdrive pacing in a small number of patients with central or OSA, most of whom demonstrated impaired cardiac output.16 Pacing reduced the AHI in patients with both forms of sleep apnoea. Improvement in CSA may be caused by pacing induced change in autonomic tone and subsequent central effects on respiration. Cardiac vagal afferents form synapses in the medullary respiratory control centre, although their effect remains uncertain. The demonstrated effect of pacing on OSA is difficult to explain. It is certainly not intuitive that cardiac pacing should reduce pharyngeal collapse and obstruction to airflow. Pharyngeal muscle tone may be dependent, to some degree, on autonomic effects and thereby influenced by cardiac afferent innervation. It is interesting to note that an early study17 of patients with pacemakers for bradycardia showed no difference in prevalence of sleep disordered breathing when compared to the age matched general population. In light of more recent studies, it is tempting to speculate that a difference may have existed before pacemaker implantation, which was subsequently masked by pacing. The role of pacing for sleep apnoea has yet to be discerned, especially in patients without a conventional indication for pacemaker implantation. Larger scale clinical trials are currently underway to assess the impact of this treatment.
It remains unclear whether monitoring of cardiac rhythm and heart rate variability can be used to effectively diagnose or exclude OSA. Although it is tempting to suggest 24 hour Holter monitoring as a screening technique, Gillis2 describes an unpublished study of 93 patients which revealed that criteria for scoring Holter results that would produce both high sensitivity and specificity for a diagnosis of OSA was elusive. Our centre has found that examination of Holter data obtained during sleep studies has not been a successful means of identifying OSA patients due to the relatively low incidence of CVHR. This may be a result of earlier identification of less severely affected OSA patients, or increased awareness of the diagnosis and a reasonable sensitivity of clinical examination.
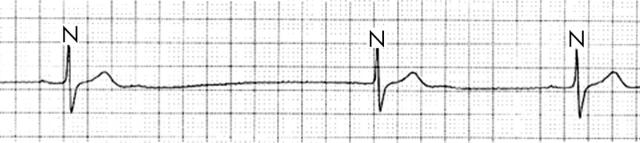
The utility of Holter monitoring in the diagnosis of OSA lies in the investigation of an incidental discovery of CVHR, sinus pauses (fig 4), ventricular ectopy, or heart block that occurs predominantly at night. With such a finding, the diagnosis of OSA should be considered and the patient questioned regarding symptoms. Information regarding snoring, episodic breathing or choking during the night, and sleep position should also be sought from the patient’s bed partner. Blood pressure should be obtained, and measurements of the oral cavity, as detailed elsewhere,w12 may also contribute. The result of this clinical assessment will predict with a high degree of accuracy whether the patient suffers from OSA, and arrangements for an overnight sleep study can then be made as appropriate.
Figure 4.
Sinus pause during episode of apnoea in an OSA patient.
Sudden infant death syndrome
Sudden infant death syndrome (SIDS) is marked by unexpected sudden death in infants up to six months of age, usually during sleep. The primary cause of SIDS has long been a source of debate, with a multifactorial postulate relating to the pulmonary or cardiovascular systems, possibly caused by failure of neural control. The abrupt nature of death points toward an arrhythmic mechanism. Recent evidence suggests that QT interval prolongation during the first week of life is a significant risk factor for SIDS.18 The odds ratio determined by this study (41) was, in fact, higher than those of the traditional risk factors of sleeping in the prone position, maternal smoking, and bed sharing. The same authors found that the QT interval tends to increase during the second month of life and subsequently return to the value recorded at birth by six months of age.w19 This pattern parallels the incidence of SIDS. A later reportw20 describes the case of a 6 week old infant with near SIDS who was resuscitated from ventricular fibrillation. The QT interval was prolonged and genetic testing revealed a mutation in the SCN5A gene, responsible for the LQT3 subtype of long QT syndrome. A subsequent studyw21 examined myocardial DNA from 93 victims of SIDS. Mutations in SCN5A were found in two of the 93 infants, and in none of 400 controls. The persistent sodium current resulting from this mutation may predispose to lethal arrhythmias, often occurring during sleep.
Other genes responsible for long QT syndrome have also been implicated in SIDS, as in the case of an infant who succumbed and was found to have a de novo mutation in the KVLQT1 gene.w22 The clinical utility of this information remains controversial. SIDS only occurs in a very small percentage of infants with a long QT interval. Furthermore, screening for QT interval prolongation in newborns would be useful only if preventative measures were acceptable in those at high risk. Since the leading known cause of death among those with congenital long QT intervals is torsades de pointes, and this arrhythmia is initiated by surges in sympathetic tone, β blockade has been suggested as a therapeutic option.w19 There are obvious concerns, however, regarding potential side effects and the ratio of benefit to risk with regard to such treatment in infants. These issues will undoubtedly be the subject of future research in the area of SIDS.
Congestive heart failure
Severe chronic congestive heart failure (CHF) is a common disease with a poor prognosis. CHF patients are susceptible to dangerous arrhythmias, and a relation between CHF and nocturnal arrhythmias has long been a topic of investigation. These rhythm disturbances often coincide with CSA. This disorder of complex pathophysiology results in the absence of respiratory effort periodically during sleep (in contrast to OSA), and has a high prevalence among CHF patients.19 The mechanism of apnoea may be related to hyperventilation induced hypocapnia caused by activation of lung receptors by pulmonary congestion.w23 Among prospectively studied CHF patients,20 a low daytime arterial carbon dioxide partial pressure (Paco2) had a positive predictive value of 78% for central sleep apnoea. Furthermore, the risk of nocturnal VT was 20 times higher among hypocapnic patients than among those who were eucapnic. The authors suggested that patients with daytime hypocapnia undergo Holter monitoring and sleep studies to prevent potentially dangerous arrhythmias. Indeed, among patients with a history of life threatening arrhythmia, central sleep apnoea has been associated with increased mortality.w15
A study of 81 male patients with stable heart failure caused by systolic dysfunction recently provided further characterisation of sleep disordered breathing in heart failure.19 Forty per cent of these patients were diagnosed with central sleep apnoea, and 11% with OSA. Both forms of sleep apnoea resulted in sleep disruption and nocturnal hypoxia, with a higher incidence of atrial fibrillation and ventricular arrhythmias among those with sleep apnoea than among controls. There is clearly a connection between CHF and sleep related arrhythmias, and the nature of this association continues to be explored and defined. New therapeutic manoeuvres, including the use of CPAP, for the prevention of central sleep apnoea and resulting arrhythmias, are currently being tested.
Clinical relevance of arrhythmias during sleep: key points.
Although most arrhythmias detected during sleep are benign, they may indicate an underlying disorder in need of investigation and treatment
Sinus bradycardia, sinus pauses up to two seconds in duration, and type 1 second degree atrioventricular block are commonly observed during sleep, and are generally benign
Nocturnal arrhythmias associated with structural heart disease or associated symptoms warrant investigation and consideration for specialist referral
Rates of sudden cardiac death, ventricular arrhythmias, and shocks delivered by implantable cardioverter-defibrillators are highest upon awakening in the morning hours, likely related to changes in autonomic tone
Obstructive sleep apnoea, a condition which may predispose to serious cardiovascular sequelae, can cause cyclic variation of the heart rate during sleep. This may be detected incidentally and provide a clue to the diagnosis
Sudden infant death syndrome is characterised by sudden death during sleep which is likely arrhythmic in origin. Recent evidence implicates repolarisation abnormalities as a possible underlying aetiology in a small proportion of patients
Sleep apnoea—central and obstructive—is prevalent among patients with chronic congestive heart failure. This predisposes to further cardiovascular events, including arrhythmias, which may be prevented by recognition and treatment of sleep disordered breathing.
CONCLUSION
Sleep is associated with a broad spectrum of arrhythmias, from benign sinus bradycardia to hypoxia associated ventricular ectopy. Numerous arrhythmias may therefore be detected during sleep, and the majority are benign. These rhythm disturbances usually reflect changing autonomic tone, characteristic of the various sleep stages. When symptoms and haemodynamic changes do not accompany low grade AV block and bradycardia, interventions such as pacing are generally not warranted. These rhythms result from changes in impulse formation, and not primary electrical disease. Current recommendations for permanent pacing acknowledge this concept by specifying that criteria for bradycardia should be met while awake.21 In some situations, however, further consideration is indicated on an individualised basis. This would pertain to patients with underlying structural heart disease, in need of medications which slow heart rate, with repolarisation syndromes, or with symptoms of coronary insufficiency or haemodynamic instability.
Whereas asymptomatic supraventricular and ventricular ectopy is generally benign, tachycardia documented during sleep should prompt the clinician to ensure the absence of structural heart disease. Management should be individualised according to the specific arrhythmia and associated symptoms or haemodynamic effects. In some cases, treatment may not be indicated for incidental rhythms recorded on overnight monitoring, but rather they may provide a clue to underlying pathology such as OSA, warranting further investigation because of risk of long term cardiorespiratory complications. For OSA specifically, emerging tools such as heart rate variability assessment may help diagnostically, and pacing may eventually become a therapeutic option. Other disorders which predispose to dangerous nocturnal arrhythmias, such as congestive heart failure in adults and abnormal repolarisation in infants (which may predispose to SIDS), are areas which are subject to ongoing research with regard to treatment and prevention.
Supplementary Material
REFERENCES
- 1.Brodsky M, Wu D, Denes P, et al. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol 1977;39:390–5. ▸ Among the first original articles documenting nocturnal rhythm in otherwise healthy individuals. [DOI] [PubMed] [Google Scholar]
- 2.Gillis AM, Flemons WW. Cardiac arrhythmias during sleep. In: Kryger MH, Roth T, Dement WC, eds. Principles and practice of sleep medicine. Philadelphia: Saunders, 1994:847–60.
- 3.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med 1999;341:1882–90. ▸ MUSTT (multicenter unsustained tachycardia trial) is a classic trial of implanted defibrillators for primary prevention of sudden death. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med 1996;335:1933–40. ▸ MADIT (multicenter automatic defibrillator implantation trial) is another classic trial of implanted defibrillators for primary prevention of sudden death. [DOI] [PubMed] [Google Scholar]
- 5.Tofler GH, Gebara OC, Mittleman MA, et al. Morning peak in ventricular tachyarrhythmias detected by time of implantable cardioverter/defibrillator therapy. The CPI investigators. Circulation 1995;92:1203–8. ▸ This study provided reliable information on timing and diagnosis of arrhythmias by using information stored by implanted defibrillators. [DOI] [PubMed] [Google Scholar]
- 6.Horner RL. Autonomic consequences of arousal from sleep: mechanisms and implications. Sleep 1996;19:S193–5. [DOI] [PubMed] [Google Scholar]
- 7.Brugada J, Brugada R, Antzelevitch C, et al. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation 2002;105:73–8. ▸ This article first described long term follow up of patients with Brugada-type ECG, and history of syncope, cardiac arrest, or absence of symptoms. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Napolitano C, Gasparini M, et al. Clinical and genetic heterogeneity of right bundle branch block and ST segment elevation syndrome: a prospective evaluation of 52 families. Circulation 2000;102:2509–15. ▸ Rates of ventricular fibrillation in asymptomatic and symptomatic Brugada syndrome were compared. Utility of programmed electrical stimulation and sodium channel blockade for diagnosis were also examined. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo K, Kurita T, Inagaki M, et al. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J 1999;20:465–70. ▸ First description of higher incidence of ventricular fibrillation at night in patients with Brugada syndrome, using recordings from implanted defibrillators. [DOI] [PubMed] [Google Scholar]
- 10.Lavery CE, Mittleman MA, Cohen MC, et al. Nonuniform nighttime distribution of acute cardiac events: a possible effect of sleep states. Circulation 1997;96:3321–7. [DOI] [PubMed] [Google Scholar]
- 11.Shepard JW Jr. Hypertension, cardiac arrhythmias, myocardial infarction, and stroke in relation to obstructive sleep apnoea. Clin Chest Med 1992;13:437–58. ▸ Review detailing cardiac sequelae of obstructive sleep apnoea, and describing underlying pathophysiology. [PubMed] [Google Scholar]
- 12.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnoea syndrome. Am J Cardiol 1983;52:490–4. ▸ First study of cardiac arrhythmias and conduction disturbance in a large group of patients with sleep apnoea syndrome. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Connolly S, Winkle R, et al. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984;i:126–31. ▸ Among the first descriptions of heart rate pattern characteristic of obstructive sleep apnoea. This pattern was eliminated by atropine, suggesting a role of the autonomic nervous system. [DOI] [PubMed] [Google Scholar]
- 14.Harbison J, O’Reilly P, McNicholas WT. Cardiac rhythm disturbances in the obstructive sleep apnoea syndrome: effects of nasal continuous positive airway pressure therapy. Chest 2000;118:591–5. ▸ This study found that nasal CPAP, the cure for obstructive sleep apnoea, eliminates rhythm disturbances which accompany the disorder. [DOI] [PubMed] [Google Scholar]
- 15.Grimm W, Koehler U, Fus E, et al. Outcome of patients with sleep apnoea-associated severe bradyarrhythmias after continuous positive airway pressure therapy. Am J Cardiol 2000;86:688–92, A9. ▸ Prospective study demonstrating that patients with sleep apnoea-associated bradyarrhythmias and normal cardiac conduction have low risk of syncope and sudden death while using CPAP. [DOI] [PubMed] [Google Scholar]
- 16.Garrigue S, Bordier P, Jais P, et al. Benefit of atrial pacing in sleep apnoea syndrome. N Engl J Med 2002;346:404–12. ▸ This study demonstrated a surprising effect of atrial overdrive pacing on patients with central or obstructive sleep apnoea, reducing apnoeic events in both conditions. This finding led to a number of larger studies which are currently ongoing. [DOI] [PubMed] [Google Scholar]
- 17.Fietze I, Rottig J, Quispe-Bravo S, et al. Sleep apnoea syndrome in patients with cardiac pacemaker. Respiration 2000;67:268–71. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz PJ, Stramba-Badiale M, Segantini A, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med 1998;338:1709–14. ▸ Study demonstrating that prolongation of the QT interval in the first week of life is strongly associated with SIDS, thus suggesting a possible role for ECG screening. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnoea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998;97:2154–9. ▸ Prospective study describing the clinically important finding that central and obstructive sleep apnoea are prevalent among patients with chronic heart failure. [DOI] [PubMed] [Google Scholar]
- 20.Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnoea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med 1998;128:204–7. ▸ Description of an important clinical finding, that low daytime Paco2 in patients with low ejection fraction suggests a diagnosis of central sleep apnoea, which is accompanied by a relatively high incidence of VT in these patients. [DOI] [PubMed] [Google Scholar]
- 21.American College of Cardiology, American Heart Association, North American Society for Pacing and Electrophysiology. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices. Circulation 2002;106:2145–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.