Abstract
Objective: To investigate the response of very small coronary arteries to stent deployment and balloon angioplasty.
Setting: Normal porcine coronary arteries.
Methods: 24 pigs underwent intervention to two main coronary arteries, in segments 2.0 mm in diameter, with balloons whose diameter was 2.5 mm at standard pressure. Twelve arteries received a BiodivYsio small vessel (SV) stent; 12 an NIR SV stent; 12 standard BiodivYsio stent, and 12 balloon only. The arteries were harvested at 28 days, fixed, embedded in plastic, and cut and ground in cross section. The injury score and histomorphometry were assessed.
Results: The BiodivYsio SV stent was associated with 20% less injury (p = 0.16), a 30% larger lumen (p = 0.13), and a 25% smaller neointima (p = 0.03) than the NIR SV stent, despite identical oversize. The standard BiodivYsio stent exhibited less recoil but 29% greater injury (p = 0.01), 59% more neointima (p = 0.00), and 18% less lumen (p = 0.27) than the BiodivYsio SV. Of all interventions, balloon only was associated with little injury, little neointima, major vessel shrinkage, and the largest lumen.
Conclusion: Despite uniform oversize dilatation, both injury and response varied widely in very small porcine coronary arteries, depending on whether a stent or balloon was used, the stent design, and the number and/or thickness of struts. The response to different stent designs is considerable and is related to the degree of injury.
Keywords: Stent, geometry, coronary artery, restenosis
Stenting small coronary arteries has now become routine. The restenosis rate in the lowest quartile (n = 52; vessel size 1.93–2.62 mm) in the BENESTENT II (Belgian Netherlands stent II) study, which employed the Palmaz-Schatz stent (Cordis, Miami Lakes, Florida, USA), was 25%.1 When a similar group was studied in the SOPHOS A (study of phosphorylcholine coating on stents), which employed the BiodivYsio stent (Abbott Vascular Devices, Redwood City, California, USA), the equivalent rate was 20%.2 This led to the development of scaled down stents, manufactured specifically for implantation in small vessels, such as the BiodivYsio small vessel (SV) stent. In a Latin American study of 216 patients with diabetes and target vessel diameter 2.0–2.9 mm (mean 2.47 mm) randomly assigned to BiodivYsio SV stent placement or “plain old balloon angioplasty” (POBA) the restenosis rate in the POBA arm was 29% and in the BiodivYsio SV stent arm 19%.3 In a study of small vessels in 502 patients from Germany (including both diabetics and non-diabetics) the angiographic restenosis rate in the PC coated stent arm was 39% and in the POBA arm 34%.4 There is little evidence to support the use of stents in very small vessels (2.0 or 2.25 mm).
The goal of the present study was to assess the response at 28 days of very small (2 mm) porcine coronary arteries to the BiodivYsio SV stent and to compare this with the response to an SV stent of different design (the NIR SV stent, Boston Scientific Corporation, Natick, Maryland, USA (NIR is a trademark of Medinol, Jerusalem, Israel)), to a standard BiodivYsio stent (the same design as the BiodivYsio SV but containing more metal), and to POBA.
METHODS
Stents and balloons
Figure 1 shows the design of the BiodivYsio and of the NIR stents. The BiodivYsio SV stent is a flexible, balloon expandable stent composed of 316L implant grade stainless steel. The thickness of the metal is 0.0024 inches (0.06 mm) and the diameter of the uncut tube is 1.0 mm. Metal coverage is approximately 12% when expanded to 2.5 mm and there are six cells in its circumference. The BiodivYsio standard stent is cut from a 1.6 mm diameter tube, with a thickness of 0.0036 inches (0.09 mm) and the same geometric pattern. The NIR is cut from 316L stainless steel. The struts are square in cross section and allow transition from flexible geometry (unexpanded) to rigid (expanded). The struts are 0.004 inches (0.10 mm) thick. Metal coverage is 11–18%. The lengths of the stents used in this study were 10 mm for the BiodivYsio SV, 11 mm for the BiodivYsio standard, and 9 mm for the NIR. Stents were deployed with a semicompliant 2.5 mm balloon. POBA was also performed with this type of balloon. Phosphorylcholine coats the BiodivYsio stents and has been shown not to affect neointimal growth in our pig coronary artery model.5
Figure 1.
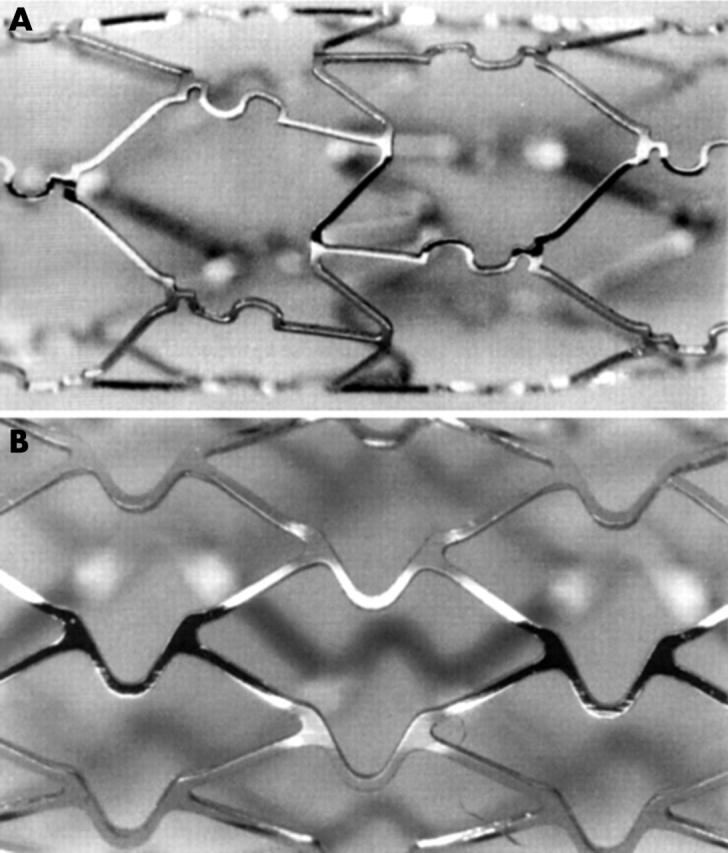
Structural details of (A) BiodivYsio stent, and (B) NIR stent.
Interventional procedure
Experimental procedures were performed according to UK Home Office regulations. Yorkshire white pigs weighing approximately 16 kg were used. Sedation was initiated with intramuscular azaperone. General anaesthesia was induced with intravenous propofol and maintained with spontaneously inhaled enflurane and oxygen. Vascular access was gained through the right carotid artery and, under fluoroscopic guidance, both the right and the left coronary arteries were intubated with 6 French Amplatz right coronary artery catheters. Quantitative angiography was used to identify segments of the left anterior descending and right coronary arteries that were 2 mm in diameter. Standard angioplasty techniques were used to perform one of four interventions at each site: BiodivYsio SV stent deployment; NIR SV stent deployment; BiodivYsio standard stent deployment; or POBA. In all cases, a 2.5 mm balloon was used, at 8 atm pressure for 30 seconds. The balloon to artery ratio was, therefore, 1.25:1, a size that we have previously shown, in this model, to produce a moderate stent related arterial injury and moderate neointimal growth.6 Adjuvant treatment comprised premedication with aspirin 150 mg, maintained for five days, and a bolus of 2500 U periprocedural intravenous heparin. No thienopyridine was used, as is routine in our model. At the end of the procedure, the angioplasty equipment was withdrawn, the carotid artery ligated, and the animal allowed to recover. At 28 days, the animals were anaesthetised and killed with an intravenous overdose of thiopentone. The stented segments of artery were excised and processed.
Tissue processing
The stented segments were processed according to our previously published technique.7 In brief, they were perfusion fixed in 10% formalin, embedded in T8100 resin, cut with a diamond tipped saw, and ground and polished. From the multiple sections produced, three from each stented segment were selected, one from each of a quarter, half, and three quarters of the distance down the segment. In the case of the angioplastied artery, in which the injured segment was not obvious macroscopically, blocks were cut at 2 mm intervals and a section cut from each. Each section was examined for evidence of injury (a breached internal elastic lamina) and three sections were selected from as close to a quarter, half, and three quarters of the distance down the segment as possible. All sections were stained with haematoxylin and eosin and prepared for microscopic examination.
Arterial measurements
Each section underwent microscopic examination and computerised histomorphometry. The cross sectional areas of the lumen, neointima, media, adventitia, and the whole vessel were measured. The percentage metal coverage was defined as the percentage of the perimeter of the stented artery that comprised stent struts. The injury score was also assessed (in the case of the stented sections) by the method of Schwartz.8
Statistical analysis
All measurements were expressed as mean (SEM). Groups were compared with analysis of variance. Significance was sought at the 95% level.
RESULTS
Procedural results
There were no problems relating to intervention and all interventional devices were deployed successfully. All vessels were angiographically patent at the end of the procedure. Of the 24 animals, one died. This was towards the end of the index procedure and was related to operator error causing an air embolism into the left anterior descending artery which resulted in intractable arrhythmia. The other animals survived to 28 days. The number of arteries (sections) that were successfully processed and available for analysis were BiodivYsio SV 12 (35); NIR SV 12 (36); BiodivYsio standard 11 (33); and POBA 11 (31).
Device sizing and vessel injury
Table 1 shows the diameter of the arterial segments and the balloon to artery ratios achieved in each group. Both the arterial diameter and the balloon to artery ratios were very similar for each group. The resulting stent induced injury, however, was much lower in the BiodivYsio SV stent group than in both the NIR SV and the BiodivYsio standard stent groups. Comparison between the severity of stent and balloon injury was not possible—the two injuries are qualitatively different and are measured in different ways. However, deep injury was rare in the balloon dilated sections.
Table 1.
Device sizing, arterial injury scores, and metal coverage for all the sections from each group of very small (2 mm) porcine coronary arteries undergoing intervention
| BiodivYsio SV | NIR SV | BiodivYsio standard | POBA | |
| Artery diameter (mm) | 2.08 (0.02) | 2.09 (0.02) | 2.01 (0.02)* | 2.02 (0.04) |
| Balloon diameter (mm) | 2.50 (0.00) | 2.50 (0.00) | 2.50 (0.00) | 2.50 (0.00) |
| Balloon/artery ratio | 1.20 (0.01) | 1.20 (0.01) | 1.25 (0.01)* | 1.24 (0.02) |
| Schwartz score | 1.15 (0.15) | 1.43 (0.12) | 1.48 (0.10)** | NA |
| Metal coverage (%) | 31 (2) | 40 (2) | 48 (2)** | NA |
Numbers are mean (SEM).
*p<0.05; **p<0.01 compared with BiodivYsio SV.
NA, not applicable; POBA, plain old balloon angioplasty; SV, small vessel.
Vessel morphometry
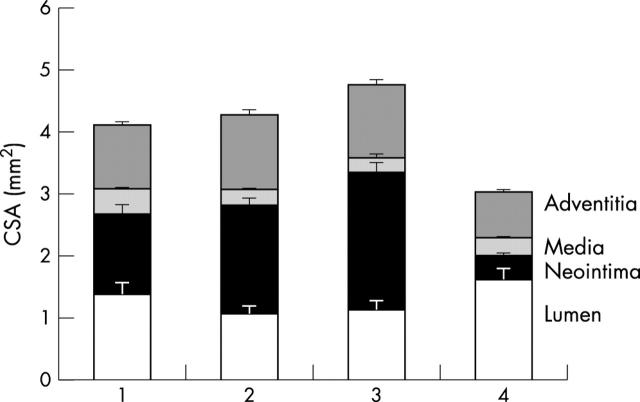
Figure 2 shows sections taken from the coronary arteries, and fig 3 and table 2 display the cross sectional areas of the arterial wall, 28 days after intervention in each group. Of note, almost identical sizing was achieved for each: mean (SEM) 3.06 (0.13) mm for the BiodivYsio SV and 3.08 (0.10) mm for the NIR (not significant). Despite this, the BiodivYsio SV was associated with a 20% lower injury score (p = 0.16), a 30% larger lumen (p = 0.13), and a 25% smaller neointima (p = 0.03). The BiodivYsio standard stent maintained a 17% larger size (p = 0.01) but produced an 18% smaller lumen (p = 0.27) and a 59% larger neointima (p < 0.01) than the BiodivYsio SV stent, despite the similarity of design. The balloon-only group was associated with an 18% larger lumen (p = 0.26) and a 73% smaller neointima (p < 0.01) than the BiodivYsio SV stent, although the vessel size at the site of injury had 25% downsize remodelling compared with that of the BiodivYsio SV (p < 0.01).
Figure 2.
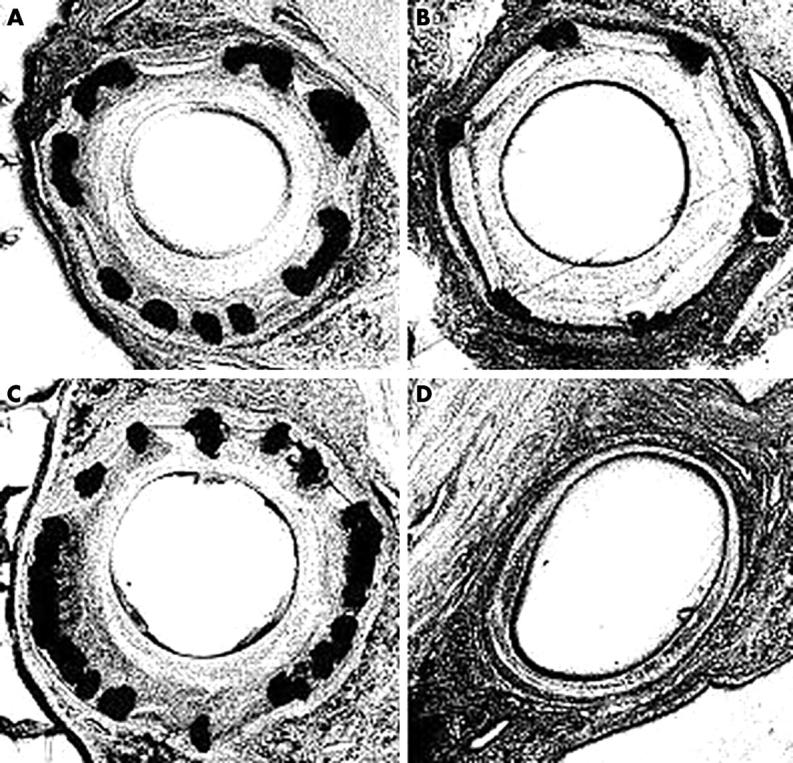
Representative sections from very small (2 mm) porcine coronary arteries 28 days after intervention with 2.5 mm devices. (A) NIR small vessel (SV) stent; (B) BiodivYsio SV stent; (C) BiodivYsio standard stent; and (D) POBA.
Figure 3.
Histograms showing the histomorphometry of very small (2 mm) porcine coronary arteries 28 days after intervention with 2.5 mm devices. (1) BiodivYsio SV stent; (2) NIR SV stent; (3) BiodivYsio standard stent; and (4) POBA.
Table 2.
Vessel morphometry
| BiodivYsio SV | NIR SV | BiodivYsio standard | POBA | |
| Lumen | 1.37 (0.18) | 1.05 (0.12) | 1.13 (0.12) | 1.62 (0.13) |
| Neointima | 1.30 (0.16) | 1.75 (0.12)* | 2.20 (0.12)** | 0.35 (0.05)** |
| Media | 0.39 (0.04) | 0.28 (0.02)* | 0.24 (0.04)** | 0.32 (0.02) |
| Adventitia | 1.04 (0.07) | 1.19 (0.08) | 1.18 (0.07) | 0.75 (0.04)** |
| Area in EEL | 3.06 (0.13) | 3.08 (0.10) | 3.58 (0.14)** | 2.29 (0.14)** |
| Total vessel | 4.65 (0.14) | 4.81 (0.13) | 5.65 (0.14)** | 3.01 (0.16)** |
Numbers are mean (SEM) and correspond to the histograms in fig 3.
*p<0.05; **p<0.01 compared with BiodivYsio SV.
EEL, external elastic lamina (the area within the EEL can be taken to be approximately the area within the stent).
DISCUSSION
In this study, we have shown that both stenting and balloon angioplasty are safe and not associated with immediate or late complications in very small (2.0 mm) pig coronary arteries. We have shown that different interventions in these arteries elicit widely differing magnitudes and types of response at 28 days, despite almost identical oversize. We have shown that one purpose-made SV stent can perform better than another, by virtue of differences in design. We have also shown that a stent with more metal (the BiodivYsio standard stent has 54% greater metal area at this size of deployment than the BiodivYsio SV stent) but a similar design performs less well than the BiodivYsio SV, despite allowing less underachievement of intended size. Simple balloon inflation, at the same oversize, is associated with little apparent injury or neointima growth and a respectable lumen, despite major downsize remodelling.
These data increase our knowledge concerning the effects of intervening on very small coronary arteries. They support the concept that stent design is important in this size of vessel; that excessive metal coverage is not helpful with respect to neointima formation (although greater percentage coverage may be necessary to confer adequate radial strength); and that balloon angioplasty alone may still have a place, by trading less scaffolding ability for less neointimal growth.
A notable finding was the substantial under-achievement of the intended lumen size, as assessed by the measurement of the in-stent area (table 2). A stent or balloon of 2.5 mm nominal diameter reaches a cross sectional area (at the moment of implantation) of 4.91 mm2. None of our interventions achieved this. The closest to this value was the cross sectional area of the BiodivYsio standard stent, although any benefit in scaffolding the artery open was more than lost with the exuberance of the resulting neointima. Both SV stent designs achieved almost identical scaffolding, at 14% less than that of the BiodivYsio standard stent.
The discrepancy in the stent area achieved between the SV stents and the standard stent may be taken to be caused by an excess of recoil with the SV designs compared with the standard design. However, the cross sectional area of an artery of circular cross section is πr2, whereas elementary geometry shows that the area enclosed by the polygon of cross section of a stent with six struts (BiodivYsio SV) fitted into the same circle is only 2.58r2 (82% of the area of the circle), and the area enclosed by a stent with 12 struts (BiodivYsio standard) fitted into the same circle is 3.00r2 (95% of the area of the circle). Therefore, a stent with more struts would, by its very design, enclose a larger area. However, even with the BiodivYsio standard stent, the area enclosed by the metal (3.58 mm2) was not 95% of that intended but only 73%. The equivalent figure for the SV stent was 63%. This underachievement may result from either undersizing or recoil. Recoil is, perhaps, less likely because the standard stent, with more scaffolding ability, may be expected to resist recoil. This was not the case: the ratio of the actual cross sectional areas achieved by the SV and the standard stents was 63%/73% = 0.86, exactly the same as the ratio predicted for six versus 12 strutted stents fitted into an artery without any recoil (82%/95% = 0.86). Hence, the underachievement observed for all stents must have been caused by a combination of stent geometry (less versus more struts) plus either recoil or (more likely) systematic undersizing.
Recoil in all the stent groups was, however, small by comparison with that found in the balloon-only group, in which the area bounded by the media was 36% smaller than that in the BiodivYsio standard stent and no less than 53% smaller than ideal (a circle of diameter 2.5 mm). Despite this considerable downsize remodelling, the lumen in the injured segments of ballooned artery was still larger than those seen in the stented arteries. This is likely to be due to the different nature of balloon and stent injury: the one transient; the other chronic, and both being (at 1.25:1 oversize) more likely to be stretch related than deep injury related.
The main limitation of the present study was that the arteries were undiseased. This was because there is no completely satisfactory large animal model of atherosclerosis. Using undiseased arteries, however, had the advantage that any changes observed were caused only by the intervention being studied.
In conclusion, the response of very small (2 mm) coronary arteries to endovascular intervention depends on the presence of a stent, the design of the stent, and the amount of metal in the design. Balloon angioplasty alone may retain a place in the treatment of these very small vessels.
Acknowledgments
We are grateful to Abbott Vascular Devices, manufacturers of the BiodivYsio stent, who sponsored this study and supplied the BiodivYsio stents.
Abbreviations
BENESTENT II, Belgian Netherlands stent II
POBA, plain old balloon angioplasty
SOPHOS, study of phosphorylcholine coating on stents
SV, small vessel
REFERENCES
- 1.Serruys PW, van Hout B, Bonnier JJRM, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary disease (BENESTENT II). Lancet 1998;352:673–81. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Buller C, Bonnier JJRM, et al. Quantitative angiographic results of the phosphorylcholine coated BiodivYsio stent in the SOPHOS study [abstract]. Eur Heart J 1999;20:1525.10529317 [Google Scholar]
- 3.Rodriguez AE. LASMAL-II: a prospective, randomised trial of PC-coated stents vs balloon angioplasty in small vessels in the diabetic patient. Oral presentation, Transcatheter Cardiovascular Therapeutics meeting, Washington DC, 15September2003.
- 4.Kastrati A. The ISAR-SMART-II study. A prospective randomised, 4-arm trial of PC-coated stenting and IIb/IIIa inhibition in patients with small coronary arteries undergoing percutaneous coronary intervention. Oral presentation, Transcatheter Cardiovascular Therapeutics meeting, Washington DC, 16September2003.
- 5.Malik N, Gunn J, Holt C, et al. Phosphorylcholine-coated stents in porcine coronary arteries: biocompatible and safe. J Invas Cardiol 2001;13:193–201. [PubMed] [Google Scholar]
- 6.Gunn J, Chan KH, Arnold N, et al. Coronary artery stretch versus deep injury in the development of in-stent neointima. Heart 2002;88:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik N, Gunn J, Holt C, et al. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry, and transmission electron microscopy. Heart 1998;80:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–74. [DOI] [PubMed] [Google Scholar]