Abstract
Objective: To examine the hypothesis that major extracellular matrix (ECM) proteins are expressed differently in the left atrial tissue of patients in sinus rhythm (SR), lone atrial fibrillation (AF), and AF with underlying mitral valve disease (MVD).
Design: Case-control study.
Patients: 118 patients with lone AF, MVD+AF, and SR.
Main outcome measures: Collagen I, collagen III, and fibronectin protein expression measured by quantitative western blotting techniques and immunohistochemical methods.
Results: Protein concentrations increased in patients with AF (all forms) compared with those in SR (all forms): collagen I (1.15 (0.11) v 0.45 (0.28), respectively; p = 0.002), collagen III (0.74 (0.05) v 0.46 (0.11); p = 0.002, and fibronectin (0.88 (0.06) v 0.62 (0.13); p = 0.08). Especially, collagen I was similarly enhanced in both lone AF (1.49 (0.15) and MVD+AF (1.53 (0.16) compared with SR (0.56 (0.28); both p = 0.01). Collagen III was not significantly increased in lone AF but was significantly increased in AF combined with MVD (0.84 (0.07) both compared with SR (0.46 (0.11); p = 0.01). The concentration of fibronectin was not significantly increased in lone AF and MVD+AF (both compared with SR). Furthermore, there was a similar degree of enhanced collagen expression in paroxysmal AF and chronic AF.
Conclusions: AF is associated with fibrosis. Forms of AF differ from each other in collagen III expression. However, there was no systematic difference in ECM expression between paroxysmal AF and chronic AF. Enhanced concentrations of ECM proteins may have a role in structural remodelling and the pathogenesis of AF as a result of separation of the cells by fibrotic depositions.
Keywords: arrhythmia, valve disease, fibrosis, extracellular matrix, remodelling
About 1% of the population suffers from atrial fibrillation (AF). Risk factors for AF are rheumatic and ischaemic heart disease, hypertension, congestive heart failure, and older age. Generally, in most cases a progression from paroxysmal AF (PAF) to chronic AF (CAF) can be detected.1–3
It is supposed that in the development of sustained AF a so called second factor is involved.1,4,5 A good candidate for such a factor would be increased tissue anisotropy due to tissue fibrosis. Fibrosis is an excessive deposition of extracellular matrix (ECM) and can occur as a result of mechanical overload of the tissue combined with the action of other profibrotic factors or as a result of tissue damage.6
The major components of the ECM are collagen I, collagen III, and fibronectin.7 Collagen I is the major collagenous product of cardiac fibroblasts and accounts for about 80% of total cardiac collagen content. Collagen type III is relatively abundant in the myocardium, with an approximate content of 10%.8 The ECM is an intricate network of macromolecules that forms a “scaffold”. In the ECM collagen is accompanied by cross linking glycoproteins. One of the most relevant cross linking glycoproteins is fibronectin. The fibrillar networks of fibronectin couple cells mechanically to their environment and to the neighbouring cells. The networks can also be cross linked to collagen.9–12
Atrial dilatation and fibrosis probably are important factors in the occurrence and maintenance of AF.13 If AF promotes fibrosis, one should expect a progressive increase in fibrosis with increased duration of the rhythm disturbance.
The differing expression of the ECM components collagen I, collagen III, and fibronectin in various forms of AF in humans has rarely been studied and is poorly understood. Therefore, we analysed the hypothesis that the expression of major ECM proteins in left atrial tissue differs in patients in sinus rhythm (SR), lone AF, and AF with underlying mitral valve disease (MVD). We also compared expression of ECM proteins in patients with PAF versus patients with CAF.
METHODS
Patients
The study group consisted of patients with lone AF (n = 56: 30 PAF, 26 CAF) and patients with both AF and MVD (n = 46: 10 MVD-PAF, 36 MVD-CAF). Patients in SR (n = 8) and patients with SR combined with MVD (n = 8) formed the control group and were matched to the AF groups according to age, left atrial size, and left ventricular function. Patients were included in the study only if they had preserved left ventricular function. The lone AF group had a left atrial size of ⩽ 45 mm as assessed by echocardiography. The surgical procedure and the concept of intraoperative ablation of AF have been described in detail.14 All patients gave written informed consent. The institutional Ethical Committee approved the study. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Atrial tissue from all patients was obtained from the left atrial free wall near the interatrial septum (about 5 × 5 mm from atriotomy) during cardiac surgery, quickly frozen in liquid nitrogen, and stored at −80°C until use.
Histology
Sirius red staining
Formalin fixed, paraffin embedded atrial tissue sections of 5 µm thickness were deparaffinised in xylol and a descending alcohol sequence (100%, 96%, 75%) and brought into distilled water. Subsequently, the slices were exposed to a pikro sirius red solution (Hollborn & Söhne, Leipzig, Germany) for one hour. Tissue sections were then washed in diluted acetic acid for 10 minutes. Slices were dehydrogenated in an ascending alcohol sequence (75%, 96%, 100%) and xylol. Lastly, slices were embedded in a mounting medium (Pertex; Histolab, Västra Frölunde, Sweden).
Immunohistology
Formalin fixed, paraffin embedded atrial tissue sections of 5 µm thickness were deparaffinised. Subsequently, slices were blocked with 5% milk powder tris buffered saline solution and incubated with primary antibodies: mouse anti-human collagen type I, mouse anti-human collagen type III (both from Medicorp, Montreal, Quebec, Canada), and mouse anti-human fibronectin (Santa Cruz Biotechnology, Santa Cruz, California, USA). The staining steps for collagen type I and fibronectin (APAAP staining method) and for collagen type III (AEC staining method) were performed as described by Dako Corp (Carpinteria, California, USA). The specificity was controlled by omitting the primary antibodies.
Western blot analysis
For electrophoresis, 20 µg of total protein was separated in a sodium dodecylsulphate polyacrylamide gel and blotted on to a cellulose membrane (Roth, Karlsruhe, Germany). Membranes were blocked in 5% milk powder (Roth) in tris buffered saline with 0.5% tween 20 for one hour. After washing, membranes were incubated with primary antibodies for two hours. Goat anti-human collagen I, goat anti-human collagen III (Santa Cruz Biotechnology) and rabbit anti-human fibronectin (DPC Biermann, Bad Nauheim, Germany) were used as primary antibodies. As a reference we used mouse anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hytest, Turku, Finland). After washing, membranes were incubated with the secondary antibodies rabbit anti-goat IgG (for collagen I and III), goat anti-rabbit IgG (for fibronectin) or rabbit anti-mouse IgG (for GAPDH), all conjugated with horseradish peroxidase, for one hour (all secondary antibodies from Sigma, Deisenhofen, Germany). Subsequently, membranes were developed with Super Signal Reagent (Pierce, Rockford, Illinois, USA).
Densitometric analysis
Immunoblots were exposed to x ray film (Eastman Kodak Co), developed, and analysed by ONE-Dscan 1.0 Software (Scanalytics, Los Angeles, California, USA). The relative amount of collagen or fibronectin (target proteins) in each sample was investigated by comparison of the grey scale value of target proteins with the grey scale signal of GAPDH. The GAPDH value was used as an adjusting factor to assure that the same amount of cellular proteins was analysed in each sample. The ratio of target protein to GAPDH from each patient was used to calculate possible differences in target protein synthesis between the patients.
Statistical analysis
All data are mean (SEM). Statistical evaluation was by two way multivariate analysis of variance with a subsequent post hoc Tukey HSD test. Values of p < 0.05 were considered significant.
RESULTS
Patients
Table 1 summarises the clinical characteristics of the patient population. Patients in the lone AF group were younger than those with MVD+AF and all patients in SR. Left atrial sizes in the lone AF and SR group were smaller than in MVD+AF group.
Table 1.
Summary of patient characteristics
| Lone AF | MVD+AF | Control group | p Value | |
| Number | 56 | 46 | 15 | |
| PAF | 30 | 10 | −1 | |
| CAF | 26 | 36 | −1 | |
| Cardiac surgery | IRAAF | MVR+IRAAF | MVR (7) | |
| MVR+CABG (1) | ||||
| CABG (6) | ||||
| AVR+CABG (2) | ||||
| Age (years) | 48 (13) | 64 (10) | 59 (7) | <0.05 lone v MVD+AF |
| <0.05 SR v lone AF | ||||
| NS SR v MVD+AF | ||||
| LVEF (%) | 60 (6) | 57 (16) | 55 (13) | NS |
| Left atrium (mm) | 43 (6) | 55 (11) | 44 (7) | 0.001 lone AF v MVD+AF |
| <0.001 SR v MVD+AF | ||||
| Calcium antagonists (all) | 8 (14%) | 6 (13%) | 3 (19%) | |
| Digitalis | 16 (29%) | 32 (69%) | 2 (13%) | |
| β Blocker | 41 (93%) | 45 (98%) | 10 (63%) | |
| Antiarrhythmic drugs | 22 (39%) | 6 (13%) | 0 | |
| ACE inhibitors | 23 (41%) | 43 (93%) | 9 (56%) | |
| Spironolactone | 1 (2%) | 8 (17%) | 0 |
Data are mean (SD) or number (%).
ACE, angiotensin converting enzyme; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CAF, chronic atrial fibrillation; IRAAF, intraoperative radiofrequency ablation of atrial fibrillation; LVEF, left ventricular ejection fraction; MVD, mitral valve disease; MVR, mitral valve repair or replacement; NS, not significant; PAF, paroxysmal atrial fibrillation; SR, sinus rhythm.
Histological results
Sirius red and immunhistochemical staining for collagen I, collage III, and fibronectin from left atrial tissue of patients in SR and patients in AF showed clear differences in the occurrence and deposition of connective tissue. In patients with AF, the muscle bundles were surrounded by thick connective tissue fibres. These thick fibres were also present between the single muscle cells or cardiomyocytes separating cells from each other (fig 1A,C,E,G). In the tissue of the control group there was only a fine network of collagen between the separate muscle bundles and no connective tissue was deposited between the single cells (fig 1B,D,F,H).
Figure 1.
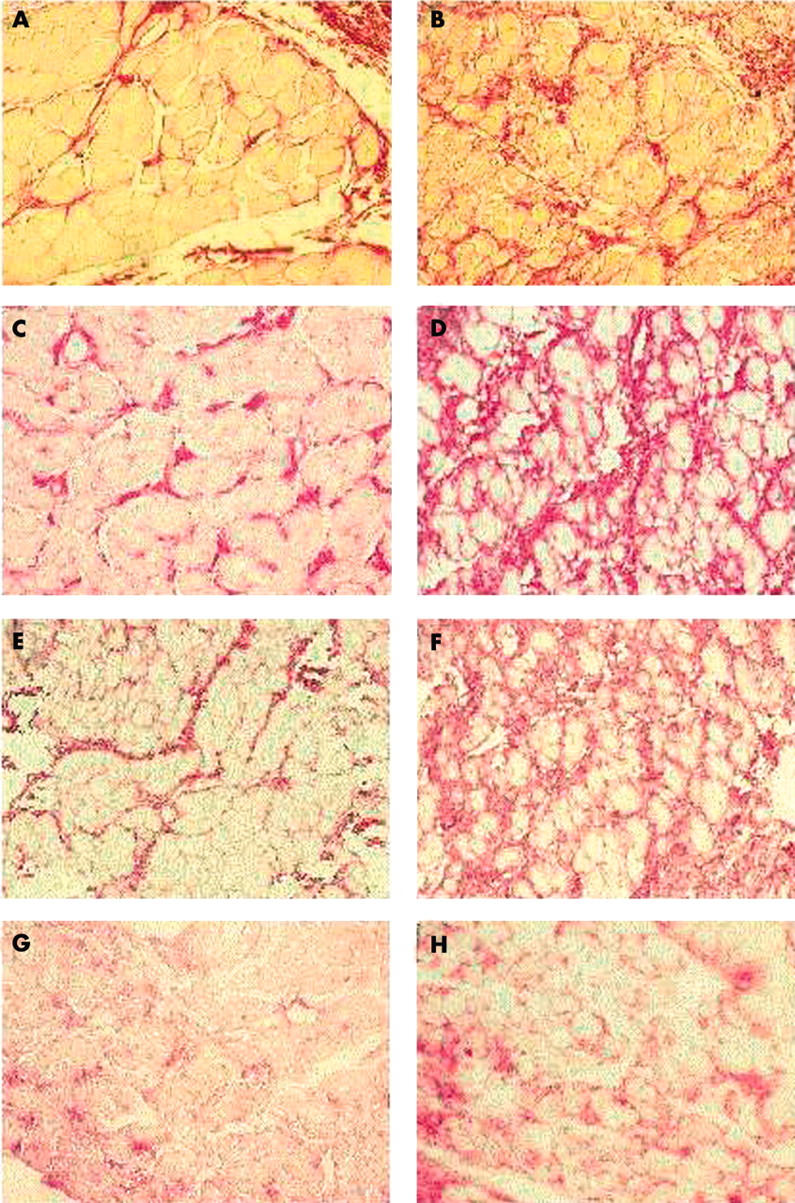
Sirius red staining of left atrial tissue for connective tissue (red staining) from patients (A) in sinus rhythm (SR) and (B) with chronic atrial fibrillation (lone CAF). Immunohistological results in left atrial tissue from (left) patients in SR and (right) patients with lone CAF for (C, D) collagen type I, (E, F) collagen type III, and (G, H) fibronectin. Original magnification ×400.
After detecting clear histological differences between patients in SR and patients with AF, we quantified the differences by Western blot techniques. Morphometric analysis of the cells showed that the mean size of the cells did not differ between SR and AF (18.5 (0.5) v 18.9 (0.4) µm, respectively).
Influence of AF on connective tissue synthesis
Western blot analysis of collagen I, collagen III, and fibronectin showed a clear increase of the collagens and to a lower extent of fibronectin in patients with AF (all AF) compared with control group patients in SR (all SR). The protein concentration of collagen type I (1.15 (0.11); n = 101) was significantly (about twofold) increased compared with the SR control group (0.45 (0.28), p = 0.002; n = 16). Likewise, type III collagen was significantly enhanced by about 60% in patients with AF (all forms) (0.74 (0.05); n = 89) in comparison with patients in SR (0.46 (0.11), p = 0.022; n = 16). Fibronectin was also, but to a lesser extent, increased in patients with AF (0.88 (0.06); n = 86) compared with the control group (0.62 (0.13); n = 14), without reaching the level of significance (p = 0.08) (fig 2A). The factors coronary heart disease and age of the patient had no significant influence on the development of fibrosis (coronary heart disease: p = 0.8; age: p = 0.62).
Figure 2.
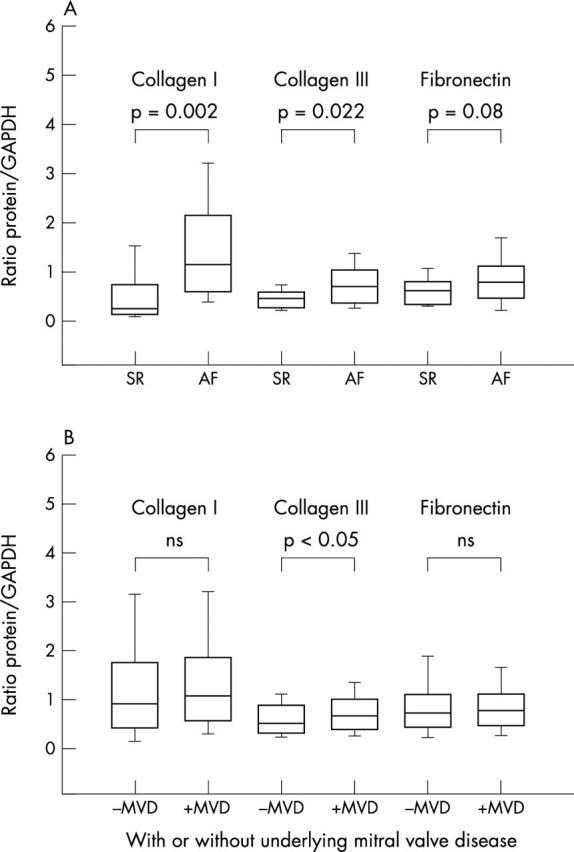
(A) Collagen I, collagen III, and fibronectin expression in atrial tissue of (left columns) patients in SR (left columns) and (right columns) patients with atrial fibrillation. (B) Comparison of all patients in the study either (left columns) with underlying mitral valve disease (MVD) or (right columns) without MVD.
Influence of MVD on ECM synthesis
We also analysed the influence of MVD on collagen and fibronectin synthesis. Comparing patients without MVD (SR and all lone AF) with patients with MVD (MVD+SR and all MVD+AF) we did not detect any significant differences either in collagen type I (1.38 (0.15); n = 64 v 1.59 (0.16); n = 54) or in fibronectin protein concentration (0.87 (0.07); n = 57 v 0.79 (0.08); n = 45). Collagen type III was only slightly increased in patients with MVD (0.79 (0.06); n = 48) compared with patients without MVD (0.62 (0.06); n = 55) with borderline significance (p = 0.045) (fig 2B).
Differences between lone AF and MVD+AF
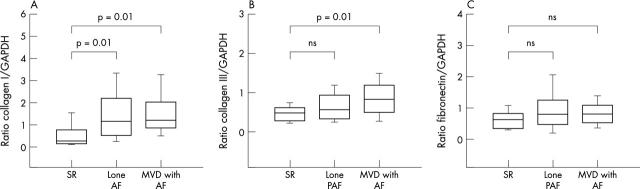
Subsequently, we determined the influence of lone AF (all forms) and MVD+AF (all forms) on collagen and fibronectin production in comparison with that in patients in SR (with and without MVD). There were significant differences in collagen type I (analysis of variance, p = 0.009). The protein concentration of collagen I was increased in patients with lone AF (1.49 (0.15), n = 56) compared with the SR group (0.56 (0.28), p = 0.01; n = 16) and was also increased in the MVD+AF group (1.53 (0.16), p = 0.01; n = 46). We did not find differences between lone AF and MVD+AF (fig 3A).
Figure 3.
(A) Collagen I, (B) collagen III, and (C) fibronectin expression in left atrial tissue of patients in SR, with lone AF, and with AF with underlying MVD (MVD+AF).
Collagen type III concentrations changed significantly in comparing SR versus lone AF versus MVD+AF by analysis of variance (p = 0.01). Especially, the increase was significant in patients with MVD+AF (0.84 (0.07); n = 41), about 80%, compared with SR (0.46 (0.11), p = 0.01; n = 14). No significant changes were detected between SR and lone AF (0.66 (0.06); n = 48) or between lone AF and MVD+AF (fig 3B).
The protein concentration of fibronectin tended to be enhanced in the lone AF group (0.93 (0.08); n = 48) and in the MVD+AF group (0.81 (0.09); n = 38) compared with SR group (0.62 (0.13); n = 16), without reaching the level of significance (analysis of variance, p = 0.13; fig 3C).
Influence of paroxysmal and chronic forms of AF
To investigate the influence of the PAF and CAF forms of AF, we compared patients with lone PAF and lone CAF with the SR group and patients with MVD+PAF and MVD+CAF with patients with MVD+SR.
The protein concentration of collagen type I increased in the left atrium of patients with lone PAF (1.31 (0.21); n = 29) and in patients with lone CAF (1.71 (0.22); n = 26) compared with the SR control group (0.57 (0.4); n = 8). Similarly, in patients with MVD collagen I concentration increased both in the MVD+PAF group (1.7 (0.36); n = 10) and in the MVD+CAF group (1.48 (0.18); n = 36) compared with the MVD+SR group (0.54 (0.4); n = 8) (fig 4A). However, the differences between the PAF and CAF subgroups did not reach significance.
Figure 4.
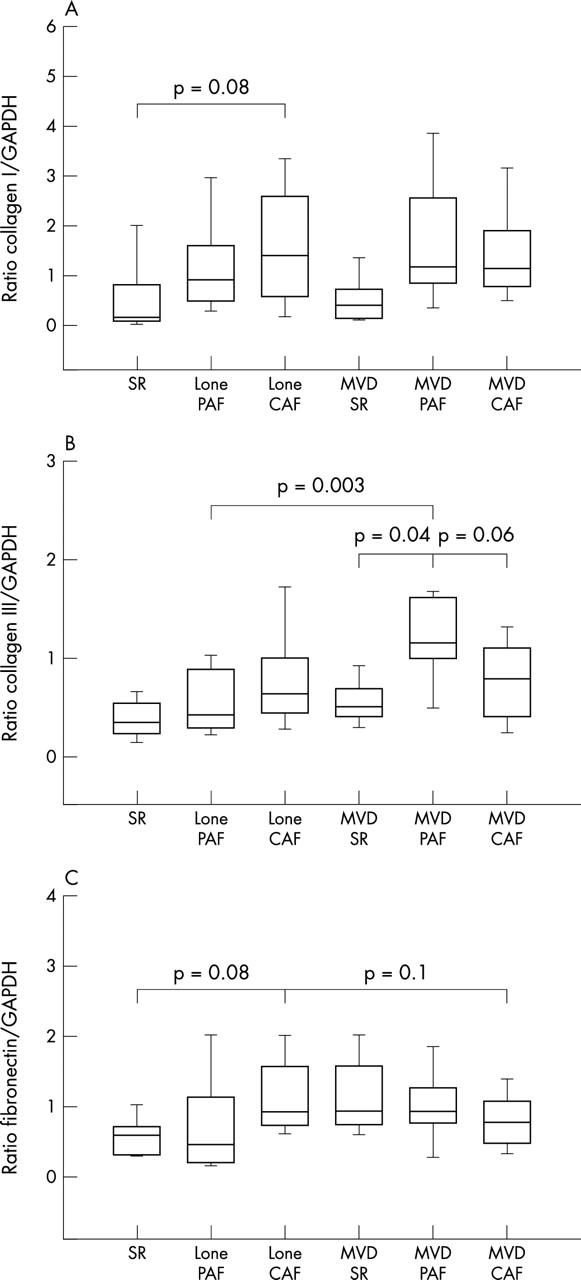
Quantitative analysis of (A) collagen I, (B) collagen III, and (C) fibronectin expression in human atrial tissue of the SR, paroxysmal atrial fibrillation (PAF), and CAF subgroups of lone AF and MVD+AF.
Regarding collagen type III, we found a (non-significant) slight rise in both lone PAF (0.57 (0.08); n = 25) and in lone CAF (0.77 (0.08); n = 23) compared with SR (0.37 (0.15); n = 7). Furthermore, collagen type III was about twofold increased in the MVD+PAF group (1.14 (0.13), p = 0.04; n = 10) compared with MVD+SR (0.55 (0.15); n = 7), whereas the increase in the MVD+CAF group (0.74 (0.07); n = 31) was only a slight and non-significant compared with patients in SR. Interestingly, in the MVD+PAF group collagen III expression was somewhat enhanced (p = 0.06) in comparison with the MVD+CAF group (fig 4B).
We estimated fibronectin within the subgroups. We did not detect changes in lone PAF (0.75 (0.1); n = 26) compared with SR (0.58 (0.17); n = 8) but there was a twofold increase in patients with lone CAF (1.14 (0.11); n = 22) compared with SR (p = 0.08). There were no significant differences between patients in MVD+SR (0.67 (0.19); n = 8), patients with MVD+PAF (0.99 (0.17); n = 10), and patients with MVD+CAF (0.75 (0.09); n = 28) (fig 4C).
DISCUSSION
The present study shows for the first time a clear relation between human AF and the expression of major ECM components such as collagen I, collagen III, and fibronectin. We found an increase of about 100% in collagen I, an increase of about 50% in collagen III (which was confined to MVD+AF), and a smaller non-significant increase in fibronectin in left atrial tissue samples of patients with AF. Thus, our data seem to confirm a relation between AF and fibrosis in both lone AF and MVD+AF. Furthermore, interstitial fibrosis may be the cause of the electrophysiological and structural changes seen in patients with AF in several investigations.15 AF is associated with an increase of connective tissue between individual cells and with the deposition of large amounts of collagen and fibronectin.16 In patients with AF, the degree of fibrosis is augmented and tends to separate myocytes from each other.17,18 Advanced interstitial fibrosis in human AF would predict an impairment of atrial conduction at the microscopic level and may render the atrial myocardium discontinuous resulting in a branching structure.19 Such types of fibrosis may alter the biophysical properties of the tissue allowing the initiation and perpetuation of AF.20
Collagen type I expression was significantly enhanced in both patients with lone AF and patients with MVD+AF compared with the control group. There were no significant differences between lone AF and MVD+AF. However, in both groups we observed a threefold increase (compared with SR) in collagen type I. Interestingly, we found an increase in collagen type III expression in patients with MVD+AF but not in patients with lone AF. The increase in patients with lone AF was only slight (about 40%) and non-significant compared with that in SR, whereas collagen III was significantly increased (about 80%) in the MVD+AF group compared with the SR group. Table 2 presents a simplified summary. Furthermore, left atrial diameter was larger in patients with MVD than in either the control patients or patients with lone AF.21,22 The left atrium in patients with MVD was significantly larger than in patients with SR and lone AF (table 1). Our data suggest that lone AF is enough to activate collagen type I expression. Progressive collagen III accumulation was caused by AF in combination with MVD. A previous study on cultured fibroblasts showed that collagen III mRNA is increased in mechanical stretch conditions, while collagen I mRNA is not altered.23 This suggests that AF with MVD has an impact on the progressive development of connective tissue in forming a fine structured connective tissue network in the atrium. Our quantitative results regarding fibronectin suggest that AF and MVD are not important factors influencing fibronectin synthesis.
Table 2.
Simplified summary of changes in extracellular matrix proteins in patients with AF compared with patients in sinus rhythm
| Collagen type I | Collagen type III | Fibronectin | |
| Lone AF | Increase | No significant change | No significant change |
| MVD+AF | Increase | Increase | No significant change |
AF, atrial fibrillation; MVD+AF, atrial fibrillation with underlying mitral valve disease (MVD+AF).
These data support the hypothesis that remodelling with increased concentrations of collagen I occurs even in lone AF and that the mechanical stress of MVD causes further changes especially in collagen III. The hypothesis that fibrosis progresses systematically from PAF to CAF was not confirmed in either the lone AF group or the MVD+AF group. Considering the data of these subgroups there was a slight increase in collagen from PAF to CAF in lone AF; however, the data varied too much to allow for a clear conclusion. It is known that systematic differences between PAF and CAF exist. Thus, patients with CAF exhibit a shorter cycle length and a higher degree of disorganised activity than do patients with PAF.24,25 Nevertheless, we could not detect significant differences between PAF and CAF regarding changes in ECM components. This is supported by other studies that also found no differences between PAF and CAF in human AF of different causes, such as AF with MVD, coronary artery disease, and lone AF, regarding other pathophysiological factors such as angiotensin and endothelin.26,27
To the best of our knowledge this is the first study that determined the relation between the degree of fibrosis and ECM components and lone AF in human left atrium in patients with PAF and CAF, with or without MVD. Previous studies based on determinations in animals or in the human right atrial appendage did not consider subgroups such as PAF and CAF.16–30 Additionally, the human studies included very mixed patient populations in most cases. Since initiation and perpetuation of AF pathophysiologically depends on the left atrium, it seems important to investigate the left atrium.31 In fact, AF itself can lead to atrial dilatation and from our data we believe that AF is associated with the fibrotic process.32
Conclusion
Forms of AF differ from each other in collagen III expression. However, there was no systematic difference in ECM expression between PAF and CAF. Furthermore, AF is associated with fibrosis. On the basis of cell-cell decoupling and isolation of the myocytes caused by fibrosis, we assume that fibrosis may be an important factor in the maintenance and progression of AF. Furthermore, the irreversible deposition of increased amounts of connective tissue in the atrial tissue, resulting in a modified electrophysiology, may support the theory of Wijffels and colleagues1 that “atrial fibrillation begets atrial fibrillation”. We conclude that the role of enhanced concentrations of ECM proteins may be important in the pathogenesis of AF due to separation of cells by fibrotic depositions.
Abbreviations
AF, atrial fibrillation
CAF, chronic atrial fibrillation
ECM, extracellular matrix
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
MVD, mitral valve disease
PAF, paroxysmal atrial fibrillation
SR, sinus rhythm
REFERENCES
- 1.Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 2.Waktare JEP. Atrial fibrillation. Circulation 2002;106:14–6. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–26. [DOI] [PubMed] [Google Scholar]
- 4.Allessie M, Ausma A, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 5.Todd DM, Walden AP, Fynn SP, et al. Repetitive one-month periods of electrical remodeling promote stability of atrial fibrillation. Circulation 2000;102:154–5. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JE. Regulation of cardiovascular collagen deposition by mechanical forces. Mol Med Today 1998;4:69–75. [DOI] [PubMed] [Google Scholar]
- 7.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev 1999;79:215–62. [DOI] [PubMed] [Google Scholar]
- 8.Lijnen PJ, Petrov VV, Fagard RH. Minireview: induction of cardiac fibrosis by transforming growth factor-beta 1. Mol Genet Metab 2000;71:418–35. [DOI] [PubMed] [Google Scholar]
- 9.Heine H. Grundregulation und extrazelluläre Matrix-Grundlagen und Systematik, 2nd ed. Stuttgart: Hippokrates Verlag, 1997:130–2.
- 10.Vogel V, Thomas WE, Craig DW, et al. Structural insights into the mechanical regulation of molecular recognition sites. Trends Biotechnol 2001;19:416–23. [DOI] [PubMed] [Google Scholar]
- 11.Shaub A. Unravelling the extracellular matrix. Nat Cell Biol 1999;1:173–5. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson MK, Mosher DF. Fibronectin: structure, assembly and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998;18:1363–70. [DOI] [PubMed] [Google Scholar]
- 13.Janse MJ. Why does atrial fibrillation occur? Eur Heart J 1997;18:12–8. [DOI] [PubMed] [Google Scholar]
- 14.Kottkamp H, Hindricks G, Autschbach R, et al. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. J Am Coll Cardiol 2002;40:475–80. [DOI] [PubMed] [Google Scholar]
- 15.Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180–4. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Fareh S, Leung TK, et al. Promotion of atrial fibrillation by heart failure in dogs. Atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 17.Kostin S, Klein G, Szalay H, et al. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res 2002;54:361–79. [DOI] [PubMed] [Google Scholar]
- 18.Schotten U, Ausma J, Stellbrink C, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 2001;103:691–8. [DOI] [PubMed] [Google Scholar]
- 19.Kucera JP, Rudy Y. Mechanistic insights into very slow conduction in branching cardiac tissue. A model study. Circ Res 2001;89:799–806. [DOI] [PubMed] [Google Scholar]
- 20.Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Cardiovasc Res 1986;58:356–71. [DOI] [PubMed] [Google Scholar]
- 21.Tse HF, Lau CP, Cheng G. Relation between mitral regurgitation and platelet activation. J Am Coll Cardiol 1997;30:1813–8. [DOI] [PubMed] [Google Scholar]
- 22.D’Olhaberriague L, Hernandez-Vidal A, Molina L, et al. A prospective study of atrial fibrillation and stroke. Stroke 1989;20:1648–52. [DOI] [PubMed] [Google Scholar]
- 23.Carver W, Nagpal ML, Nachtigal M, et al. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res 1991;69:116–22. [DOI] [PubMed] [Google Scholar]
- 24.Sih HJ, Zipes DP, Berbari EJ, et al. Differences in organization between acute and chronic atrial fibrillation in dogs. J Am Coll Cardiol 2000;36:924–31. [DOI] [PubMed] [Google Scholar]
- 25.Ndrepepa G, Karch MR, Schneider MA, et al. Characterization of paroxysmal and persistent atrial fibrillation in the human left atrium during initiation and sustained episodes. J Cardiovasc Electrophysiol 2002;13:525–32. [DOI] [PubMed] [Google Scholar]
- 26.Goette A, Arndt M, Roecken C, et al. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 2000;101:2678–81. [DOI] [PubMed] [Google Scholar]
- 27.Brundel BJ, Van Gelder IC, Tuinenburg AE, et al. Endothelin system in human persistent and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2001;12:737–42. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen VLJL, van der Velden HMW, van Ankeren EP, et al. Analysis of altered gene expression during sustained atrial fibrillation in the goat. Cardiovasc Res 2002;54:427–37. [DOI] [PubMed] [Google Scholar]
- 29.Goette A, Juenemann G, Peters B, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res 2002;54:390–6. [DOI] [PubMed] [Google Scholar]
- 30.Anyukhovsky EP, Sosunov EA, Plotnikow A, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res 2002;54:462–9. [DOI] [PubMed] [Google Scholar]
- 31.Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001;103:769–77. [DOI] [PubMed] [Google Scholar]
- 32.Thamilarasan M, Klein AL. Factors relating to left atrial enlargement in atrial fibrillation: “chicken or egg” hypothesis. Am Heart J 1999;137:381–3. [DOI] [PubMed] [Google Scholar]