Abstract
The availability of the only drug eluting stent currently approved in the USA has been limited, so that operators often resort to the deployment of multiple undersized stents and post-stenting high pressure inflations with larger balloons to achieve optimal lesion coverage and stent expansion. A case of stent fracture following percutaneous coronary intervention in which this strategy was used is reported.
Keywords: drug eluting stent, stent fracture, sirolimus, stent
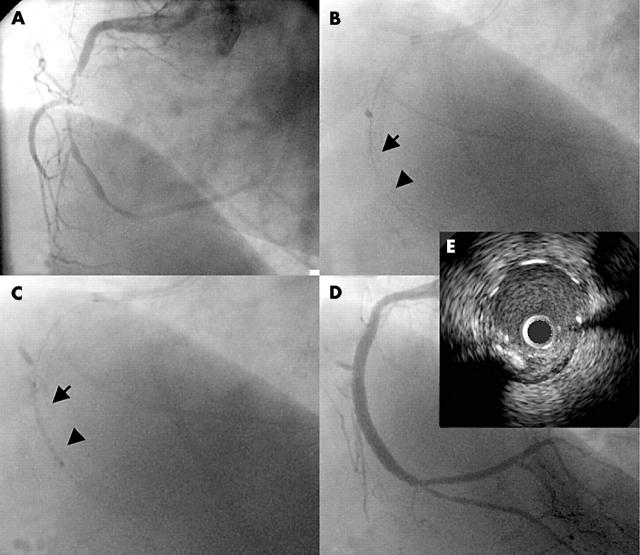
A 58 year old woman with hypertension and hypercholesterolaemia underwent an intravascular ultrasound (IVUS) guided percutaneous coronary intervention for diffuse, high grade stenosis of the right coronary artery (RCA) and occlusion of the posterior descending artery at its ostium. After balloon angioplasty of the RCA lesion and the ostium of the posterior descending artery (not shown), a 2.5/13 mm Cypher stent (Cordis, Miami Lakes, Florida, USA) was deployed in the distal RCA and a second 2.5/18 mm Cypher stent was deployed in the mid-RCA (fig 1B). A gap between the stents was completely covered by a third 2.5/18 mm Cypher stent (fig 1B,C). The proximal stents were post-dilated with a 3.5/15 mm high dilatation force balloon (Quantum Maverick, Boston Scientific, Natick, Massachusetts, USA) at 1215.9 kPa (12 atmospheres), with a good angiographic result (fig 1D). IVUS at that time showed well apposed struts throughout the entire stented segment without identifiable gaps between the stents.
Figure 1.
Right coronary artery (RCA) angiography showing (A) pre-intervention stenosis; and (B) sequential deployment of three Cypher stents in the mid-distal RCA (2.5/13 mm, 2.5/18 mm, and 2.5/18 mm), with contiguous lesion coverage. (B, C) The arrow identifies the distal edge of the proximal stent and the arrowhead points to the proximal edge of the distal stent. (D) Procedural result. (E) Segment of later interest (five months’ follow up) at the end of the index procedure. The intravascular ultrasound (IVUS) image is 2 mm proximal to the distal edge of the proximal stent.
Five months after the intervention, the patient underwent repeat coronary catheterisation (fig 2). RCA angiography showed a discrete area of luminal narrowing in the mid-RCA, estimated visually as a 30% stenosis. IVUS interrogation of this area showed a 1 mm segment uncovered by stent struts that was not considered to limit flow (cross sectional area 7.1 mm2; fig 2D). The distance between the proximal edge of the stented segment and the lesion was 16 mm by IVUS measurement, 2 mm less than the length of the proximally deployed stent. IVUS of this site at the completion of the index procedure showed contiguous strut coverage (fig 1E). Incomplete lesion coverage was thus excluded, leading to strut fracture as the cause of the angiographic finding at follow up.
Figure 2.
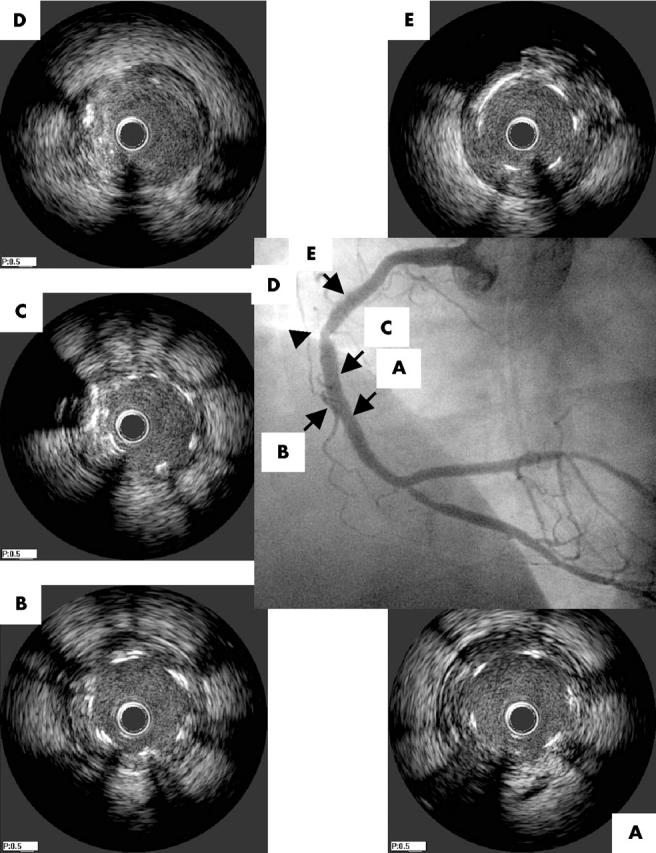
Angiography and IVUS of the RCA five months after the index intervention, showing a 30% stenosis in the mid-RCA within the stented segment (arrowhead). (A, B) Middle stent, distal to and at the overlapping segment; (C) proximal stent, distal to the gap between stent struts; (D) gap corresponding to the angiographic finding (arrowhead) with cross sectional area 7.1 mm2; (E) proximal edge of the proximally deployed stent.
DISCUSSION
Drug eluting stents greatly reduce the risk of in-stent restenosis.1,2 The use of the sirolimus eluting Cypher stent has spread rapidly since its recent approval in the United States but with high demand the availability of this stent has been limited. Consequently, multiple undersized stents and post-stenting dilatation with large balloons are frequently used to achieve complete lesion coverage and stent dimensions commensurate with those of the reference vessel. The clinical outcomes of this practice are unknown.
A small study recently reported a relatively high incidence of discontinuous lesion coverage in patients with in-stent restenosis following Cypher deployment. This was attributed to small gaps between stents at the time of their deployment or to strut fracture,3 but data pertaining to the size of the stents used, stent deployment pressures, and whether post-stenting inflations were performed were not provided. The same investigators separately reported two cases of focal strut fracture in long (33 mm) Cypher stents post-dilated with larger balloons at high inflation pressures.4 In the current case, strut fracture occurred in a shorter (18 mm), six cell stent that is mounted on 2.5–3.0 mm balloons.
Aggressive expansion of a drug eluting stent can potentially limit the effect of the device by altering drug distribution or by incomplete lesion coverage due to strut disruption. No long term data are available on the outcomes of patients treated in this manner, so whether the excellent long term patency rates observed with the Cypher stent in randomised trials can be reproduced by this practice is unknown.
In the current case, IVUS performed at the conclusion of the index procedure excluded deployment related acute stent fracture. Thus, the exact timing and mechanism of delayed strut fracture are unknown. It is possible that multiple mechanical factors such as strut stretching at the time of deployment followed by repetitive kinking of the stent during the cardiac cycle interact to disrupt the strut. Considering that significant restenosis had not developed at the site of the disrupted struts, it may be postulated that strut was fractured long enough after the intervention so that the sirolimus effect was not compromised.
In summary, physicians electing to deploy undersized drug eluting stents should be aware of the risk of strut fracture related to aggressive post-dilatation and should consider routine IVUS evaluation of the stented segment in this context. The clinical consequences of strut fracture and the importance of the temporal relation of this event to stent deployment remain to be determined.
REFERENCES
- 1.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–23. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221–31. [DOI] [PubMed] [Google Scholar]
- 3.Lemos PA, Saia F, Ligthart JM, et al. Coronary restenosis after sirolimus-eluting stent implantation: morphological description and mechanistic analysis from a consecutive series of cases. Circulation 2003;108:257–60. [DOI] [PubMed] [Google Scholar]
- 4.Sianos G , Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv 2004;61:111–6. [DOI] [PubMed] [Google Scholar]