Abstract
The 5′-3′ exoribonucleases Xrn1p and Xrn2p/Rat1p function in the degradation and processing of several classes of RNA in Saccharomyces cerevisiae. Xrn1p is the main enzyme catalyzing cytoplasmic mRNA degradation in multiple decay pathways, whereas Xrn2p/Rat1p functions in the processing of rRNAs and small nucleolar RNAs (snoRNAs) in the nucleus. Much less is known about the XRN-like proteins of multicellular eukaryotes; however, differences in their activities could explain differences in mRNA degradation between multicellular and unicellular eukaryotes. One such difference is the lack in plants and animals of mRNA decay intermediates like those generated in yeast when Xrn1p is blocked by poly(G) tracts that are inserted within mRNAs. We investigated the XRN-family in Arabidopsis thaliana and found it to have several novel features. First, the Arabidopsis genome contains three XRN-like genes (AtXRNs) that are structurally similar to Xrn2p/Rat1p, a characteristic unique to plants. Furthermore, our experimental results and sequence database searches indicate that Xrn1p orthologs may be absent from higher plants. Second, the lack of poly(G) mRNA decay intermediates in plants cannot be explained by the activity of the AtXRNs, because they are blocked by poly(G) tracts. Finally, complementation of yeast mutants and localization studies indicate that two of the AtXRNs likely function in the nucleus, whereas the third acts in the cytoplasm. Thus, the XRN-family in plants is more complex than in other eukaryotes, and, if an XRN-like enzyme plays a role in mRNA decay in plants, the likely participant is a cytoplasmic Xrn2p/Rat1p ortholog, rather than an Xrn1p ortholog.
The 5′-3′ exoribonucleases play key roles in many RNA processing pathways, including mRNA degradation and the processing of rRNA and small nucleolar RNAs (snoRNAs). In the yeast Saccharomyces cerevisiae, Xrn1p and Xrn2p/Rat1p are particularly prominent in these processes. These two exoribonucleases are highly related in sequence and enzymatic activity, but they differ with respect to their main in vivo substrates, intracellular locations, and relative abundance. Xrn1p catalyzes the degradation of the majority of mRNAs, is cytoplasmic, and is highly expressed (1, 2). It is the main enzyme catalyzing mRNA degradation of decapped mRNAs in both the deadenylation-dependent-decapping, as well as the nonsense-mediated decay (NMD) pathways (3). In the deadenylation-dependent decapping pathway, transcripts are deadenylated by an unknown activity, decapped by Dcp1p, then degraded 5′-3′ by Xrn1p. The NMD pathway is similar, except that decapping and subsequent 5′-3′ degradation by Xrn1p is not dependent on prior deadenylation. In addition to mRNA degradation, Xrn1p functions in the maturation of the 5′ ends of rRNAs and degrades rRNA processing intermediates (4, 5). Processing of rRNA 5′ ends is a function shared with Xrn2p/Rat1p. In contrast to Xrn1p, Xrn2p/Rat1p is located primarily in the nucleus, is essential, and is expressed at lower levels than Xrn1p (6, 7). It is believed to be the major activity responsible for trimming the 5′ ends of several rRNAs and also trims the 5′ ends of many snoRNAs during their maturation (8, 9).
Studies of Xrn1p's role in mRNA degradation were aided by the analyses of mRNA decay intermediates. Whereas mRNA degradation intermediates usually do not accumulate to detectable levels in eukaryotic cells, it is possible to trap them in yeast by the insertion of a poly(G) tract into a mRNA. Expression of poly(G)-containing genes in yeast cells results in the accumulation of mRNA decay intermediates that begin at the 5′ end of the poly(G) tract and end at the poly(A) tail, an indication that mRNA degradation is catalyzed from the 5′ end in yeast (10–12). The demonstration that Xrn1p cannot progress through poly(G) tracts in vitro (13), and genetic studies that showed that the generation of the poly(G)-stabilized mRNA decay intermediates in vivo is almost exclusively dependent on Xrn1p (12), implicated Xrn1p as a major enzyme in mRNA degradation. Thus, the ability to generate mRNA decay intermediates by insertion of poly(G) tracts, in combination with studies of xrn1 mutants, was crucial in determining Xrn1p's role in mRNA degradation in yeast cells. However, expression of poly(G)-containing genes in plant or animal cells does not result in the accumulation of poly(G)-stabilized mRNA decay intermediates, despite the presence of XRN-like enzymes in these organisms (refs. 14 and 15; L. Maquat, A.-B. Shyu, G. Goodall, personal communications). The absence of poly(G)-stabilized mRNA decay intermediates in plant and animal cells may indicate that there is likely a difference in the mechanism by which mRNAs are degraded in multicellular eukaryotes compared with yeast.
To investigate this difference, we cloned three members of the Arabidopsis XRN family and examined their enzymatic activities through heterologous expression in yeast. Because the absence of poly(G)-stabilized mRNA decay intermediates in plant cells could most easily be explained by the AtXRNs progressing directly through poly(G) tracts, we investigated their activity on poly(G)-containing mRNAs. All three AtXRNs are blocked by poly(G) tracts when expressed in yeast, indicating that the absence of poly(G)-stabilized mRNA decay intermediates in plant cells is likely due to a novel mechanism. Beyond addressing the absence of poly(G)-stabilized mRNA decay intermediates in plant cells, our experiments provide evidence that the number, type, and intracellular distribution of these Xrn2p/Rat1p orthologs is unique in Arabidopsis, observations that may have important implications for the mechanism of mRNA turnover in higher plants.
Materials and Methods
Cloning of AtXRN3 and AtXRN4 cDNAs and Analyses of Sequences.
The expressed sequence tag (EST) H4B9T7 (accession no. W43714), which contained the entire AtXRN2 ORF, was obtained from the Arabidopsis Biological Resource Center (http://aims.cps.msu.edu/aims/). The 3′ end of a cDNA clone for AtXRN3 was isolated by using the internal SacI to ClaI fragment of H4B9T7 as a probe to screen the PRL2 library (16). The 5′ end of the AtXRN3 cDNA was obtained by rapid amplification of cDNA ends (RACE), using as a template cDNAs generated from 7-day-old Arabidopsis seedlings grown on plates containing 1× Murashiga and Skoog medium and 3% sucrose. These template cDNAs were produced with a Marathon 5′ 3′ RACE kit (CLONTECH). The primers used for amplification were the Marathon AP1 primer and an AtXRN3 cDNA-specific primer PG469 5′-GCTCTGGAAGTGCATGCGAACTTGC-3′. The full-length AtXRN3 sequence was constructed by ligation of 5′ RACE product and partial cDNA. The AtXRN4 cDNA was generated by reverse transcription-PCR and 3′ RACE using the above described seedling cDNAs as template. The 5′ end of the AtXRN4 cDNA was obtained with PG676 5′-CCTTCAAGCTCGAGACCAC-3′, and PG704 5′-CCCGAAGCCGCACCAGTAGAGGA-3′, the 3′ end with PG734 5′-CCCATACCATTATGCTCC-3′ and AP1. The full-length AtXRN4 sequence was constructed by ligation of 5′ and 3′ RT-PCR products into the yeast expression vector PG1 (described below) to generate p2038. The sequences of all PCR products were determined and matched the corresponding genomic sequences.
Complementation of Yeast Mutants.
All of the studies in yeast used derivatives of the shuttle vector pG1 (17) with the AtXRN cDNAs inserted between the BamHI and SalI sites. These plasmids were p1846 (AtXRN2), p1958 (AtXRN3), and p2038 (AtXRN4). Yeast strains yRP841 (MATα, trp1-Δ1, ura3-52, leu2-3,112, lys2-201, cup∷LEU2 pm) and yRP884 (MATa, trp1-Δ1, ura3-52, leu2-3,112, lys2-201, cup∷LEU2 pm, XRN1∷URA3), generously provided by Dr. Roy Parker (Department of Molecular and Cellular Biology, University of Arizona, Tucson, AZ), were used to study the activity of the AtXRNs on poly(G) mRNAs as described (18).
Yeast strains FY86 (MATα, ura3-52, his3Δ200, leu2Δ1) and DAt1-1 (MATα, ura3-52, leu2Δ1, trp1Δ63, rat1-1), generously provided by Dr. Charles Cole (Department of Biochemsitry, Dartmouth Medical School, Hanover, NH), were used to study rat1-1ts complementation (19). Over-night liquid cultures of rat1-1ts transformants were diluted to a similar OD600 and streaked on duplicate plates; one plate was incubated at 26°C and the other at 37°C for 3 days.
Northern Blot Analyses of RNA from Arabidopsis Plants.
Total RNA from most tissues was isolated as described in (20) from Arabidopsis thaliana ecotype Columbia grown in soil for 30 days under standard conditions. The root tissue was harvested from seedlings grown on Murashiga and Skoog medium for 14 days. The gene-specific probes used were the XhoI to NotI fragment of H4B9T7(AtXRN2), the XbaI to NotI fragment of p1958 (AtXRN3), and the XhoI to NotI fragment of p2038 (AtXRN4).
Construction of Green Fluorescent Protein (GFP) Fusions and Localization Studies.
The ORFs of AtXRN2 and AtXRN4 were amplified by PCR and inserted into the NcoI site of pAVA393 (21) for studies in onion epidermal cells. The correct sequence of all PCR products was verified. The primers used were: PG773 5′-CCATGGAACTGTTTTGGGAGG-3′ and PG774 5′-CCATGGGTGTACCGTCGTTTT-3′ for AtXRN2, and PG766 5′-GGAATCCGCCATGGGAGTACCGGC-3′ and PG767 5′-CCATGGACAAGTTTGCACCTGC-3′ for AtXRN4.
For localization studies in yeast, the AtXRN4-GFP fusion was expressed in rat1-1ts from p2039, a pG1 derivative containing an AtXRN4-GFP fusion. Transformed cells were grown overnight at the permissive temperature, diluted to an OD600 similar to that used for rat1-1ts complementation, and photographed with a Kodak DC120 camera (Kodak) and a Zeis Axiophot fluorescence microscope (Zeis) using appropriate filters. Treatment with 20% ethanol was used to facilitate 4′,6-diamidino-2-phenylindole (DAPI) staining and did not effect AtXRN4-GFP localization (data not shown).
Bombardment of onion epidermal cell layers was carried out as described (22), with the exception that 1.0-μm gold particles were used and the amounts of DNA were as indicated in Fig. 5. The plasmids used were: pGFP-GUS and pAVA 367(GFP-NIa) (21), p2046 (AtXRN2-GFP), and p2042 (AtXRN4-GFP). Transformed onion epidermal layers were incubated on plates containing 1× Murashiga and Skoog medium and 3% sucrose for 20–24 h in the dark and then photographed as described above.
Figure 5.
AtXRN2-GFP and AtXRN4-GFP localization. (A) AtXRN4-GFP was expressed in the ratl-lts strain, and GFP fluorescence was compared with DAPI-stained nuclear DNA. The GFP and DAPI images of the boxed cells are shown superimposed (Overlay). (B) Plasmids encoding AtXRN2-GFP and AtXRN4-GFP were used to transform onion epidermal cells by particle bombardment. GFP fluorescence of AtXRN2-GFP and AtXRN4-GFP was compared with that of GFP-GUS, a primarily cytoplasmic protein, and GFP-NIa, which is targeted to the nucleus. The onion epidermal cell images were obtained with a ×20 objective. A ×40 image of AtXRN4-GFP 1 μg is also shown. The arrows indicate the nuclei. (A larger version of Fig. 5. is published as supplemental data on the PNAS web site, www.pnas.org.)
Results
AtXRN2, AtXRN3, and AtXRN4 Are Orthologs of the Saccharomyces cerevisiae Protein Xrn2p/Rat1p.
To identify XRN-like sequences of Arabidopsis, we conducted a search of the GenBank database for sequences similar to Xrn1p. Portions of three chromosomal sequences, TAC K16E1 (accession no. AB022210), BAC F10A5 (accession no. AC006434), BAC F20D21 (accession no. AC005287) and two ESTs, H4B9T7 (accession no. W43714) and H4B8T7 (accession no. W43713), contained sequences highly similar to Xrn1p. The two ESTs correspond to the XRN-like gene present on TAC K16E1. Analysis of the entire sequence of the EST H4B9T7 revealed an ORF highly similar to Xrn1p, as well as to Xrn2p/Rat1p. Due to its greater similarity to Xrn2p/Rat1p, the protein encoded by H4B9T7 was designated AtXRN2 (accession no. AF286720). cDNAs corresponding to the remaining XRN-like sequences were obtained by cDNA library screening and RT-PCR as described in Materials and Methods. The protein encoded by the cDNA corresponding to the XRN-like gene on BAC F10A5 was designated AtXRN3 (accession no. AF286719), and the protein encoded by the cDNA corresponding to the XRN-like gene on BAC F20D21 was designated AtXRN4 (accession no. AF286718).
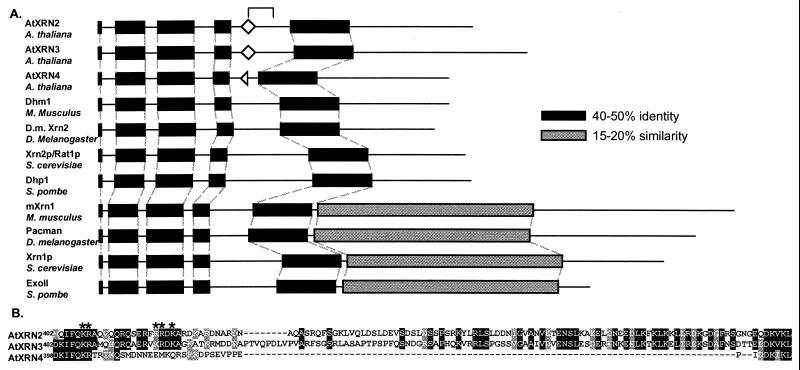
By comparing amino acid sequences, it is possible to distinguish between Xrn1p-like and Xrn2p/Rat1p-like proteins because members of the Xrn1p-like class have a carboxyl-terminal domain specific to this class (Fig. 1A, gray boxes). The AtXRNs lack this carboxyl-terminal domain. An additional characteristic of Xrn1p-like class is the closer spacing of the four N-terminal-most conserved regions relative to that of the Xrn2p/Rat1p-like class (Fig. 1A, black boxes). The spacing of these N-terminal conserved regions in the AtXRNs is most like members of the Xrn2p/Rat1p-like class. Based on these sequence features, we classified the AtXRNs as Xrn2p/Rat1p orthologs.
Figure 1.
The Arabidopsis proteins AtXRN2, AtXRN3, and AtXRN4 are orthologs of the Xrn2p/Rat1p protein of S. cerevisiae. (A) Members of the XRN family were aligned with the program clustalw (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html), revealing conserved regions of the XRN family (black regions) and an Xrn1p-specific domain (gray regions). Bipartite NLS consensus motifs (diamonds), in the regions indicated by the bracket, were identified with the program psort (http://psort.nibb.ac.jp). The half diamond in AtXRN4 indicates the lack of the C-terminal portion of a consensus bipartite, NLS. The AtXRNs, M. musculus Dhm1 (accession no. I49635), S. pombe Dhp1 (accession no. S43891), S. cerevisiae Xrn2p/Rat1p (accession no. NP 014691), D. melanogaster gene product (accession AAF52452), Mus musculus mXrn1 (accession no. CAA62820), D. melanogaster Pacman (accession no. CAB43711), S. cerevisiae Xrn1p (accession no. P22147), and S. pombe ExoII (accession no. P40383) were aligned. (B) The amino acid sequences of the AtXRNs, which correspond to the bracket in A, are shown. Identical residues are shown in black; similar residues are in gray. The basic residues constituting a bipartite NLS found in AtXRN2 and AtXRN3, only part of which is conserved in AtXRN4, are indicated by asterisks.
Additional experiments were carried out to identify sequences from Arabidopsis and other plant species that were more similar to Xrn1p than to Xrn2p/Rat1p; however, no evidence for XRN1-like sequences in plants was obtained. Low stringency Southern blots using the AtXRN2 cDNA as a probe did not indicate the presence of additional XRN-like genes in Arabidopsis other than those reported here (data not shown). Because Xrn1p is highly expressed in yeast (1), we anticipated that an XRN1 ortholog from Arabidopsis might also be highly expressed and be abundant in a cDNA library. However, the screening of the PRL2 library used to clone the AtXRN3 cDNA did not result in the isolation of any XRN1-like sequences. Furthermore, anti-Xrn1p antibodies did not indicate that an Xrn1p ortholog is present in Arabidopsis (P. A. Bariola and P.J.G., unpublished). Similarly, Heyer et al. (1) reported that anti-Xrn1p antibodies did not crossreact with protein extracts from cauliflower, whereas such antibodies crossreacted with proteins from Schizosaccharomyces pombe, and Mus Musculus, organisms known to have Xrn1p-like proteins (1). Finally, extensive searches of sequence databases have not yielded evidence for an XRN1-like gene in Arabidopsis or any other plant species. The absence of XRN1-like sequences from the Arabidopsis genome, over 90% of which has been sequenced, and from the variety of sequences available from other plant species, makes it unlikely that Xrn1p orthologs are present in higher plants.
AtXRN2, AtXRN3, and AtXRN4 Are Expressed in the Major Organs of Arabidopsis.
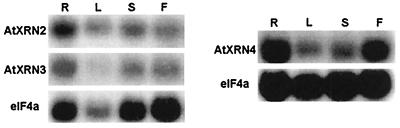
The 3′ ends of the AtXRN cDNAs, including a portion of the ORFs and 3′ untranslated regions, are not conserved and were used to generate gene-specific probes to study the expression of the AtXRNs using Northern blots. These probes were able to specifically recognize the individual AtXRN genes on Southern blots (data not shown). As seen in Fig. 2, all three AtXRN transcripts were detected in roots, leaves, stems, and flowers. The levels of the AtXRN transcripts were similar to each other relative to the control eIF4A (quantitation not shown).
Figure 2.
AtXRN2, AtXRN3, and AtXRN4 are expressed in the major Arabidopsis organs. Northern blot analysis was carried out on 20 μg total RNA isolated from the indicated organs. Gene-specific probes for the AtXRNs, as well as a probe against the eIF4A transcript that served as a control, were used in the hybridization. AtXRN4 was analyzed separately and is shown relative to the eIF4A control for that experiment. R, roots; L, leaves; S, stems; F, flowers.
AtXRN2, AtXRN3, and AtXRN4 Function as 5′-3′ Exoribonucleases and Are Blocked by Poly(G) Tracts When Expressed in Yeast.
The enzymatic activity of the AtXRNs could explain the absence of poly(G)-stabilized mRNA decay intermediates in plants. The simplest explanation could be that the XRN-like enzymes of plants (and possibly other multicellular eukaryotes) can progress directly through poly(G) tracts degrading poly(G)-containing mRNAs to completion. To examine this possibility, and to determine whether the AtXRNs were functional as exoribonucleases, the activity of each of the AtXRNs on poly(G) mRNAs was tested through heterologous expression in yeast.
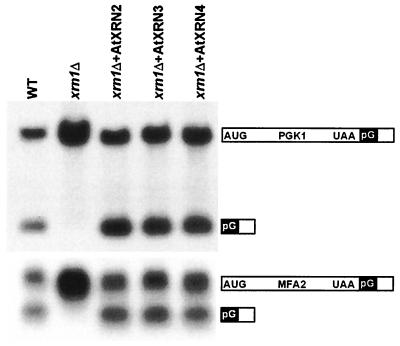
The AtXRN2, AtXRN3, and AtXRN4 proteins were expressed from a multicopy plasmid in wild-type and xrn1Δ yeast strains. These strains expressed two poly(G)-containing mRNAs, PGK1 and MFA2, under the control of the GAL1 upstream activating sequence. For each gene, two transcripts, full-length and poly(G) intermediate, were readily detected in RNA gel blots from wild-type yeast (Fig. 3). In contrast, little or no poly(G) intermediate accumulated for either reporter transcript in xrn1Δ cells as previously observed (Fig. 3, and ref. 11). Expression of AtXRN2, AtXRN3, or AtXRN4 in the xrn1Δ cells resulted in a decrease in the abundance of the full-length reporter mRNAs, and in an increase in the accumulation of the poly(G) intermediates for both reporter transcripts (Fig. 3). This result indicates that all three AtXRNs function as 5′-3′ exoribonucleases, which are able to degrade mRNAs, and that they are blocked by poly(G) tracts when expressed in yeast. Therefore, the absence of poly(G)-stabilized mRNA decay intermediates in plant cells is unlikely due solely to an AtXRN progressing through poly(G) tracts.
Figure 3.
All three AtXRNs function as exoribonucleases, which are blocked by poly(G) tracts when expressed in yeast. The AtXRNs were expressed in the xrn1Δ strain, and the accumulation of the poly(G) reporter mRNAs PGK1 (Top) and MFA2 (Bottom) was analyzed by Northern blot. The structure of the poly(G) reporters is shown at Right, and the AtXRN expressed in the xrnΔ strain is shown above.
AtXRN2 and AtXRN3 but Not AtXRN4 Complements the rat1-1ts Mutation.
All three of the AtXRNs are structurally more similar to Xrn2p/Rat1p than to Xrn1p, indicating that they may have a nuclear function as does Xrn2p/Rat1p. Xrn2p/Rat1p is encoded by an essential gene, and cells harboring a temperature-sensitive allele, rat1-1ts, rapidly arrest growth at the nonpermissive temperature (19). The function of Xrn2p/Rat1p required for cell viability is unknown but is thought to be exoribonuclease activity within the nucleus (7). Coupled with our observation that the AtXRNs have exoribonuclease activity when expressed in yeast (Fig. 3), successful complementation of rat1-1ts would imply nuclear localization in yeast cells.
The AtXRN yeast expression plasmids were introduced into the rat1-1ts strain, and the growth of the transformants on solid medium was monitored. Expression of the AtXRNs did not alter the growth of the rat1-1ts strain at the permissive temperature (Fig. 4). At the nonpermissive temperature, expression of AtXRN2 and AtXRN3 in the rat1-1ts strain restored growth, albeit to a lesser extent than the XRN2/RAT1 control (Fig. 4). This indicated that AtXRN2 and AtXRN3 likely entered the nucleus and replaced the essential function of Xrn2p/Rat1p. In contrast, expression of AtXRN4 did not rescue the growth arrest of the rat1-1ts strain, indicating that it likely did not enter the yeast nucleus.
Figure 4.
AtXRN2 and AtXRN3 but not AtXRN4 complement the rat1-1ts mutation. The rat1-1ts strain was transformed with the expression plasmids used in Fig. 3, and the growth of the transformants was monitored at the permissive (Top) and nonpermissive (Bottom) temperatures.
AtXRN2-GFP Is Targeted to the Nucleus, Whereas AtXRN4-GFP Is Cytoplasmic.
Complementation of rat1-1ts indicated that the AtXRNs likely differ regarding nuclear targeting, and therefore might differ with respect to nuclear localization sequences (NLS). The AtXRNs are about 65% identical to each other in the regions encompassing the XRN family conserved domains (Fig. 1A, black boxes); however, AtXRN4 lacks about 90 amino acids present in AtXRN2 and AtXRN3 (indicated by bracket in Fig. 1A). These 90 amino acids of AtXRN2 and AtXRN3 contain an obvious bipartite NLS beginning at amino acid 407 (Fig. 1A, diamonds). The bipartite NLS is a well characterized motif that targets proteins to the nucleus in plants and other eukaryotes and consists of two basic regions separated by a variable spacer (23). The N-terminal domain of the NLS in also conserved in AtXRN4, but, as a result of the sequence deletion (relative to the other AtXRNs), the C-terminal region of this NLS is absent (Fig. 1B). If AtXRN2 and AtXRN3 were targeted to the nucleus, but AtXRN4 was not, this localization would explain the AtXRNs differential ability to complement rat1-1ts and could indicate that AtXRN4 has a cytoplasmic function.
To examine this possibility, an AtXRN4-GFP fusion was expressed and characterized first in yeast and subsequently in plant cells. The RNase activity of the AtXRN4-GFP fusion was confirmed by its ability to generate a poly(G)-stabilized mRNA decay intermediate when expressed in xrn1Δ cells, and, like AtXRN4, AtXRN4-GFP did not complement rat1-1ts (data not shown). Yeast cells expressing AtXRN4-GFP exhibited two expression patterns, uniform cytoplasmic fluorescence and spots that varied in both size and number (Fig. 5). The uniform fluorescence was distributed evenly across the yeast cells but appeared to be excluded from the nucleus. Exclusion from the nucleus is illustrated by the cells within the box, where a region without fluorescence (indicated by the arrow) corresponds to DAPI-stained nuclear DNA (Fig. 5A, Overlay). Similarly, the spots did not correspond to the nucleus (Fig. 5A, Overlay). Although we cannot rule out that some AtXRN4-GFP may enter the nucleus, these results indicate that the most likely reason for the inability of AtXRN4 to complement rat1-1ts is due to its exclusion from the nucleus.
To examine the intracellular location of the AtXRNs in plant cells, AtXRN-GFP fusion proteins were transiently expressed in onion epidermal cells by particle bombardment (22). As expected, based on successful rat1-1ts complementation, AtXRN2-GFP showed high fluorescence in the nucleus, an expression pattern similar to the nuclear GFP-NIa protein (21) (Fig. 5B). In addition to a general nuclear localization, AtXRN2-GFP accumulated in bright spots within the nucleus, which may represent the nucleoli. As an ortholog of Xrn2p/Rat1p of yeast, AtXRN2 may also function in rRNA processing and could be targeted to this subnuclear region. Similar to AtXRN2-GFP, transient expression of AtXRN3-GFP in onion epidermal cells revealed a nuclear localization (data not shown). In contrast to the intense nuclear fluorescence of AtXRN2-GFP, AtXRN4-GFP appeared more similar to GFP-GUS, a protein known to accumulate in the cytoplasm (21). In addition, similar to its expression in yeast, AtXRN4-GFP accumulated as both uniform fluorescence and as spots that were not detected within the nucleus (Fig. 5B). Because the AtXRN4-GFP spots may have been cytoplasmic inclusions (24), we tested whether lowering AtXRN4-GFP expression would diminish the number of spots. As seen in Fig. 5B, (AtXRN4-GFP 1 μg), reducing the amount of DNA used in the bombardment resulted in a reduction in the number and size of the spots. A higher magnification shows uniform AtXRN4-GFP fluorescence and the reduction in the number of spots (AtXRN4-GFP 1 μg, ×40). Thus, reducing the amount of DNA decreases the number and size of the spots, while not effecting the cytoplasmic localization of AtXRN4-GFP.
Discussion
In this study, we investigated the XRN-family in Arabidopsis and discovered it has several features not found in other eukaryotes. The Arabidopsis genome encodes an unusual number and distribution of XRN enzymes. All three AtXRNs are Xrn2p/Rat1p orthologs, and Xrn1p orthologs are apparently absent from higher plants. The absence of an Xrn1p ortholog is surprising because they have been described from S. pombe (25), M. musculus (26), and D. melanogaster (27) and are present in sequence databases from a variety of other eukaryotes (unpublished data). The presence of Xrn1p orthologs in many eukaryotes indicates that Xrn1p-like function may be conserved in eukaryotes; however, the apparent lack of an Xrn1p ortholog from plants would require that plants carry out this function with a different protein. This study indicates that Xrn1p-like function may be carried out by AtXRN4-like enzymes in higher plants. AtXRN4 functions as an exoribonuclease and is cytoplasmic where it could function in the degradation of mRNAs.
A potential difference in the mechanisms of mRNA decay between yeast and multicellular eukaryotes is indicated by the accumulation of poly(G)-stabilized mRNA decay intermediates in yeast, but not in higher eukaryotes (refs. 14 and 15; L. Maquat, A.-B. Shyu, and G. Goodall, personal communications). In plant cells, expression of mRNAs containing poly(G) tracts does not lead to the accumulation of poly(G)-stabilized mRNA decay intermediates like those observed when Xrn1p is blocked by the insertion of poly(G) tracts in mRNAs in yeast (14). One possible explanation for the absence of poly(G)-stabilized mRNA decay intermediates in higher eukaryotes may be that the XRNs from multicellular eukaryotes are more robust than their yeast counterparts and can progress directly through poly(G) tracts. Alternatively, these XRNs may be blocked by poly(G) tracts, but additional proteins, such as RNA helicases, resolve the structures formed by poly(G) tracts, thus allowing XRN-like enzymes to complete degradation. It is also possible that a highly active 3′-5′ mRNA degradation pathway in plants degrades through poly(G) tracts from the 3′ end. However, whereas our experiments in plant cells were designed to detect poly(G)-stabilized mRNA decay intermediates because of the action of a 3′-5′ exoribonuclease-mediated pathway, or intermediates because of degradation from both ends of the mRNA simultaneously [“poly(G) stub” (28)], such intermediates were not detected. A 3′-5′ pathway in plants would therefore also likely require the activity of an RNA helicase to degrade poly(G)-containing mRNAs. RNA helicases are likely associated with mRNA degradation machinery in eukaryotes (29), and, because it is known that greater than 30 RNA helicase-like genes are present in the Arabidopsis genome (30), such activities could be quite prevalent in the cytoplasm. It is unlikely that blockage of XRN proteins by poly(G) tracts in yeast depends on cellular factors particular to yeast, because poly(G) tracts and other stable secondary structures inhibit both Xrn1p and Xrn2p/Rat1p in vitro with only purified components present (13). Although the XRNs from animals have not been directly tested for their ability to progress through poly(G) tracts, the five XRNs that have so far been tested do not progress through poly(G). These results indicate that blockage by poly(G) tracts is likely an inherent property of XRN enzymes, and that the absence of poly(G)-stabilized mRNA decay intermediates in animal cells is likely due to a similar reason as in plants.
Several observations indicate that some mRNAs in plants may be degraded, presumably in the cytoplasm, by 5′-3′ exoribonuclease-mediated pathways that could involve AtXRN4 in Arabidopsis, or AtXRN4-like enzymes of other plants. The most compelling evidence comes from studies of degradation intermediates of phyA mRNA in oat seedlings. The majority of these intermediates exist as a series of transcripts lacking increasing amounts of the 5′ end, consistent with degradation mediated by a 5′-3′ exoribonuclease (31). Degradation of at least some of mRNAs from the 5′ end in plants may resemble the major mRNA decay pathway in yeast in which mRNAs are degraded from the 5′ end by Xrn1p after their deadenylation and decapping. In addition to AtXRN4, orthologs of other components relevant to this pathway exist in the Arabidopsis genome (32), including PAB2. PAB2 is a poly(A) binding protein that is able to function in the coupling of deadenylation to mRNA decay when expressed in yeast (33). Alternatively, endoribonuclease cleavage may initiate mRNA decay, with AtXRN4 catalyzing the degradation of the products of endoribonuclease cleavage. Such a mechanism has been proposed for the degradation of SRS4 mRNAs in petunia (34), as well as for the degradation of mRNAs in a variety of plant species targeted by posttranscriptional gene silencing (35–37). Posttranscriptional gene silencing is a phenomenon which occurs in plants, animals, and fungi in which mRNAs that share a high degree of similarity are selectively degraded (reviewed in ref. 38). Because both cytoplasmic and nuclear mRNA decay may be involved in this process, any of the AtXRNs could participate.
The presence of three Xrn2p/Rat1p orthologs in Arabidopsis is a particularly interesting aspect of the XRN family in Arabidopsis. Because AtXRN2 and AtXRN3 are both targeted to the nucleus, they may have functions similar to Xrn2p/Rat1p, such as rRNA or snoRNA processing (8, 9). These functions may be redundant, substrate-specific, or specific to particular cell types. Insight into these possibilities may be gained by a more detailed analysis of their expression patterns in different cells, intranuclear distributions, and enzymatic activities. Another implication of multiple XRN2/RAT1 orthologs in Arabidopsis is the potential to gain insight into the evolution of gene function. The three AtXRN genes are likely due to gene duplication. The deletion of a bipartite NLS during duplication may have given rise to an AtXRN4-like protein. This protein could then function in the cytoplasm, and may have eventually replaced a Xrn1p ortholog. That a Xrn2p/Rat1p protein can replace Xrn1p function is supported by the observation that Xrn2p/Rat1p expressed in the cytoplasm of yeast can complement an xrn1Δ strain (7). Therefore, mRNA degradation in plants may resemble the major mRNA decay pathway of yeast; however, in contrast to yeast, the final degradation of mRNAs could be catalyzed by an Xrn2p/Rat1p-like enzyme such as AtXRN4. mRNA degradation catalyzed by an Xrn2p/Rat1p ortholog may be unique to plants, because this is the only eukaryotic kingdom from which XRN1-like genes are apparently absent.
Ultimately, it should be possible to determine the substrates and functions of the AtXRNs in Arabidopsis by the isolation and characterization of xrn mutants. Analyses of these mutants may aid in dissecting the potentially overlapping roles of AtXRN2 and AtXRN3 in the nucleus. It should be noted that analyses of xrn1 and xrn2/rat1 mutants has implicated Xrn1p as having a meiosis-specific role independent from its exoribonuclease activity (39), and Xrn2p/Rat1p in nucleocytoplasmic mRNA trafficking (19). Thus, analysis of Arabidopsis xrn mutants may provide further insight not only into mRNA decay and RNA processing in plants, but also into functions of the XRN-family in general.
Supplementary Material
Acknowledgments
We thank Dr. Mark Johnson and Dr. Ambro van Hoof for helpful comments on this manuscript, Chris Behrens for contributing to the localization studies, and Linda Danhof for expert technical assistance. This work was supported by the National Science Foundation (Grant IBN 9408052), U.S. Department of Energy (Grant DE-FG02-91E920021), and the U.S. Department of Agriculture (Grant 9801498).
Abbreviations
- snoRNA
small nucleolar RNA
- EST
expressed sequence tag
- RACE
rapid amplification of cDNA ends
- GFP
green fluorescent protein
- DAPI
4′,6-diamidino-2-phenylindole
- NLS
nuclear localization sequence
Footnotes
References
- 1.Heyer W-D, Johnson A W, Reinhart U, Kolodner R D. Mol Cell Biol. 1995;15:2728–2736. doi: 10.1128/mcb.15.5.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu C L, Stevens A. Mol Cell Biol. 1993;13:4826–4835. doi: 10.1128/mcb.13.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tharun S, Parker R. In: mRNA Metabolism & Post-Transcriptional Gene Regulation. Harford J B, Morris D, editors. New York: Wiley-Liss; 1997. pp. 181–200. [Google Scholar]
- 4.Henry Y, Wood H, Morrissey J P, Petfalski E, Kearsey S, Tollervey D. EMBO J. 1994;13:2452–2463. doi: 10.1002/j.1460-2075.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens A, Hsu C L, Isham K R, Larimer F W. J Bacteriol. 1991;21:7024–7028. doi: 10.1128/jb.173.21.7024-7028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenna M, Stevens A, McCannon M, Douglas M G. Mol Cell Biol. 1993;13:341–350. doi: 10.1128/mcb.13.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson A W. Mol Cell Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petfalski E, Dandekar T, Henry Y, Tollervey D. Mol Cell Biol. 1998;18:1181–1189. doi: 10.1128/mcb.18.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villa T, Ceradini F, Presutti C, Bozzoni I. Mol Cell Biol. 2000;18:3376–3383. doi: 10.1128/mcb.18.6.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vreken P, Raué H A. Mol Cell Biol. 1992;12:2986–2996. doi: 10.1128/mcb.12.7.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker C J, Parker R. Genes Dev. 1993;7:1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- 12.Muhlrad D, Decker C J, Parker R. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- 13.Poole T L, Stevens A. Biochem Biophys Res Commun. 1997;235:799–805. doi: 10.1006/bbrc.1997.6877. [DOI] [PubMed] [Google Scholar]
- 14.Johnson M A. Dissertation. East Lansing, MI: Michigan State University; 2000. [Google Scholar]
- 15.Kastenmayer J P, van Hoof A, Johnson M, Green P J. In: Plant Molecular Biology. Raikhel N, Last R, Morelli G, LaShavo F, editors. Berlin: Springer; 1998. pp. 125–133. [Google Scholar]
- 16.Newman T C, de Bruijn F, Green P J, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N V, Somerville S C, Tomashow M, et al. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schena M, Picard D, Yamamoto K R. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- 18.Caponigro G, Parker R. Genes Dev. 1995;9:2421–2432. doi: 10.1101/gad.9.19.2421. [DOI] [PubMed] [Google Scholar]
- 19.Amberg D C, Goldstein A L, Cole C N. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C B, Bariola P A, DelCardayré S B, Raines R T, Green P J. Proc Natl Acad Sci USA. 1993;90:5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Arnim A G, Deng X W, Stacey M G. Gene. 1998;221:35–43. doi: 10.1016/s0378-1119(98)00433-8. [DOI] [PubMed] [Google Scholar]
- 22.Vargona M J, Schmidt R J, Raikhel N. Plant Cell. 1992;4:1213–1227. doi: 10.1105/tpc.4.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks G R, Raikhel N. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- 24.Stacey M G, Hicks S N, von Arnim A G. Plant Cell. 1999;11:349–363. doi: 10.1105/tpc.11.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szankasi P, Smith G R. Curr Genet. 1996;30:284–293. doi: 10.1007/s002940050134. [DOI] [PubMed] [Google Scholar]
- 26.Bashkirov V I, Scherthan H, Solinger J A, Buerstedde J-M, Heyer W-D. J Cell Biol. 1997;136:761–773. doi: 10.1083/jcb.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Till D D, Linz B, Seago J E, Elgar S J, Marujo P E, Elias M D, Arraiano C M, McClellan J A, McCarthy J E G, Newbury S F. Mech Dev. 1998;79:51–55. doi: 10.1016/s0925-4773(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs Anderson J S, Parker R. EMBO J. 1998;17:1497–1506. doi: 10.1093/emboj/17.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs Anderson J S, Parker R. Curr Biol. 1996;6:780–782. doi: 10.1016/s0960-9822(02)00593-6. [DOI] [PubMed] [Google Scholar]
- 30.Aubourg S, Kreis M, Lecharny A. Nucleic Acids Res. 999;27:628–636. doi: 10.1093/nar/27.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgs D C, Colbert J T. Plant Cell. 1994;6:1007–1019. doi: 10.1105/tpc.6.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez R A, MacIntosh G C, Green P J. Trends Plant Sci. 1999;4:429–438. doi: 10.1016/s1360-1385(99)01484-3. [DOI] [PubMed] [Google Scholar]
- 33.Palanivelu R, Belostotsky D A, Meagher R B. Plant J. 2000;22:187–198. doi: 10.1046/j.1365-313x.2000.00721.x. [DOI] [PubMed] [Google Scholar]
- 34.Tanzer M M, Meagher R B. Mol Cell Biol. 1995;15:6641–6652. doi: 10.1128/mcb.15.12.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanzer M M, Thompson W F, Law M D, Wernsman E A, Uknes S. Plant Cell. 1997;9:1411–1423. doi: 10.1105/tpc.9.8.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Eldik G J, Litiere K, Jacobs J J, Van Montagu M, Cornelissen M. Nucleic Acids Res. 1998;26:5176–5181. doi: 10.1093/nar/26.22.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzlaff M, O'Dell M, Cluster P D, Flavell R B. Cell. 1997;88:845–854. doi: 10.1016/s0092-8674(00)81930-3. [DOI] [PubMed] [Google Scholar]
- 38.Sijen T, Kooter J M. BioEssays. 2000;22:520–531. doi: 10.1002/(SICI)1521-1878(200006)22:6<520::AID-BIES5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Solinger J A, Pascolini D, Heyer W D. Mol Cell Biol. 1999;9:5930–5942. doi: 10.1128/mcb.19.9.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.