Coarctation of the aorta was described pathologically in the 1700s and clinical recognition occurred from the early 1900s.w1 w2 Postmortem data suggested a median age of death of 31 years—mainly from complications of the coarctation (cardiac failure in 26%, aortic rupture in 21%, bacterial endarteritis in 18%, and intracranial haemorrhage in 12%.w3 w4 The presurgical “natural” history data were similar accepting the case selection bias prevalent in that era.w5 Surgical repair was first described in 1945, balloon dilation in 1982, and stent implantation in 1991.w6–8
Currently, the clinical diagnosis (murmur, upper limb hypertension, and absent or diminished femoral pulses) can rapidly be confirmed non-invasively by echocardiography and magnetic resonance imaging (MRI), and monitored by these techniques following treatment (fig 1). Radio-femoral delay is a poor marker for follow up. 1w9 The vast majority of coarctations are identified and treated in the first year of life and adult presentation is becoming less frequent.
Figure 1.
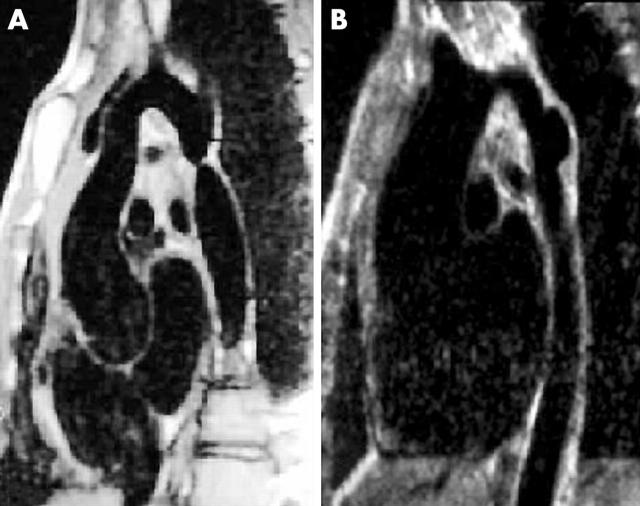
Magnetic resonance imaging scans of native coarctation in a 32 year old man (A) and aneurysm at balloon dilation site in a 14 year old boy (B).
Coarctation of the aorta accounts for approximately 7% of all live births with congenital heart disease and is 1.7 times more common in males.w10 w11 Hypoplasia of the aortic arch to various degrees is a common accompanying feature.w12 There is a significant association with other cardiac lesions (ventricular septal defect, patent arterial duct, and bicuspid aortic valve (in up to 50%) commonly but also with complex defects); in some the prognosis depends more on the outcome of the associated lesions which requires simultaneous management with the coarctation while in others the associated lesion is mild and can be treated on its own merits. In Turner’s syndrome (46 XO) coarctation is present in 10% of patients.w13
While coarctation is readily diagnosed and treated in general, there are a few areas of contention and uncertainty. A serious concern is the failure to diagnose reliably the condition antenatally or at birth, so that even in the current era neonates who are clinically normal remain undiagnosed until they present with a sudden collapse and are at risk of considerable morbidity and mortality. While choice of the primary therapeutic intervention may generate some controversy, perhaps the most uncertain area is the definition of satisfactory correction and whether this can be considered a cure. It is unclear how important it is to obtain a “perfect result” with treatment and whether a mild degree of recoarctation can be accepted.2 w14 There is doubt as to whether all mild native coarctations should be treated.
FETAL DIAGNOSIS
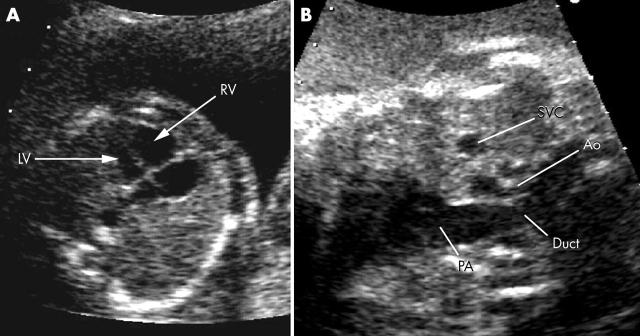
Before birth the fetal circulation is able to cope with coarctation (indeed it also copes with complete interruption at the coarctation site). Venous return to the left ventricle is via the foramen ovale and its output is via the ascending aorta to the head and arms. Pressure in the right ventricle is the same as in the left ventricle and the majority of its output is directed via the arterial duct to the descending aorta to supply the abdomen and legs. Thus blood pressure above and below the potential coarctation site is identical with little flow across the isthmus between the left subclavian artery and the arterial duct (fig 2). It is therefore surprising that the cardiovascular system responds to the coarctation in utero, with right ventricular dilation that can be detected by fetal echocardiography (fig 3A).w15 Right ventricular dilation is, however, not a specific sign of postnatal coarctation with a significant number of false positives—especially when based on right ventricular dilation only in late gestation.3 In those with more severe coarctation or with additional transverse arch hypoplasia, there is a significant disproportion in the size of the aortic arch (smaller than normal) and main pulmonary artery (larger than normal) (fig 3B).
Figure 2.
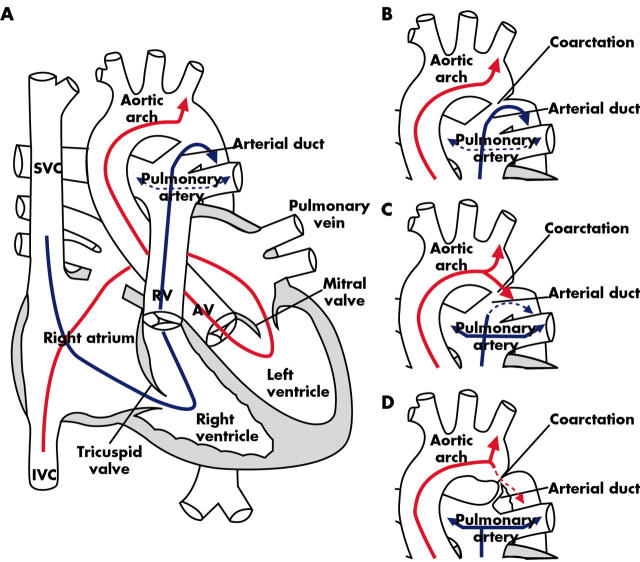
Diagram of normal fetal circulation. (A) Superior vena cava (SVC) blood flow (blue arrow) is directed through the tricuspid valve to the pulmonary artery and via the arterial duct to the lower body segment. Inferior vena cava (IVC) blood flow (red arrow) containing oxygenated blood from the placenta is directed across the foramen ovale to the left ventricle, ascending aorta and upper body segment with little flow across the isthmus between the left subclavian artery and the arterial duct. (B) Coarctation of the aorta in utero does not affect the fetal blood flow pattern. (C) After birth there is a fall in pulmonary resistance with increased pulmonary blood flow (blue arrows) and forward flow from the aortic arch to the descending aorta (red arrow). (D) As the duct constricts, the narrowing of the coarctation is accentuated and the increasing obstruction leads to a gradient (red dotted line).
Figure 3.
(A) Disproportion between the right and left ventricles is striking. (B) Great vessel disproportion between the aorta (Ao) and pulmonary artery (PA) is more specific for coarctation than ventricular disproportion. LV, left ventricle, RV, right ventricle; SVC, superior vena cava.
Thus the antenatal findings are of ventricular disproportion (earlier in gestation being more important) and great vessel disproportion (more sensitive than ventricular disproportion alone). The coarctation site itself is not easily identifiable because of the lack of flow and the presence of the arterial duct that overlies it (figs 2B and 3B). The ability to detect these changes is variable, with a high success rate in fetal cardiology units but only a low rate in general obstetric units where great vessel assessment is not routine and mild ventricular disproportion alone is ignored.w16 w17 The presence of significant extracardiac fetal abnormalities or markers of chromosomal anomalies improves the detection rate as detailed fetal echocardiography is then undertaken.w18 In a large fetal echocardiography series of 144 fetuses with suspected coarctation, two out of three were normal at birth. Of those suspected to have a coarctation and a ventricular septal defect, two thirds had a ventricular septal defect only postnatally.3 In some fetuses, the ventricular disproportion is so pronounced that it is difficult to be certain that the fetus is not progressing to a hypoplastic left heart syndrome. In these there may be reverse shunting across the foramen ovale and continued evaluation is required until term.
NEONATAL AND INFANTILE PRESENTATION
Antenatally diagnosed
Where there is strong suspicion antenatally that a severe coarctation with aortic arch hypoplasia is likely (and the neonate is at risk from duct closure), the fetus should be delivered in a paediatric cardiology centre and a prostaglandin infusion commenced immediately to maintain ductal patency. The early echocardiogram is revealing and when it confirms arch hypoplasia and a posterior shelf at the coarctation site, prostaglandins are maintained until surgical repair a few days later. Where the suspicion is less strong, the fetus is delivered and observed closely without prostaglandins. As long as there are no features on echocardiography as above, the duct is allowed to close spontaneously with daily or alternate daily echocardiograms and regular monitoring of the four limb blood pressure and femoral pulses until it is clear whether a coarctation is present or not. Those without a coarctation are allowed home after duct closure but should be reviewed again in 4–6 weeks and finally at six months as there is occasional late detection of coarctation in this cohort.3
Postnatally diagnosed
Neonates who collapse are fully resuscitated before transfer and surgery. Intubation, mechanical ventilation, inotropes, and correction of the severe metabolic acidosis are needed. Prostaglandins are given in an attempt to reopen the arterial duct whose closure usually precedes the collapse. Duct closure causes collapse by a number of mechanisms. In some, where the coarctation is very severe, all lower body blood flow has been via the duct; once it closes, lower body hypoperfusion leads to profound acidosis and ventricular dysfunction. In others the ampulla of the duct serves as a bypass channel past the coarctation shelf—obstruction increases as the duct closes. There is also ductal tissue in the coarctation segment of the aorta, which constricts the aorta further as the duct closes. Closure of the duct does not always lead to instantaneous collapse, but with severe coarctation the raised left ventricular afterload is eventually not tolerated any longer.
Neonates and infants in whom the diagnosis is established because of absent femoral pulses, a murmur, or development of mild cardiac failure are admitted at the time of diagnosis for surgery. Rarely the infant presents with the appearance of a dilated cardiomyopathy and a low gradient across the coarctation (caused by poor cardiac output), which can cause diagnostic difficulty.
CHILDHOOD, ADOLESCENT, AND ADULT PRESENTATION
In later life the finding of a coarctation is usually an incidental discovery, although in some symptoms do occur which may even be life threatening (table 1).w19
Table 1.
Presentation of coarctation
| • Fetus |
| - ventricular disproportion |
| - great vessel disproportion |
| - associated with other congenital heart disease |
| - nuchal thickening/chromosomal abnormality (Turner’s syndrome) |
| • Neonate |
| - collapse, acidosis |
| - heart failure |
| - systolic/continuous murmur conducted to back |
| - weak or absent femoral pulses |
| - upper limb hypertension |
| • Infant |
| - heart failure |
| - systolic/continuous murmur conducted to back |
| - weak or absent femoral pulses |
| - upper limb hypertension |
| - cardiomyopathy rarely |
| • Child, adolescent, and adult |
| - systolic/continuous murmur conducted to back (collateral murmurs over scapula rarely) |
| - weak or absent femoral pulses (radio-femoral delay in older patients) |
| - upper limb hypertension |
| - exercise intolerance |
| - leg fatigue and claudication |
| - cold feet |
| - cardiac arrest (left ventricular hypertrophy and arrhythmia) |
| - hypertensive retinopathy |
| - intracranial bleed |
| - aortic dissection/rupture |
| - infective endocarditis |
Diagnostic findings
Findings on chest x ray and ECG range from none to signs of ventricular hypertrophy and dilation. Rib notching from collateral vessels and the “3” sign of indentation in the aortic shadow are seen on the chest x ray in older children and adults.
TREATMENT
Surgical repair
Surgical repair is usually performed via a left lateral thoracotomy and involves clamping the aorta above and below the coarctation segment so that lower body blood flow is only via collaterals (if they have developed). The three standard approaches were resection and end-to-end anastomosis, subclavian flap repair (ligation of distal subclavian artery to use the proximal portion to overlay patch the coarctation segment), and patching with foreign material (usually a Dacron patch), while interposition grafting or carotid to descending aorta shunting were used much less frequently.w20–24 All three techniques were accompanied by a low rate of morbidity and mortality but a definite rate of recoarctation that was highest when performed in neonates (up to 19%) and infants.4 The Dacron patch technique was also accompanied by a high incidence of aneurysms at the repair site and is no longer used.w25 w26 The subclavian flap technique resulted in loss of the pulses in the left arm, which were eventually reconstituted via collaterals. Blood pressure remained lower in this limb and occasionally there was a reduction in arm length.w27 It was not used in adults for fear of limb ischaemia. Very rarely claudication or a subclavian steal syndrome could occur in later life.w28
While resection and end-to-end anastomosis remains the mainstay of surgery, modifications to treat simultaneously the hypoplastic transverse arch have developed. These include the end-to-side anastomosis and extended aortic arch repairs.5w29 w30 In addition to resecting the coarctation segment, the descending aorta is anastomosed onto the underside of the aortic arch thereby shortening the arch and simultaneously correcting the coexistent arch hypoplasia—during surgery the upper clamp needs to include the left carotid artery. Currently this can be performed with a low morbidity and mortality and a recoarctation rate as low as 3%.6 Where the arch is particularly hypoplastic, homograft tissue can be used to augment the repair, which in that situation may need to be performed via a midline sternotomy on cardiopulmonary bypass (which is also used when additional cardiac lesions are repaired at the same time). Whether this approach is necessary for localised coarctation with mild transverse arch hypoplasia or even at all is subject to controversy. Many believe that adequate treatment of the coarctation site alone results in rapid remodelling of the hypoplastic arch though others have found a significant incidence of arch obstruction after successful coarctation repair.7w31–33
Paraplegia as a complication of surgery occurs in 0.3% and is related to dissection and interruption of collateral and intercostal vessels or when collateral vessels around the coarctation are absent with low distal pressure during aortic cross clamping (table 2). It seems to be more of a problem for repeat surgery involving extensive dissection when the collaterals have diminished and may be as high as 2.6%.w34–36 Paraplegia can occur weeks after surgery.8 There has been a move to perform late complicated repeat surgery with cardiopulmonary bypass and deep circulatory arrest or with left heart bypass through a lateral thoracotomy, reducing the earlier morbidity and mortality and allowing full rather than palliative treatment.w36–39
Table 2.
Complications of treatment
| • Left thoracotomy approach |
| - bleeding, haemothorax |
| - chylothorax |
| - recurrent laryngeal nerve palsy |
| - phrenic nerve palsy |
| - Horner’s syndrome |
| - paradoxical hypertension (risk of suture leak) |
| - paraplegia |
| - left arm impaired growth/ischaemia (subclavian flap repair) |
| - vertebro-basilar ischaemia (subclavian flap repair) |
| - cerebral ischaemia (extended arch repair) |
| • Cardiopulmonary bypass |
| - bleeding |
| - cerebral ischaemia |
| - myocardial dysfunction |
| - recurrent laryngeal nerve palsy |
| - Horner’s syndrome |
| - paradoxical hypertension (risk of suture leak) |
| • Catheter intervention |
| - femoral artery occlusion/pseudoaneurysm |
| - aortic dissection/aneurysm/rupture |
| - paraplegia (? with covered stent) |
| - stent migration/malposition |
Catheter intervention
Balloon dilation was introduced for the treatment of recoarctation to avoid the risks of repeat surgery and subsequently used to treat native coarctation.w40 Its mechanism of action is by stretching and tearing of the thickened intimal shelf and adjacent media layers. Tears of the adjacent “normal” aortic wall also occur, perhaps reflecting an inherent weakness in the aorta in patients with coarctation.w41 Limiting the damage to the coarctation shelf could not be guaranteed and it gained only modest acceptance in native coarctation where there was less surgical scarring to support the aortic wall and where the results of surgery were felt to be superior. Acute rupture and dissection is rare but aneurysms can develop later.w42 It is generally avoided in the first 6–12 months of life because of a high incidence of recoarctation and femoral artery damage.w43 A single randomised trial comparing balloon dilation with surgery revealed aneurysms in 0% of the surgical patients and 20% in the patients undergoing balloon dilation, although none were significant enough to need an intervention.w44 There are numerous reports, however, of balloon dilation series without any aneurysm formation and equally of surgical series with aneurysms, so the influence of this one small study has been small.9w45–47 Overall the long term results are favourable and balloon dilation remains a first line option in many centres.10 w48
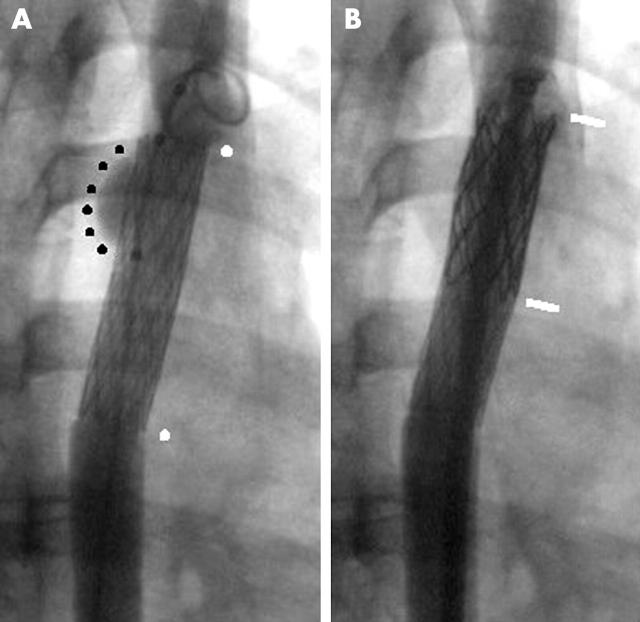
The safety and efficacy of catheter intervention were considerably increased with the advent of percutaneous stent implantation.w8 The rationale for stent implantation is that over dilation of the coarctation segment is unnecessary, thus avoiding major transmural tears, while at the same time the stent struts will splint any smaller tears against the aortic wall preventing progressive dissection and aneurysm formation. The acute elastic recoil of the coarctation segment that contributes to a suboptimal initial result and later recoarctation is prevented by stent implantation, leading to a greater relief of obstruction than with balloon dilation alone. The size of the delivery sheath that is required, however, precludes use in most children under 25 kg. While mild recoarctations were not amenable to balloon dilation because of the need for large balloons to overcome the natural recoil and carrying greater risks of tearing, stent implantation allowed this type of lesion to be easily treated albeit with a small risk of stent migration.w49 w50 Excessive stretching of a tight coarctation, however, could still lead to aneurysm formation and rupture so graded dilation, allowing for healing before further dilation, and covered stents are now employed to prevent this (fig 4).11w51 A particular role for stent implantation is in the situation where aortic valve or root replacement or coronary bypass grafting is needed. Presurgical stenting of the coarctation segment removes the need to dissect the coarctation area or try to recover from bypass in the face of an aortic obstruction (fig 5).w52 w53 Covered stents now allow endovascular repair of associated aneurysms whether native, after surgery or catheter intervention. For localised aneurysms and those in association with residual coarctation, stent delivery is via a percutaneous approach (fig 6). For extensive aneurysm formation, large custom made self expanding stents are used which are mounted on 22–24 French delivery systems and require femoral or iliac exposure.w54
Figure 4.
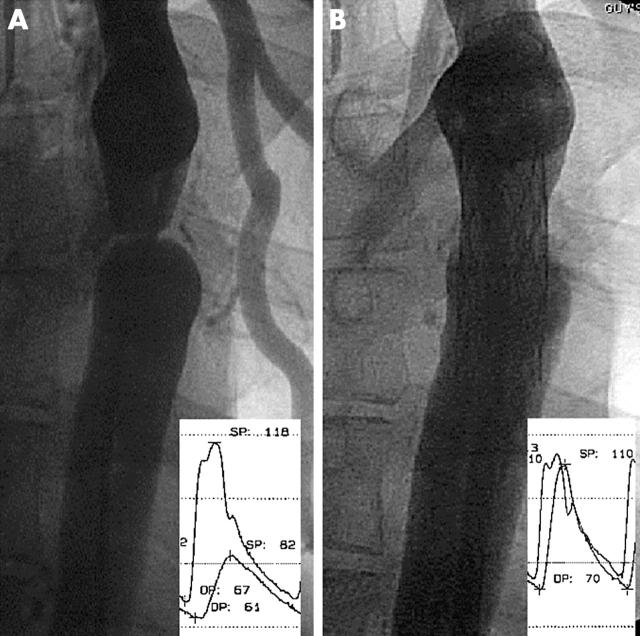
(A) Severe native coarctation in adult with large gradient and collateral vessels. (B) Stent implantation to 80% of the final aortic diameter abolishes the gradient.
Figure 5.
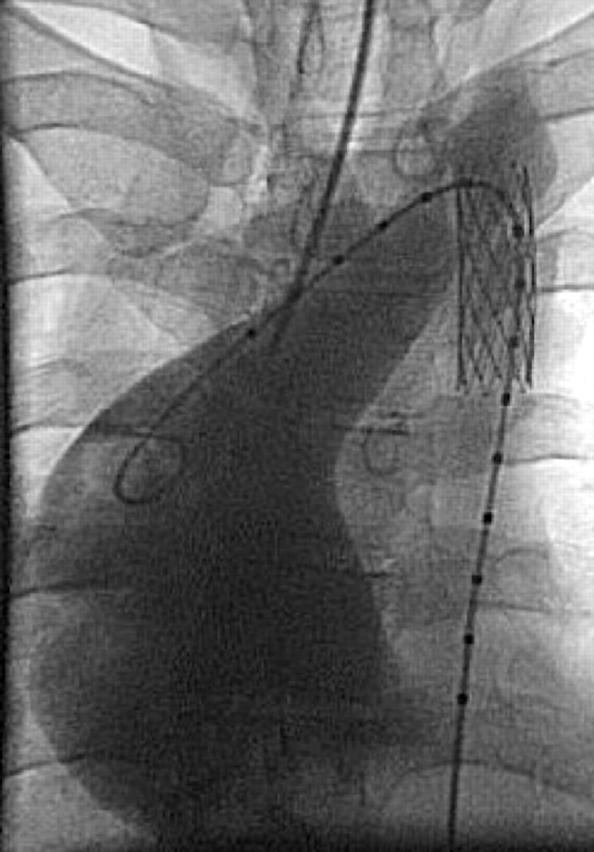
Gross dilation of the ascending aorta (> 7 cm) in an adult patient with “mild” coarctation and a bicuspid aortic valve, followed for 20 years without treatment. Stent implantation has been performed before aortic root replacement.
Figure 6.
(A) Small aneurysm (black dots) at the top end of an uncovered stent (white dots) placed in a severe native coarctation presenting with an out of hospital cardiac arrest. (B) A covered stent (white lines) has been expanded inside the previous stent with exclusion of the aneurysm.
Surgery or catheter intervention?
While many hold strong views—addressed by Hanley in 1996—in most centres repair is by open surgery in the first year of life.11w55–61 In childhood, surgery or balloon dilation are common approaches depending on the views of individual centres, while in adolescents and adults balloon dilation and stent implantation are more likely to be used than surgery. Recurrences after surgery are invariably treated by catheter intervention in the first instance.
FOLLOW UP
While originally viewed as a simple disease process that could be cured by effective surgery, it became clear that this was not so. The first large long term series in 1987 of 226 survivors of coarctation surgery with 15–30 year follow up showed a 12% mortality and a freedom from hypertension of only 32% (despite normal blood pressure after surgery).w62 The Mayo clinic series of patients operated on from 1946 to 1981 revealed a disturbing incidence of morbidity and mortality in the 571 survivors with a median follow up of 20 years. Further surgery was required in 11%, hypertension required treatment in 25%, and death occurred in 15% at a mean age of 38 years. Deaths were caused by coronary artery disease, sudden death, heart failure, cerebrovascular accidents, and ruptured aortic aneurysm. The best long term survival occurred in those operated on before the age of 9 years. Systolic hypertension was predictive of late death.w63 A further long term study with 24–53 year follow up of 254 survivors revealed a late cardiovascular mortality of 18% (coronary artery disease and reoperation being the most common causes of death) at a mean age of 34 years after surgery, and 35% were hypertensive.12
All these studies suffer from the limitations of retrospective analysis with incomplete follow up, learning curve, changes in the surgical approach, and a non-uniform cohort with a wide age range at first operation and a differing duration and intensity of follow up. A fundamental problem in this field is the imprecise definition of terms such as recoarctation and aneurysm. Significant coarctation or recoarctation has traditionally been defined as a gradient (strictly a catheter withdrawal gradient but also interchanged with arm/leg cuff blood pressure and Doppler) of > 20 mm Hg together with hypertension.w64 Recently a more conservative definition has been proposed of hypertension (rest or exercise induced) and a non-invasive gradient > 30 mm Hg.w65 Some have ignored “mild” hypertension, which would impose long term treatment on young asymptomatic patients. Some authors include gradients at the level of the hypoplastic arch as “recoarctation” even when the actual coarctation site is devoid of any narrowing. Aneurysm may be defined as a diameter > 1.5 times the adjacent “normal” diameter, but some mean dilation that mandates intervention. Long term outcome of a single surgical strategy in a uniform age group (for example, extended arch repair in neonates) is still awaited.
Hypertension
The upper limb hypertension associated with untreated coarctation usually responds dramatically to treatment. Paradoxical rebound hypertension may be present for hours to days in a small proportion with more severe coarctation, but this usually subsides over a few weeks. Although a majority of patients will have a normal blood pressure after their initial intervention in the short term, some patients continue to manifest a mildly elevated blood pressure, and some with an initial good response go on to develop hypertension—as many as one in three in long term studies.12w62 w63 In an adult cohort of 49 patients undergoing balloon dilation 63% were normotensive at a median of 10 years.10
The cause for ongoing hypertension is likely to be multifactorial. In some patients there is a “mild” degree of residual coarctation (< 20 mm Hg), which conventionally has been considered to be acceptable, and in others there is mild hypoplasia of the transverse arch, which was not considered to be important or a surgical target initially. Careful MRI assessment of the aortic arch geometry confirms a higher incidence of hypertension in those with tortuous aortic arches (possibly due to lack of remodelling/growth of the originally hypoplastic arch segment) despite similar degrees of residual narrowing at the original repair site. This has also been associated with an increased left ventricular mass.2,13w66 Other postulates include early renal hypoperfusion has “reset” the renin–angiotensin–aldosterone system or upper limb hypertension has “reset” the aortic baroreceptors.w67 w68
Even in those with acceptable resting blood pressures, exercise appears to unmask an exaggerated response in some—both with and without mild residual gradients. In a series of 35 adult patients undergoing surgical repair, 66% were normotensive off treatment although of these one third had an exaggerated blood pressure response to exercise.14 In these patients an apparently adequate size repair site is postulated to be non-compliant following surgery and fails to dilate during exercise.w69 Exercise induced hypertension correlates with resting blood pressure but is not an independent predictor of increased left ventricular mass.w70
Practical point.
Blood pressure should always be measured in the right arm unless it is known that the right subclavian artery has an anomalous origin or was ligated surgically. The left subclavian artery may be amputated during subclavian flap repair, with a combined end-to-end resection and flap procedure or even with an extended arch repair to allow mobilisation of the arch. In rare patients it may originate at the level or even below the coarctation site but even when normally placed, it is distal to the transverse arch, which may be narrowed or tortuous, and the pressure “seen” by the left ventricle and cerebral circulation will be underestimated. Many oscillometric sphygmomanometers are designed for use on the left arm and ambulatory monitors are deliberately placed on the non-dominant arm (usually left) to improve recordings leading to inaccurate measurements of the true blood pressure.
Many have blamed late or imperfect surgery as the cause of the persistent hypertension.w71 Disappointingly therefore, O’Sullivan et al found a 28% incidence of hypertension 10 years after repair in children.15 Although the confounding effects of small Doppler gradients were present (1 of 3 < 3.5 m/s on Doppler, 2 of 3 < 2.5 m/s) it does suggest that the perceived benefits of early surgery to prevent late hypertension may be to some extent due to the younger age of these patients and shorter duration of follow up.
Left ventricular function
Left ventricular hypertrophy after coarctation repair is likely to be caused by a combination of several factors: increased afterload from small residual gradients, ongoing mild hypertension, exercise induced gradients or hypertension, bicuspid aortic valve with progressive stenosis, and premature coronary disease. Left ventricular dilation is usually associated with bicuspid aortic valve regurgitation possibly accentuated by arch or coarctation obstruction and hypertension.w14
Coronary artery intimal thickening has been documented in children with coarctationw72 and this may be accentuated by persisting elevation in left ventricular afterload from mild hypertension or recoarctation.
Aneurysm formation
Aneurysm formation after surgical repair has been observed most frequently in relation to repair with a Dacron patch, and this type of repair is no longer employed. The incidence after other forms of surgery is low when there is no associated infection, which in itself is rare. It has been noted after balloon dilation acutely and even after stent implantation.9,10w44–48
A bicuspid aortic valve is present in 50% of patients with a coarctation and recently has been found to have an independent association with ascending aorta dilation.w73 Minor increases in systemic blood pressure and/or a mild coarctation site gradient may accentuate the ascending aorta dilation process in repaired coarctation patients with a bicuspid aortic valve (fig 5).
A recent report of 235 adults (182 surgical repair, 28 catheter intervention, and 26 untreated) revealed a 16% incidence of ascending or descending aortic wall complications causing death or requiring a surgical or catheter intervention.16 Ascending aorta complications were three times as common as descending aorta (coarctation site) complications. The age at repair and the type of surgical repair were surprisingly not predictive of aortic wall complications, but age at the time of the study and a bicuspid aortic valve were predictive. Interestingly, those with mild coarctation who had not been treated also had a 15% incidence of aortic wall complications. In a smaller study of 124 adults after coarctation repair, a bicuspid aortic valve was found in 62% and 28% required an aortic valve intervention. Ascending aorta dilation was present in 28%—defined as an ascending aorta greater than 4.0 cm.w74
Generalised vasculopathy
Evidence for a more generalised vasculopathy is confounded by definitions of a satisfactory repair and coexistent hypertension. There is evidence that the peripheral vascular beds proximal and distal to the coarctation site behave differently. Typically there is an elevated forearm resistance and reduced hyperaemic response, which is absent in the lower limb, and the magnitude of impairment seemed to correlate with the age at surgical repair.w75 w76 The differential vascular response is also postulated as a cause of exercise induced gradients and hypertension after satisfactory coarctation repair—indeed arm leg gradients of 20–100 mm Hg can develop on exercise in those with normal resting blood pressure and “normal” Doppler flow over the coarctation repair site.17w77 The pulsatility of the poststenotic aorta, which is severely impaired before repair, does not return to normal when compared to controls and the degree of impairment may correlate with the age at surgery.w78 w79 In adults, intima–media thickness in the precoarctation segment (carotid artery) is greater than in the postcoarctation segment (femoral artery) after repair. Carotid intima–media thickness is similar to normal controls in those with a good repair but thicker in those with residual hypertension, while the femoral intima–media thickness is thinner in both compared to normals.w80 There are no studies following these changes serially in well stratified cohorts of patients after coarctation repair.
Pregnancy
Detection for the first time during a pregnancy—often a murmur but occasionally severe hypertension or even aortic dissection—still occurs.w81 w82 In general, pregnancy is tolerated well by the mother and fetus as long as the hypertension is controlled and management of the pregnancy is altered little.18 Despite a higher incidence of pre-existing hypertension, there does not seem to be an increased risk for pre-eclampsia. Uncontrolled hypertension together with the oestrogen induced weakening of the arterial walls and the inherent abnormality thought to be present in the aorta may lead to rupture, particularly during straining in the second stage but also up to six weeks postpartum. If the pregnant patient is found to have an aneurysm then consideration should be given to a termination or early induction of labour and management of labour with an epidural to avoid straining during the second stage.w83 Antibiotic prophylaxis against infective endocarditis is required for complicated deliveries. There is also a risk of intracranial bleeding from berry aneurysms in this setting (table 3).w84
Table 3.
Complications in adults
| • Local site |
| - recoarctation, aneurysm, dissection, rupture, fistula, endarteritis, mycotic aneurysms |
| • Ascending aorta |
| - aneurysm, dissection, rupture, sinus of Valsalva fistula |
| • Left ventricle |
| - hypertrophy, dilation |
| • Aortic valve |
| - (bicuspid valve), stenosis and regurgitation |
| • Coronary arteries |
| - premature atherosclerosis |
| • Cerebral vessels |
| - berry aneurysms, intracranial bleeds |
| • Abdominal vessels |
| - renal artery stenosis |
| • Systemic hypertension |
Coarctation of the aorta from fetus to adult: key points.
Antenatal diagnosis relies on detection of cardiovascular changes, but the coarctation itself is rarely identified
Despite a normal postnatal examination, neonatal coarctation may remain undetected until presentation with profound collapse after duct closure
There is evidence for a generalised vasculopathy but it is not known whether it develops in response to the haemodynamic changes in utero, is a complication of late or imperfect treatment, or whether it is an inherent part of the disease process
Hypertension and aortic dilatation, dissection, and rupture remain a risk throughout life so that life long surveillance is required.
Ideally the woman with coarctation, whether previously treated or not, should be fully evaluated before pregnancy for the severity of the coarctation/recoarctation, aneurysm formation, ascending aorta dilation, aortic valve disease, and ventricular dysfunction so that any interventions can be performed electively. Where there is evidence of an aneurysm (native or after previous treatment) pregnancy is best avoided until it has been treated. Patients with Turner’s syndrome are at increased risk from aortic dissection and those who have assisted conception with egg donation and exogenous oestrogen may be at even greater risk.w85
CONCLUSIONS
There are no large, long term studies of outcome for surgical repair in infancy. That the recoarctation rate appears to be lower and acute morbidity and mortality are less for patients treated over the past 20 years is encouraging. Until further evidence accrues, it would be prudent to consider the patient with coarctation to have an ameliorated but not cured condition even after a “perfect” repair in infancy.w86 Ongoing surveillance of the coarctation site, ascending aorta, aortic valve, blood pressure, and left ventricular function will be required for the foreseeable future. Blood pressure should be checked on a 6–12 month basis with impeccable control, left ventricular and aortic valve function every 1–3 years by echocardiography, and coarctation site and ascending aorta every 2–5 years by echocardiography and occasionally by MRI. At the very least, a baseline MRI should be performed in all adolescents and young adults who have undergone childhood intervention and before a pregnancy.
We still do not know whether the ongoing degree of morbidity and mortality reflect inadequacies in the initial treatment either as a result of late diagnosis, acceptance of imperfect outcomes of treatment because they are practical, or whether there is an independent underlying local or generalised vasculopathy that is possibly the cause of coarctation in the first place and leads to a continuous effect on the cardiovascular system. Life long surveillance of both treated and untreated coarctation patients is required to monitor and intervene for hypertension, aortic wall, and other cardiovascular complications.
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Supplementary Material
Acknowledgments
I am grateful to Dr JM Simpson for fig 2 and Dr SA Qureshi for fig 6.
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
REFERENCES
- 1.Nielsen JC, Powell AJ, Gauvreau K, et al. Magnetic resonance imaging predictors of coarctation severity. Circulation 2005;111:622–8. ▸ Combination of MRI derived flow velocity and aortic narrowing indexed to body surface area predicts catheter gradients in excess of 20 mm Hg. [DOI] [PubMed] [Google Scholar]
- 2.Vriend J, Zwinderman A, de Groot E, et al. Predictive value of mild, residual descending aortic narrowing for blood pressure and vascular damage in patients after repair of aortic coarctation. Eur Heart J 2005;26:84–90. ▸ Mild residual coarctation is associated with increased blood pressure and carotid intimal thickness. Should the threshold for intervention from current guidelines be lowered? [DOI] [PubMed] [Google Scholar]
- 3.Head CEG, Jowett VC, Sharland GK, et al. Timing of presentation and postnatal outcome of infants suspected of having coarctation of the aorta during fetal life. Heart 2005;91:1070–4. ▸ Largest series of fetuses with suspected coarctation—the diagnosis was confirmed in one third postnatally. Coarctation was only detected late after duct closure in 7%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfammatter JP, Ziemer G, Kaulitz R, et al. Isolated aortic coarctation in neonates and infants: results of resection and end-to-end anastomosis. Ann Thorac Surg 1996;62:778–82. ▸ Significant re-coarctation rate in neonates of 19%. [DOI] [PubMed] [Google Scholar]
- 5.Younoszai AK, Reddy VM, Hanley FL, et al. Intermediate term follow-up of the end-to-side aortic anastomosis for coarctation of the aorta. Ann Thorac Surg 2002;74:1631–4. ▸ Recoarctation rate of only 5.5% by two years when performed in neonates—with good response to balloon dilation. [DOI] [PubMed] [Google Scholar]
- 6.Wood AE, Javadpour H, Duff D, et al. Is extended arch aortoplasty the operation of choice for infant aortic coarctation? Results of 15 years’ experience in 181 patients. Ann Thorac Surg 2004;77:1353–8. ▸ Excellent results for repair of coarctation and hypoplastic arch during a single operation in infancy. [DOI] [PubMed] [Google Scholar]
- 7.DiBardino DJ, Heinle JS, Kung GC, et al. Anatomic reconstruction for recurrent aortic obstruction in infants and children. Ann Thorac Surg 2004;78:926–32. ▸ Sternotomy and cardiopulmonary bypass approach to repair arch hypoplasia not addressed during initial coarctation surgery. Suggests that an initial complete repair including the arch is preferable to a local repair. [DOI] [PubMed] [Google Scholar]
- 8.Peters P, Brennan JW, Hughes CF, et al. Late quadriplegia after adult coarctation repair. Ann Thorac Surg 2003;75:268–70. ▸ Management of late spinal cord ischaemia caused by thrombosis in spinal artery collateral with spinal decompression. [DOI] [PubMed] [Google Scholar]
- 9.von Kodolitsch Y, Aydin MA, Koschyk DH, et al. Predictors of aneurismal formation after surgical correction of aortic coarctation. J Am Coll Cardiol 2002;39:617–24. ▸ Aneurysms are more frequent with patch repair and older age at repair. [DOI] [PubMed] [Google Scholar]
- 10.Fawzy ME, Awad M, Hassan W, et al. Long-term outcome (up to 15 years) of balloon angioplasty of discrete native coarctation of the aorta in adolescents and adults. J Am Coll Cardiol 2004;43:1062–7. ▸ Long term follow up in 49 patients. Repeat dilation was required and successful in four patients. Small aneurysms not requiring treatment remained stable in four patients. Blood pressure was normal in 63% without medication. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi ZM. Catheter intervention for adult aortic coarctation: be very careful! Cathet Cardiovasc Intervent 2003;59:536–7. ▸ Argument for covered stents as first line treatment of coarctation/recoarctation. [DOI] [PubMed] [Google Scholar]
- 12.Toro-Salazar OH, Steinberger J, Thomas W, et al. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 2002;89:541–7. ▸ Results of surgery are not as favourable as anticipated: 18% cardiovascular mortality and 35% remain hypertensive. [DOI] [PubMed] [Google Scholar]
- 13.Ou P, Bonnet D, Auriacombe L, Pedroni E, et al. Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. Eur Heart J 2004;25:1853–9. ▸ MRI analysis of the aortic arch long after coarctation repair and its correlation with hypertension and increased left ventricular mass. Despite adequate coarctation repair, an acutely angled aortic arch is associated with increased blood pressure. [DOI] [PubMed] [Google Scholar]
- 14.Bouchart F, Dubar A, Tabley A, et al. Coarctation of the aorta in adults: surgical results and long-term follow-up. Ann Thorac Surg 2000;70:1483–8. ▸ Significant incidence of exercise induced hypertension after “satisfactory” repair of coarctation when performed in adults. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24-hour blood pressure measurement. Heart 2002;88:163–6. ▸ Early onset of hypertension in children with “good” results from surgical repair—need for long term follow up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver JM, Gallego P, Gonzalez A, et al. Risk factors for aortic complications in adults with coarctation of the aorta. J Am Coll Cardiol 2004;44:1641–7. ▸ Aneurysm incidence of 16% with two thirds in the ascending aorta. Aneurysms occurred after surgery, catheter intervention, and in untreated patients. [DOI] [PubMed] [Google Scholar]
- 17.Markham LW, Knecht SK, Daniels SR, et al. Development of exercise-induced arm-leg blood pressure gradient and abnormal arterial compliance in patients with repaired coarctation of the aorta. Am J Cardiol 2004;94:1200–2. ▸ Patients with a “satisfactory” repair and normotension at rest can develop arm leg gradients up to 100 mm Hg. [DOI] [PubMed] [Google Scholar]
- 18.Beauchesne L, Connolly H, Ammash N, et al. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol 2001;38:1728–33. ▸ Follow up of pregnancies in 50 women. Hypertension is common and related to coarctation gradients while cardiovascular complications are infrequent. Fetal outcome is good. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.