Abstract
Objectives
The aim of this study was to observe the effects of mannitol, administered topically at the round window (RW), on cochlear blood flow (CBF) and distortion-product otoacoustic emission (DPOAE) after repeated episodes of cochlear ischemia.
Methods
Ten young rabbits were used for this study. Reversible ischemic episodes within the cochlea were induced by directly compressing the internal auditory artery (IAA). CBF was measured using a laser-Doppler (LD) probe positioned at the RW niche. DPOAEs were measured at 4, 8, and 12 kHz geometric mean frequency (GMF) using 60 dB sound pressure level (SPL) primary tone stimuli. In five test ears, mannitol was administered topically at the RW for 30 minutes before the IAA compressions. In five control ears, the IAA compressions were undertaken without application of RW medication. Each ear underwent three 5 minute IAA compressions with a 60 minute rest period between compressions.
Results
In the control animals, it was observed that a progressive reduction in DPOAE level followed each successive IAA compression at all three test frequencies. The reduction in DPOAE amplitudes was consistently greater at the higher test frequencies. In the test rabbits, the RW administration of mannitol resulted in significantly less reduction in DPOAE level measures after repeated IAA compressions. For example, 30 minutes after reperfusion at 12 kHz GMF, DPOAE levels in the control ears were reduced by 1.5, 6.0, and 16 dB, compared with 1.5, 4.0, and 6.0 dB in the mannitol test ears.
Conclusions
Mannitol appears to exert a protective effect on cochlear function after periods of ischemia. The RW appears to be an efficacious route for topical administration of mannitol into the inner ear.
Keywords: Otoacoustic emissions, mannitol, cochlear blood flow, cochlear damage
INTRODUCTION
It has been reported that reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radical can damage different tissues in the cochlea.1,2 Some reports have shown that the capacity of the enzymatic antioxidant system changes with age, making the auditory system more sensitive to oxidative insults.3 Recently, many studies have provided data showing that hearing loss induced by noise and ototoxic drugs are associated with ROS production.1–5 During ischemia-reperfusion episode, ROS are thought to be responsible for cochlear damage, the severity of which has been related to duration of ischemic period.6–10 Mild oxidative stress can be tolerated by up-regulating the synthesis of anti-oxidant defense system,11 but severe oxidative stress leads to cell death through apoptosis or necrosis by directly damaging cellular proteins, lipids, nucleic acids, and carbohydrates.12,13
Defense mechanisms are present in cells to detoxify or scavenge the ROS. Antioxidant defense systems involve intracellular enzymes (e.g., superoxide dismutase, gluthatione peroxidase, gluthatione reductase, and catalase) and low–molecular-mass antioxidants (e.g., gluthatione, ascorbic acid, α-tocopherol, uric acid) acting intra- and extracellularly.1,2,14,15 Substances such as radical scavengers (RS), nitric oxide synthase (NOS) inhibitors, and iron chelators have been shown to attenuate the ROS damaging effect following ischemia-reperfusion episodes, noise overexposure, and ototoxic drug application.4,5,16–18
Mannitol (molecular weight [MW] 182 D) is one of the osmotic drugs reported to change cochlear blood flow (CBF) and microvascular resistance as well as to increase oxygen pressure in perilymph within the cochlea.19–21 More recently, mannitol was reported to be a selective hydroxyl RS and weak iron chelator in ischemia-reperfusion experiments.7,22–24
Auditory evoked responses such as otoacoustic emissions, cochlear microphonics, and endocochlear potentials measure the functional status of the cochlea after both reversible and irreversible inner ear damage. Distortion-product otoacoustic emissions (DPOAEs) have been shown to be a sensitive tool to measure cochlear function during transient local ischemia of the inner ear.25–28 Laser-Doppler (LD) measurements CBF is another method objectively reflecting the status of cochlear circulation, specifically useful when cochlear ischemia-reperfusion episodes are recorded.26,29,30 Monitoring of the cochlear status during reversible ischemic episodes using both CBF and DPOAE techniques simultaneously has been described previously.26,30,31
The purpose of this study was to observe the effect of mannitol on cochlear function and CBF after ischemia. To avoid the systemic vascular effects, mannitol was applied topically at the round window (RW). Our hypothesis was that DPOAE would recover more completely in mannitol-treated ears than in untreated control ears.
MATERIAL AND METHODS
Ten young (6-month-old) albino rabbits weighing between 3.5 and 4.0 kg were used for this study. The protocol for the care and use of rabbits was approved by the institutional animal care and use committee. Several days before experimentation, DPOAEs at 2f1-f2 were obtained from each ear to assess adequate cochlear function using standard techniques.25,26 Two equilevel (L1 = L2) primary tones at an optimal f2/f1 ratio of 1.25 were acoustically mixed and presented to the ear by way of a custom-designed probe sealed tightly into the external auditory canal. DPOAE levels as a function of frequency (i.e., DP-grams) were plotted from 1 to 12 kHz for equilevel primary tones from 45 to 75 dB sound pressure level (SPL) at 5 dB intervals. During the actual experimental manipulations of CBF, DP tracking functions at 4, 8, and 12 kHz were obtained by monitoring DPOAE levels in response to L1 = L2 = 60 dB SPL primary tones. For both DP-grams and the DP tracking functions, DPOAE frequencies were converted to the geometric mean frequency (GMF) to adequately describe the generator site in rabbits for 2f1-f2 DPOAEs at the stimulus levels used here. These steps were repeated just before surgery and also after the opening of the middle ear cavity to ensure continued normal cochlear function.
The animals were anesthetized with an intramuscular injection of ketamine hydrochloride (50 mg/kg) mixed with xylazine hydrochloride (10 mg/kg). The anesthetized animals were placed on a feedback-controlled heating blanket set to maintain a core body temperature of 38°C within a standard double-walled, walk-in, sound-treated chamber (Industrial Acoustics Corp., Bronx, NY). After otoscopic examination to confirm normal ears, a head stabilization device was placed according to a standard procedure previously described.25,26 Surgical anesthesia was maintained by administration of supplemental doses of ketamine/xylazine as needed according to regular monitoring for the elicitation of an eye-blink or pain reflex.
To measure CBF, a commercial LD device (LaserFlo BPM, Vasamedics, Eden Prairie, MN), with a wavelength of 780 nm and an optical power of 2 mW at the probe tip, was used. A rigid needle sensor probe directed the laser beam. The sample rate for recording the CBF data were 2 points per second, with the on-line display consisting of a running average that was computed over a 10 second interval. LD flow measurements are described by arbitrary units. The numerical values are normalized to baseline levels, however, because no meaningful absolute values can be obtained. Therefore, the baseline CBF measures were normalized to 100% and changes expressed as percent values greater or less than 100%.
Before performing an occlusion experiment, the timing clocks of the LD device and the microcomputer’s digital signal processing board were synchronized. DPOAE levels and phases, as well as CBF, were then plotted as a function of time using customized software installed on a personal microcomputer (Macintosh Quadra 700, Apple Computers, Cupertino, CA). The data were then transferred off-line and analyzed using a commercially available spreadsheet (Microsoft Excel v. 2000; Microsoft Corp., Redmond, WA).
Experimental Procedures
The subsequent surgical steps for exposing the RW and internal auditory artery (IAA) have been previously described in detail for a protocol developed in this laboratory.25,26 After confirming that a particular rabbit’s DPOAEs were consistent with the normal rabbit database for our laboratory, the first surgical step under visualization by way of an operating microscope was to expose the cochlea and RW through a postauricular approach for placement of the LD probe to measure CBF. Toward this end, by exposing the external surface of the auditory bulla with a curette and then drilling with a diamond burr, the middle ear was entered between the bulla’s thick and thin posterior walls. The cochlea was further exposed using either the diamond burr or rongeurs until the RW was clearly visualized. The opening into the middle ear was then sealed using surgical gauze, and a postsurgical DP-gram at L1 = L2 = 60 dB SPL was obtained to confirm that no changes in cochlear function occurred after the surgical exposure.
After exposure of the middle ear, the cerebellopontine angle was approached by way of a suboccipital posterior craniotomy detailed previously.25,26 A posterior midline incision was made inferior to the occipital process. The dorsal neck muscles were then sectioned and retracted away from the midline. Using rongeurs to remove parts of the posterior calvarium, the cerebellum and brainstem were visualized through the operating microscope. Throughout this procedure, viability of the animal was monitored by observing changes in respiratory pattern, and additional doses of the ketamine/xylazine anesthetic regimen were administered as necessary. After exposing this area, the LD probe was placed into the RW niche on the side to be tested by way of the opening created by the previously described middle-ear surgery. The probe was adjusted using a micromanipulator so that an optimal level of CBF was detected. Then, a small piece of surgical gauze was placed in the middle ear to ensure a dry area and to reduce the opening.
One ear in five animals to be investigated was tested after the administration of 25% mannitol (American Pharmaceutical Partners, Los Angeles, CA). Under microscopic control, using an insulin syringe, the RW niche was filled with mannitol at a concentration of 250 mg/mL. Usually, one drop was enough to maintain contact with the RW membrane. Every 5 minutes, the RW was assessed with the microscope and, if needed, an additional drop was added. After 30 minutes, using a small suction canula, mannitol was evacuated gently. All of these manipulations to the RW were performed under ×25 magnification to prevent damage to the membrane. Just after RW cleaning, the LD probe was placed on the edge of the RW niche. No coupling material, such as petroleum jelly or surgical lubricant, was used between the RW and the LD probe tip because measurements were made through a thin (RW) membrane and not over bone or skin. Previous RW LD flow measurements in this laboratory have been made without the use of coupling agents. Also, application of these foreign materials may have interfered with mannitol crossing the RW membrane. Then, the porus of the internal auditory canal and the eighth nerve complex were identified. Baseline DPOAE tracking functions were obtained for each ear for GMFs at 4, 8, and 12 kHz GMF by measuring the emissions level as a function of the primary tones at 60 dB SPL for 60 to 90 seconds. A glass microprobe was used to compress the exposed eighth nerve complex. The first compression was maintained for 5 minutes and then released. DPOAE levels at each of the 4, 8, and 12 kHz monitoring frequencies and CBF were recorded from the onset of ischemia through 40 minutes after release of the compression. Usually, 5 minutes were needed to prepare and clear blood from the operating field between the end of the first monitoring session and the beginning of the second session. The IAA compressions were repeated twice for a total of three 5 minute compressions per ear. Five control ears were tested in the same manner by using saline instead of mannitol.
For each ear, the amount of reduction of DPOAE level at 4, 8, and 12 GMF after postblockage release for all three ischemic episodes was calculated. This reduction value was defined as the difference between DPOAE levels recorded after postblockage release for subsequent ischemic episodes from the value of pre-blockage baseline recorded before the first compression. The calculated medians of reduction values for control and investigated ears at each frequency for all three ischemic episodes were compared using Friedmann’s nonparametric analysis of variance (ANOVA) test. Comparisons of the reduction values between control and investigated ears at a given frequency for subsequent ischemic episodes were performed applying the nonparametric Mann-Whitney test. P values less than 0.05 were considered statistically significant.
RESULTS
Ears treated topically at the RW with mannitol exhibited less reduction of cochlear function, as measured using DPOAEs, than control ears after multiple 5 minute ischemic episodes induced by IAA occlusions. In every subject, CBF followed appropriate time-course patterns for transient ischemia (Fig. 1). Following IAA compression, LD measurements decreased rapidly and remained stable at low background levels. After release, CBF recovered rapidly to baseline levels within several seconds and then proceeded to exceed the baseline (i.e., overshoot), reaching a maximum approximately 1 minute later. The maximum CBF values reached 200% to 220% of the precompression baseline levels. LD measures stabilized back down to baseline within 7 to -11 minutes after release of IAA compression. In general, CBF overshoot tended to decrease with each successive ischemic episode. These CBF time-course patterns were consistent in both the control and the experimental groups. Comparing CBF overshoot values of the control and mannitol ears using the Mann-Whitney test showed no statistically significant differences, although after second and third ischemia, CBF overshoot in mannitol ears was higher.
Fig. 1.
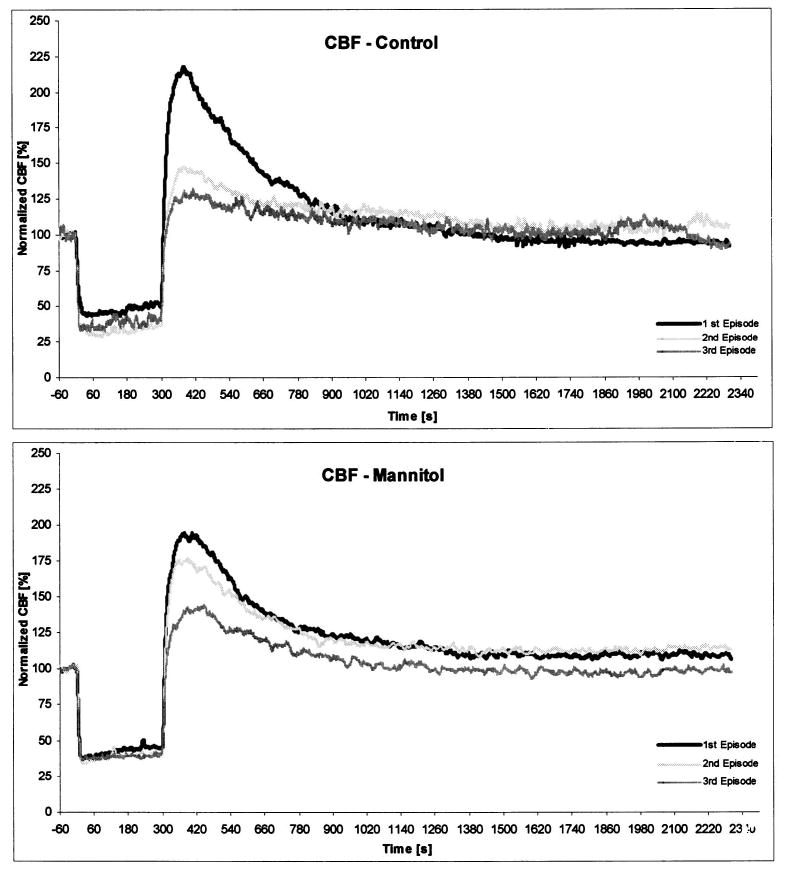
The time courses of median values for all subjects of cochlear blood flow (CBF) during and following the subsequent 5 minute internal auditory artery (IAA) compressions in five control ears pretreated with saline (upper) and five experimental ears pretreated with mannitol (lower). All values were normalized and shown as percent values. The comparison of CBF median values between the control and mannitol ears for adequate ischemic episodes using Mann-Whitney test showed no statistically significant differences.
Unlike CBF, DPOAE level time courses varied according to treatment group and measurement frequency. For 8 kHz and 12 kHz, DPOAE levels in saline-treated ears stabilized at significantly lower levels (compared with precompression baseline measures) after successive compression-release manipulations than in mannitol-treated ears. In general, the degree of DPOAE recovery varied inversely with measurement frequency. Recovery after ischemia was uniformly below precompression baseline values at 12 kHz, although the DPOAEs in the mannitol group eventually returned to baseline levels (Fig. 2).
Fig. 2.
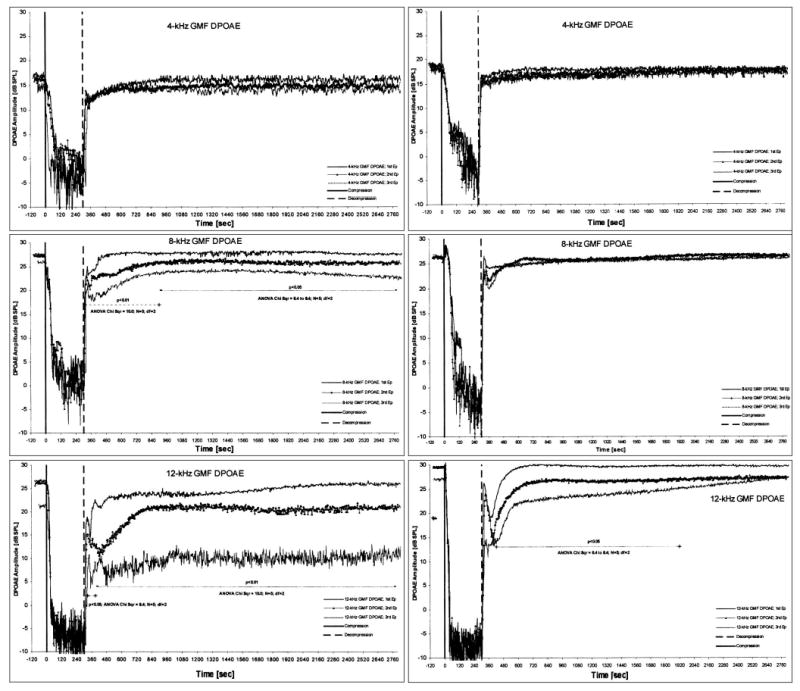
The time courses of median values for all subjects of distortion-product otoacoustic emissions (DPOAEs) during and following the subsequent 5 minute internal auditory artery (IAA) compressions in five control ears pretreated with saline (left) and five experimental ears pretreated with mannitol (right) recorded at 4 , 8 , and 12 kHz geometric mean frequency (GMF) DPOAE. Results of comparisons of DPOAE values measured during and following the subsequent ischemic episodes for given frequencies using Friedman’s analysis of variance (ANOVA) test.
In the control group, the reduction values (i.e., the difference between precompressions DPOAE measures and postrecovery levels of DPOAEs) were statistically different at the 8 and 12 kHz DPOAEs (Friedmann’s ANOVA test P < .05 and P < .01, respectively) (Fig. 2). In other words, DPOAE levels decreased significantly after successive ischemic events at the higher frequencies. In the experimental group, the reduction values were significantly different only at 12 kHz (Friedmann’s ANOVA test P < .05) (Fig. 2).
Comparing reduction values of the control and the experimental groups using the Mann-Whitney test resulted in no statistically significant differences for 4 kHz DPOAEs. Reduction values were significantly different for the third ischemic events at 8 kHz (P < .05) and for the second (P < .05) and third (P < .01) ischemic events at 12 kHz (Fig. 3).
Fig. 3.
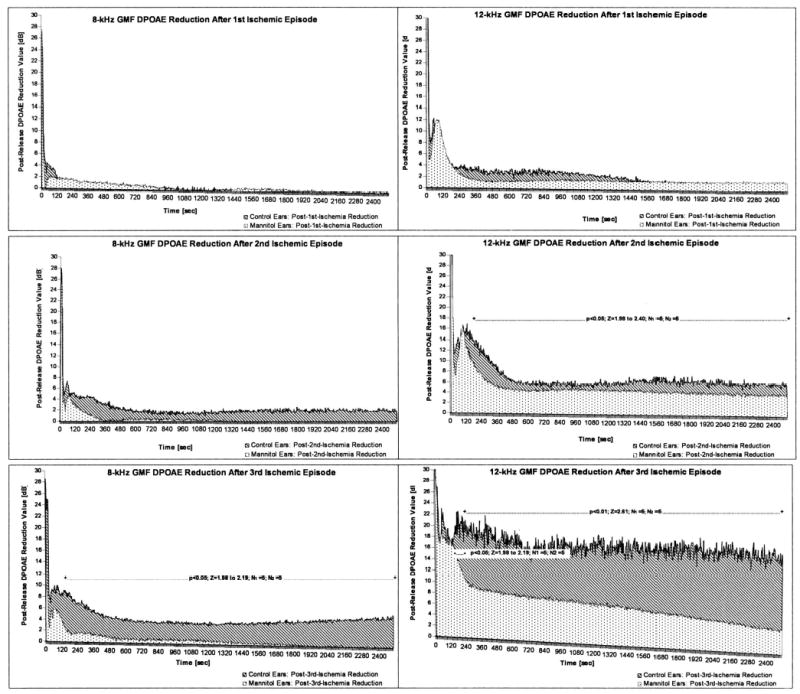
Comparison of median values for all subjects of postrelease distortion-product otoacoustic emission (DPOAE) reduction (release = 0 s) following the subsequent 5 minute internal auditory artery (IAA) compressions between five control ears pretreated with saline and five experimental ears pretreated with mannitol calculated for 8 kHz geometric mean frequency (GMF) DPOAE (left) and 12 kHz GMF DPOAE (right). Results of comparisons of DPOAE values calculated after release of the subsequent compressions for given frequencies using the Mann-Whitney test.
DISCUSSION
The primary finding of the present study was that DPOAEs recovered nearer to baseline values after repetitive 5-minute ischemic events when mannitol was applied topically to the RW membrane. CBF responses to the experimental manipulations of the IAA were comparable in the control (saline-treated) and experimental (mannitol-treated) ears. Thus, the differences in DPOAE measures after IAA compression–release were not related to changes in blood flow to the cochlea. These data suggest that mannitol applied topically to the RW membrane acted locally within the cochlea to protect against the damaging effects of multiple anoxic insults without significantly affecting CBF. The differences between the experimental and control groups were more pronounced and reached statistical significance for 8 and 12 kHz DPOAEs. Thus, mannitol appeared to exert a protective effect against repetitive periods of ischemia on the generation of evoked otoacoustic emissions in the experimental group.
The basal turn of the cochlea, subserving higher frequency audition, is known to be more sensitive to a variety of insults such as ototoxic drugs and noise exposure.4,5,16 Previous studies have shown that during ischemia-reperfusion episodes, the higher frequencies are more severely affected and that the severity of damage depends on the degree and duration of hypoxia, resulting in damage to both hair cells and afferent fibers within the cochlea.6–10,17,18,30 In the present study, for the control group, recovery was significantly less at 12 kHz DPOAEs than at 4 and 8 kHz DPOAEs. This frequency dependent response after ischemia has been noted in previous experiments. Billet et al.32 described the early ultrastructural changes within the Corti organ following total cochlear ischemia indicating that outer hair cells (OHCs) became swollen and showed alterations to mitochondria, endoplasmic reticulum, and the nucleus. Also inner hair cells (IHCs) and afferent nerve endings beneath IHCs were described as damaged. These changes along the cochlea developed earlier at the base of the cochlea than at the apex.8,32,33 Increase in ROS production, mostly hydroxyl radical, after arterial compression was found to be responsible for the presence of the above ultrastructural changes.6,8
Sha et al.34 showed that different susceptibilities of the apical and basal hair cells is caused by differential levels of natural free RS in basal and apical parts of the cochlea. These authors demonstrated that the level of natural glutathione within the OHCs at the basal cochlea was significantly lower than that at the apical turn, confirming a key role of the glutathione-dependent antioxidant system in protection against various factors damaging cochlear function. RS, iron chelators, and NOS inhibitors attenuated the damaging effects of ROS after ischemia-reperfusion episodes by enhancing efficacy of the antioxidant system within the cochlea.7,10,17,18
Mannitol works as both an osmotic agent and a hydroxyl RS. It has been shown to moderate injury during ischemia-reperfusion insults in a variety of tissues.7,22–24 The rheologic properties, reducing blood viscosity, and vasodilatory activity of mannitol combined to reduce cerebral edema and protect neural tissues.22,35 When administered systemically in animals by way of intravenous and intraperitoneal routes, mannitol has been reported to alter CBF by changing microvascular resistance as well as to enhance the level of oxygen pressure in the cochlear perilymph.19–21 Mannitol has also been shown to protect against functional and morphologic injuries caused by damaging levels of noise, anoxia, and ototoxic agents.4,7,16
The data from the present study suggest that RW application may be a suitable method of drug delivery for mannitol. Three mechanisms of penetration of drugs into the scala tympani in vivo through the three-layer RW membrane have been described: diffusion through the cytoplasm, traversing by the pinocytotic vesicles, and directly through channels between cells.36,37 These active and passive mechanisms are efficient ways to transport relatively high MW substances such as dexamethasone (MW 516) or cefmetazole sodium (MW 493) across the RW membrane into the scala tympani.36–39 The relatively low MW of mannitol (MW 182) suggests that it will cross the RW membrane in concentrations suitable for clinical applications.16,36 In vitro, mannitol appeared not to pass through the RW membrane unless combined with streptolysin O.40 Evidence from our and other in vivo studies suggest that mannitol does cross the RW in pharmacologically sufficient quantities.4,7
Although the CBF time course was similar across all subjects, cochlear function as measured with DPOAEs was qualitatively and, for the higher frequencies, statistically different between control and experimental groups. DPOAE amplitude functions demonstrated slower and less complete recovery to precompression baseline levels in the saline-treated control ears. The damaging effects of ischemia-reperfusion on DPOAEs were more pronounced at the higher frequencies. Even in the mannitol group, the 12 kHz DPOAEs returned very gradually toward precompression baseline values after successive ischemic events compared with the rapid recovery noted at 4 and 8 kHz (Fig. 2).
The initial drop in DPOAE levels after reperfusion, more pronounced at 8 and 12 kHz, coincided with the overshoot of precompression baseline CBF (Figs. 1 and 2). This phenomenon may represent an exaggerated reperfusion injury caused by the near doubling of CBF during the overshoot period after the 5 minute ischemic interval. Cytotoxic reperfusion injuries are well described in the brain and heart literature and are now thought to involve the production of damaging ROS and free radical molecules. The studies in these tissues have shown two peaks in concentrations of reactive oxygen free radicals after reperfusion, one each at approximately 5 and 20 minutes.41–43 The results of the present study, and those of other partial reperfusion blood flow experiments, suggest that postischemic depression of cochlear function (as reflected by DPOAEs) occurs almost immediately after resumption of CBF and recovers shortly thereafter to a preischemic baseline or to a new, lower level.26,30,31
CONCLUSIONS
After 5 minute IAA compressions, DPOAEs measured from ears treated with mannitol returned to preischemic baseline values, whereas those from saline-treated control ears recovered only partially.
Mannitol appeared to cross the RW membrane in quantities sufficient to protect cochlear OHC function from transient ischemia.
The free radical scavenging properties were probably responsible for the protective effect because CBF patterns were not appreciably altered after topical RW membrane administration of mannitol.
Mannitol may find clinical applications in protecting against, as well as treatment of, inner ear damage caused by ROS injuries such as ischemia, noise overexposure, and ototoxicity. Ongoing studies in this laboratory are investigating mannitol’s protective effect on these cochlear insults.
Footnotes
This study was supported by the National Institute of Health (2 R44 DC 04344-02), the Fullbright Commission, and the University of Miami Ear Institute.
References
- 1.Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98(1–2):116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- 2.El Barbary A, Altschuler RA, Schacht J. Glutathione S-transferases in the organ of Corti of the rat: enzymatic activity, subunit composition and immunohistochemical localization. Hear Res. 1993;71(1–2):80–90. doi: 10.1016/0378-5955(93)90023-t. [DOI] [PubMed] [Google Scholar]
- 3.Lautermann J, Crann SA, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res. 1997;114(1–2):75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 4.Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- 5.Husain K, Scott RB, Whitworth C, et al. Dose response of carboplatin-induced hearing loss in rats: antioxidant defense system. Hear Res. 2001;151(1–2):71–78. doi: 10.1016/s0300-2977(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 6.Seidman MD, Quirk WS, Nuttall AL, Schweitzer VG. The protective effects of allopurinol and superoxide dismutase-polyethylene glycol on ischemic and reperfusion-induced cochlear damage. Otolaryngol Head Neck Surg. 1991;105:457–463. doi: 10.1177/019459989110500318. [DOI] [PubMed] [Google Scholar]
- 7.Tabuchi K, Ito Z, Wada T, et al. The effect of mannitol upon cochlear dysfunction induced by transient local anoxia. Hear Res. 1998;126(1–2):28–36. doi: 10.1016/s0378-5955(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 8.Ohlemiller KK, Dugan LL. Elevation of reactive oxygen species following ischemia-reperfusion in mouse cochlea observed in vivo. Audiol Neurootol. 1999;4:219–228. doi: 10.1159/000013845. [DOI] [PubMed] [Google Scholar]
- 9.Hara A, Serizawa F, Tabuchi K, et al. Hydroxyl radical formation in the perilymph of asphyxic guinea pig. Hear Res. 2000;143(1–2):110–114. doi: 10.1016/s0378-5955(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 10.Tabuchi K, Tsuji S, Ito Z, et al. Does xanthine oxidase contribute to the hydroxyl radical generation in ischemia and reperfusion of the cochlea? Hear Res. 2001;153(1–2):1–6. doi: 10.1016/s0378-5955(00)00247-1. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–266. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 12.Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32(Suppl):S22–S27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 13.Huang T, Cheng AG, Stupak H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000;18(2–3):259–270. doi: 10.1016/s0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 14.Chaudiere J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37(9–10):949–962. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 15.Evans P, Halliwell B. Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- 16.Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res. 1996;94(1–2):87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 17.Tabuchi K, Okubo H, Fujihira K, et al. Protection of outer hair cells from reperfusion injury by an iron chelator and a nitric oxide synthase inhibitor in the guinea pig cochlea. Neurosci Lett. 2001;307:29–32. doi: 10.1016/s0304-3940(01)01919-x. [DOI] [PubMed] [Google Scholar]
- 18.Tabuchi K, Tsuji S, Asaka Y, et al. Ischemia-reperfusion injury of the cochlea: effects of an iron chelator and nitric oxide synthase inhibitors. Hear Res. 2001;160(1–2):31–36. doi: 10.1016/s0378-5955(01)00315-x. [DOI] [PubMed] [Google Scholar]
- 19.Quirk WS, Dengerink HA, Bademian MJ, Wright JW. Mannitol and dextran increase cochlear blood flow in normotensive and spontaneously hypertensive rats. Acta Otolaryngol. 1990;109(5–6):383–388. doi: 10.3109/00016489009125159. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Uemura T. Effect of glycerol and mannitol on perilymphatic PO2 in guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104:495–498. doi: 10.1177/019459989110400412. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin DL, Ohlsen KA, Miller JM, Nuttall AL. Cochlear blood flow and microvascular resistance changes in response to hypertonic glycerol, urea, and mannitol infusions. Ann Otol Rhinol Laryngol. 1992;101(2 Pt 1):168–175. doi: 10.1177/000348949210100212. [DOI] [PubMed] [Google Scholar]
- 22.Magovern GJ, Jr, Bolling SF, Casale AS, et al. The mechanism of mannitol in reducing ischemic injury: hyperosmolarity or hydroxyl scavenger? Circulation. 1984;70(Suppl 1):91–95. [PubMed] [Google Scholar]
- 23.Kobayashi H, Ide H, Kabuto M, et al. Effect of mannitol on focal cerebral ischemia evaluated by somatosensory-evoked potentials and magnetic resonance imaging. Surg Neurol. 1995;44:55–61. doi: 10.1016/0090-3019(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 24.Luvisotto TL, Auer RN, Sutherland GR. The effect of mannitol on experimental cerebral ischemia revisited. Neurosurgery. 1996;38:131–139. doi: 10.1097/00006123-199601000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Widick MP, Telischi FF, Lonsbury-Martin BL, Stagner BB. Early effects of cerebellopontine angle compression on rabbit distortion-product otoacoustic emissions: a model for monitoring cochlear function during acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1994;111:407–416. doi: 10.1177/019459989411100404. [DOI] [PubMed] [Google Scholar]
- 26.Mom T, Telischi FF, Martin GK, Lonsbury-Martin BL. Measuring the cochlear blood flow and distortion-product otoacoustic emissions during reversible cochlear ischemia: a rabbit model. Hear Res. 1999;133:40–52. doi: 10.1016/s0378-5955(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 27.Telischi FF, Mom T, Agrama M, et al. Comparison of the auditory-evoked brainstem response wave I to distortion-product otoacoustic emissions resulting from changes to inner ear blood flow. Laryngoscope. 1999;109:186–191. doi: 10.1097/00005537-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Morawski K, Namyslowski G, Lisowska G, et al. Intraoperative monitoring of the cochlear function using distortion product otoacoustic emissions in patients with cerebellopontine angle tumors. Abstr Assoc Res Otolaryngol. 2002;25:204. doi: 10.1097/00129492-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Miller JM, Ren TY, Nuttal AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol Head Neck Surg. 1995;112:101–113. doi: 10.1016/S0194-59989570308-X. [DOI] [PubMed] [Google Scholar]
- 30.Morawski K, Telischi FF, Merchant F, et al. Preventing internal auditory artery vasospasm using topical papaverine: an animal study. Otol Neurotol. 2003 doi: 10.1097/00129492-200311000-00017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mom T, Telischi FF, Martin GK, et al. Vasospasm of the internal auditory artery: significance in cerebellopontine angle surgery. Am J Otol. 2000;21:735–742. [PubMed] [Google Scholar]
- 32.Billett TE, Thorne PR, Gavin JB. The nature and progression of injury in the organ of Corti during ischemia. Hear Res. 1989;41:189–198. doi: 10.1016/0378-5955(89)90010-5. [DOI] [PubMed] [Google Scholar]
- 33.Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res. 1995;84(1–2):30–40. doi: 10.1016/0378-5955(95)00010-2. [DOI] [PubMed] [Google Scholar]
- 34.Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155(1–2):1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 35.Otsubo K, Katayama Y, Kashiwagi F, et al. Comparison of the effects of glycerol, mannitol, and urea on ischemic hippocampal damage in gerbils. Acta Neurochir Suppl (Wien) 1994;60:321–324. doi: 10.1007/978-3-7091-9334-1_86. [DOI] [PubMed] [Google Scholar]
- 36.Juhn SK, Hamaguchi Y, Goycoolea MV. Review of round window membrane permeability. Acta Otolaryngol (Stockh) 1988;457(Suppl):43–48. doi: 10.3109/00016488809138883. [DOI] [PubMed] [Google Scholar]
- 37.Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc Res Tech. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Kopke RD, Hoffer ME, Wester D. Targeted topical steroid therapy in sudden sensorineural hearing loss. Otol Neurotol. 2001;22:475–479. doi: 10.1097/00129492-200107000-00011. (Suppl) [DOI] [PubMed] [Google Scholar]
- 39.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109(7 Pt 2):1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 40.Engel F, Blatz R, Kellner J, et al. Breakdown of the round window membrane permeability barrier evoked by streptolysin O: possible etiologic role in development of sensorineural hearing loss in acute otitis media. Infect Immun. 1995;63:1305–1310. doi: 10.1128/iai.63.4.1305-1310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto A, Ohnishi ST, Ohnishi T, Ogawa R. Relationship between free radical production and lipid peroxidation during ischemia-reperfusion injury in the rat brain. Brain Res. 1991;554(1–2):186–192. doi: 10.1016/0006-8993(91)90187-z. [DOI] [PubMed] [Google Scholar]
- 42.Kramer JH, Misik V, Weglicki WB. Magnesium-deficiency potentiates free radical production associated with post-ischemic injury to rat hearts: vitamin E affords protection. Free Radic Biol Med. 1994;16:713–723. doi: 10.1016/0891-5849(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 43.Numagami Y, Ohnishi ST. S-allylcysteine inhibits free radical production, lipid peroxidation and neuronal damage in rat brain ischemia. J Nutr. 2001;131:1100S–1105S. doi: 10.1093/jn/131.3.1100S. [DOI] [PubMed] [Google Scholar]


