Abstract
APS reductase catalyzes the first committed step of reductive sulfate assimilation in pathogenic bacteria, including Mycobacterium tuberculosis, and is a promising target for drug development. We report the 2.7 Å resolution crystal structure of Pseudomonas aeruginosa APS reductase in the thiosulfonate intermediate form of the catalytic cycle and with substrate bound. The structure, high-resolution FT-ICR mass spectrometry, and quantitative kinetic analysis, establish that the two chemically discrete steps of the overall reaction take place at distinct sites on the enzyme, mediated via conformational flexibility of the C-terminal 18 residues. The results address the mechanism by which sulfonucleotide reductases protect the covalent but labile enzyme-intermediate prior to release of sulfite by the protein cofactor thioredoxin. Pseudomonas aeruginosa APS reductase contains an [4Fe-4S] cluster that is essential for catalysis. The structure reveals an unusual mode of cluster coordination by tandem cysteines and suggests how this arrangement might facilitate conformational change and cluster interaction with substrate. Assimilatory PAPS reductases are evolutionarily related, homologous enzymes that catalyze the same overall reaction, but do so in the absence of an [Fe-S] cluster. The APS reductase structure reveals adaptive use of a phosphate-binding loop for recognition of the APS O3′ hydroxyl, or alternatively, the PAPS 3′-phosphate.
Introduction
Metabolic assimilation of sulfate (SO42−) from the environment requires its reduction to sulfite (SO32−). In many species of bacteria and plants this pathway, which culminates in the biosynthesis of cysteine and methionine, proceeds via adenosine 5′-phosphosulfate (APS)1,2,3 (Supplementary Figure 1). This intermediate is produced by the action of ATP sulfurylase, which condenses sulfate and adenosine 5′-triphosphate (ATP) to form APS4,5. The activated sulfonucleotide is reduced to sulfite and adenosine 5′-phosphate (AMP) by APS reductase (Figure 1)6,7. This reaction requires reducing equivalents provided by the protein cofactor thioredoxin (Trx).
Figure 1.
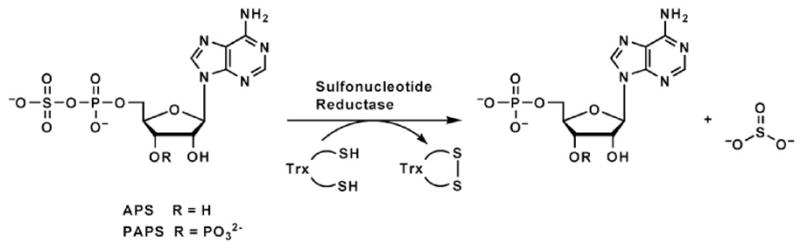
Reaction catalyzed by sulfonucleotide reductases.
Humans lack the enzymes required for sulfate reduction. Thus, APS reductase may be an attractive drug target if the enzyme is required for bacterial survival or virulence in vivo3. NO and superoxide are produced in response to Mycobacterium tuberculosis infection8,9,10,11 and it is likely that the bacterium has a mechanism of protection against these reactive oxidants. Products of the reductive sulfate assimilation pathway, such as mycothiol (biosynthesized from cysteine), are excellent candidates for this function. Consistent with this hypothesis, APS reductase was identified in a screen for essential virulence genes in Mycobacterium bovis BCG12. Moreover, it was recently demonstrated that the APS reductase gene (CysH) is essential for the bacteria to survive during the persistence phase in a murine model of tuberculosis infection13. No antibiotics are currently available to target this part of the bacterial lifecycle, so inhibitors of APS reductase could represent the first drugs that address the latent phase. Toward this end, it is essential to obtain high-resolution structural information for this enzyme in complex with its sulfonucleotide substrate.
Interestingly, not all organisms that assimilate sulfate reduce APS as the source of sulfite. Through divergent evolution some organisms such as Escherichia coli and Saccharomyces cerevisiae reduce the related metabolite 3′-phosphoadenosine 5′-phosphosulfate (PAPS), which is produced by APS kinase from ATP and APS (Figure 1, Supplementary Figure 1)14,15,16,17. Biochemical, spectroscopic and mass spectrometry investigation of sulfonucleotide reductases in both APS- and PAPS-dependent bacteria have recently established a two-step mechanism, in which the sulfonucleotide undergoes nucleophilic attack to form an enzyme-thiosulfonate (E-Cys-Sγ–SO3−) intermediate, followed by release of sulfite in a thioredoxin-dependent manner (Figure 2)6,18. Hence, APS and PAPS reductases perform the same chemistry, and overall, their primary sequences are homologous (Figure 3, Supplementary Figure 2) despite their difference in substrate specificity6,18.
Figure 2.
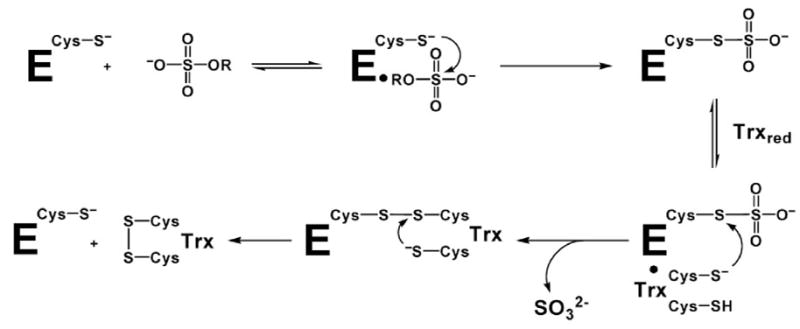
Mechanism of sulfonucleotide reduction.
Figure 3.
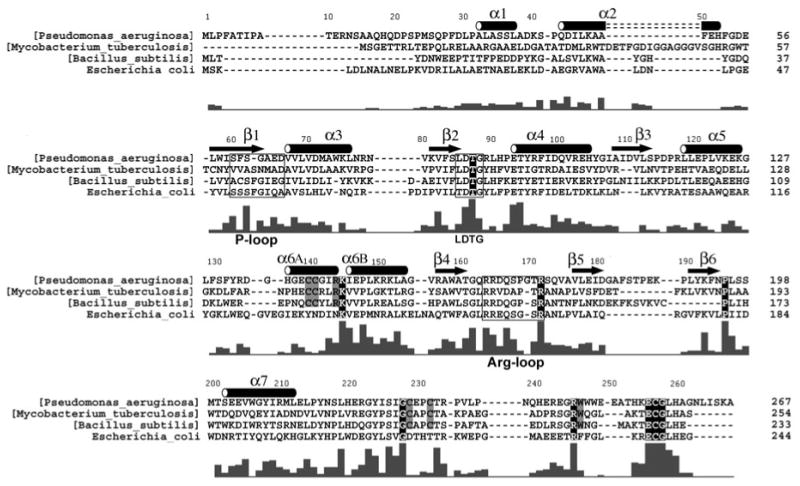
Primary sequences for APS reductases from P. aeruginosa, M. tuberculosis, and B. subtilis, and for PAPS reductase from E. coli, are shown based on alignment of 38 APS and 34 PAPS reductases (Supplementary Figure 2). Secondary structure elements are denoted for P. aeruginosa APS reductase, and residues within the P-loop (60–66), LDTG motif (85–88), and Arg-loop (163–171), are boxed. The bar graph indicates the degree of conservation based on all 72 sequences (Supplementary Figure 2). Strictly conserved residues are outlined in black; six additional residues conserved in 38 APS reductases that ligate or interact with the [4Fe-4S] cluster are boxed in gray.
Efficient reduction of the thiosulfonate bond requires the protein reductant Trx. Thus, in the absence of thioredoxin, the sulfite remains covalently attached to an essential cysteine residue near the C-terminus6,19. Small-molecule reductants with redox potentials comparable to Trx, such as dithiothreitol (DTT) and β-mercaptoethanol, release the intermediate from the unfolded polypeptide at elevated temperatures, but do not support multiple turnover for the active, folded catalyst6 (and data herein). These data suggest that the second half of the catalytic cycle is dependent upon protein-protein interaction between Trx and APS reductase, most likely to facilitate conformational rearrangements. The molecular details of such changes have remained unknown, but are essential for understanding how sulfonucleotide reductases preserve the thiosulfonate intermediate until interaction with Trx and subsequent reduction.
A central feature that distinguishes APS from PAPS reductases is the presence of a conserved cysteine motif, CC-X~80-CXXC, which occurs in addition to the universally conserved catalytic cysteine (Figure 3, Supplementary Figure 2)3,16. The presence of this motif is correlated with the presence of a [4Fe-4S] cluster, and when the cluster is present, it is required for catalytic activity2,6,18,20,21. PAPS-specific reductases lack the cysteine motif and therefore the [Fe-S] cluster1,16. As a result, the evolutionary divergence that has segregated specificity toward APS vs. PAPS, but maintained a common two-step chemical transformation, has also resulted in APS-specific enzymes that contain an [Fe-S] cluster. This raises the question whether the cluster is involved in the chemical mechanism of APS reduction2,6,18,20,31. In this regard, it is interesting to note that the occurrence of sequential cysteines in the sequence motif associated with the [4Fe-4S] cluster in APS reductase is highly unusual, and has led to doubts as to whether both cysteines coordinate the cluster2,20. Structural definition of cluster coordination is therefore an important step toward understanding its functional role.
Herein, we address outstanding questions regarding substrate recognition, protein dynamics during the catalytic cycle, and cluster coordination through structure determination of Pseudomonas aeruginosa APS reductase with APS bound, and through biochemical, kinetic and FT-ICR mass spectrometry experiments with both P. aeruginosa and M. tuberculosis APS reductases. The features of the structure, and the dynamic properties of the enzyme, indicate that APS reductase must undergo significant conformational rearrangement in formation of the thiosulfonate intermediate, and in subsequent reduction by thioredoxin, and that the two discrete steps of the overall reaction occur at distinct sites on the enzyme. Furthermore, the structure establishes coordination of the [4Fe-4S] cluster by a tandem cysteine motif, Cys139 and Cys140, together with Cys228 and Cys231. We propose that the unusual conformation associated with ligation by adjacent cysteine residues might allow for flexible coordination at an iron atom and be associated with conformational changes during the catalytic cycle. Finally, the structure reveals a role for a phosphate-binding loop (P-loop) in substrate recognition and provides a molecular rationale for substrate specificity by sulfonucleotide reductases.
Results
Protein fold, APS site, and [4Fe-4S] cluster
The crystal structure of P. aeruginosa APS reductase is the first sulfonucleotide reductase to be determined with a substrate bound, and it is the first containing a [Fe-S] cluster. The protein monomer folds as a single domain with a central six-stranded β sheet with five parallel strands, and one strand (β5) anti-parallel (Figures 3, 4(a), 4(b)). Strands of the central β sheet are interleaved with seven α-helices that pack against both sides of the β sheet. Residues 220–249 comprise a pair of consecutive β-loops; this structural element caps the space between α-helices flanking the β-sheet to create a deep active site cleft. The substrate APS extends across the C-terminal ends of the β strands (Figure 4(a)). Opposite the nucleotide at one end of the active site cleft is the [4Fe-4S] cluster with standard tetrahedral geometry. The cluster is ligated by four cysteines, Cys228 and Cys231, positioned at the tip of a β-loop, and the tandem pair, Cys139 and Cys140, within a long, kinked helix, α6A. Three additional elements define the active site: the “P-loop” between β1 and α3 (residues 60–66), the “LDTG motif” following β2 (residues 85–88), and the “Arg-loop” between β4 and β5 (residues 162–173). The C-terminal segment of residues 250–267, which carries the catalytically essential Cys256, is disordered, but could be positioned above the active site cleft.
Figure 4.
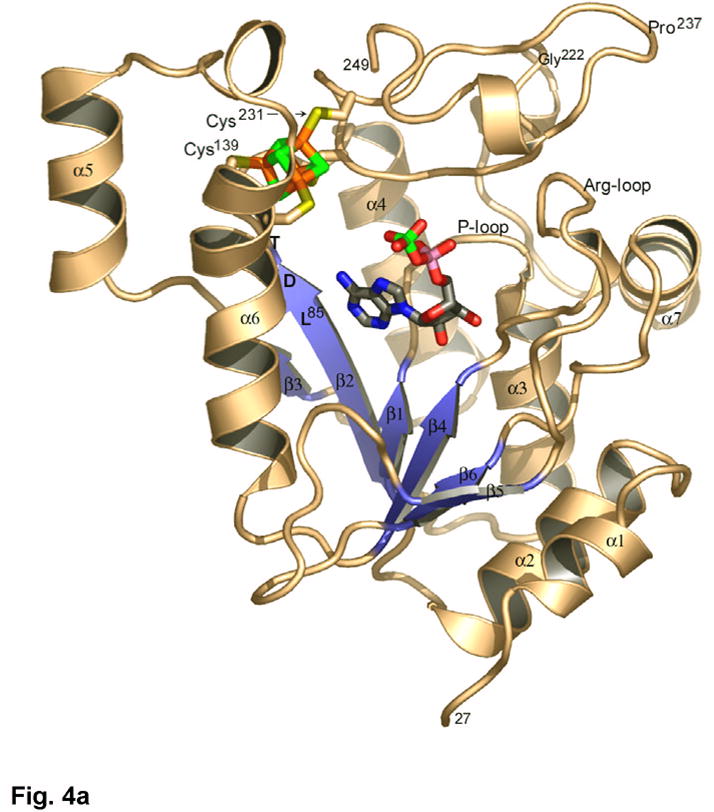
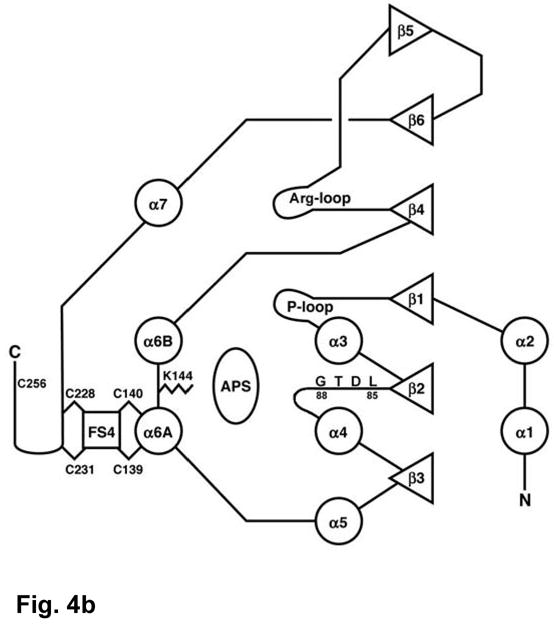
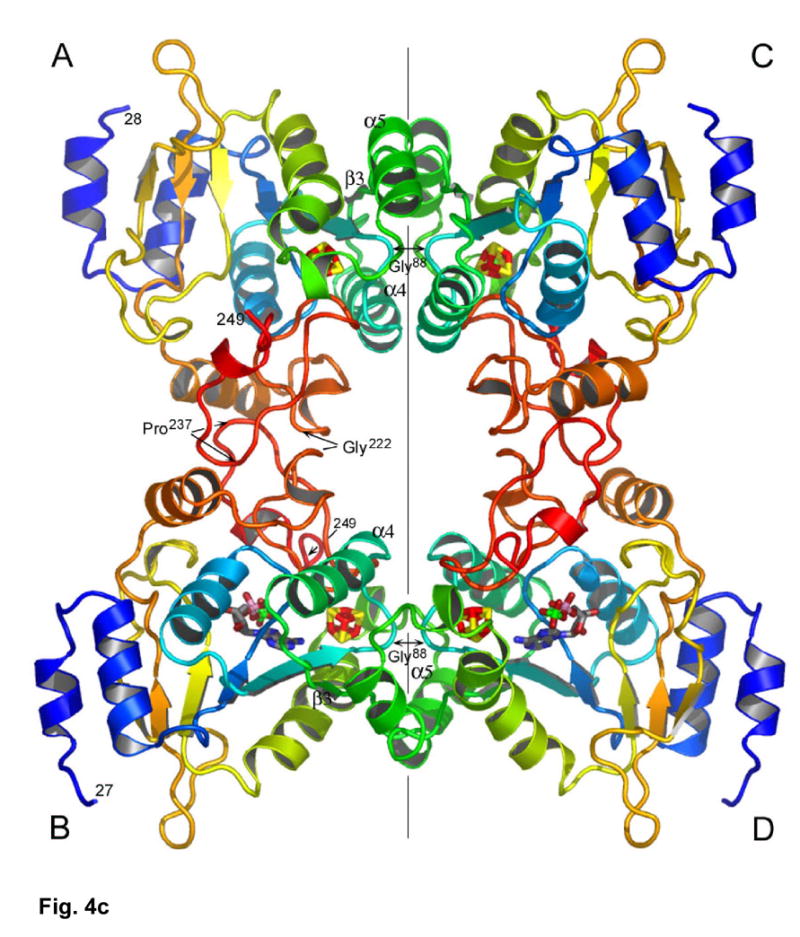
(a) The structure of P. aeruginosa APS reductase comprises a central 6-stranded β sheet flanked by α-helices and loops that create a deep active site cavity. The substrate APS binds across the C-terminal ends of the β-strands, and occupies the active site in half of the independent copies of the protein in the crystal (subunit B is shown). The [4Fe-4S] cluster is ligated by four cysteines at residues 139 and 140 on the long, kinked helix α6, and at residues 228 and 231 at the tip of a β-turn. (b) Secondary structure topology of P. aeruginosa APS reductase indicating α-helix and β-strand elements, active site loops, conserved residues Lys144 and Cys256, the LDTG motif, and Cys ligand connectivity to the [4Fe-4S] cluster. (c) Triclinic crystals of P. aeruginosa APS reductase contain two tetramers in the asymmetric unit. Each chain of the ABCD tetramer is colored blue->red from N- to C-terminus (residues 27–249). Labeled residues are involved in inter-subunit contacts.
Quaternary structure
P. aeruginosa APS reductase crystallized as tetramer, consistent with its oligomeric state in solution6 (Figure 4(c)). In accord with the non-crystallographic symmetry (NCS) observed for the [Fe-S] clusters, the tetramer has strict 2-fold symmetry but only pseudo-222-fold symmetry. The strict 2-fold relates the AB dimer onto the CD dimer (rmsd 0.14 Å for Cα atoms) (vertical axis in Figure 4(c)), while the pseudo-2-fold axes relate individual subunits, i.e. A onto B, A onto D, etc., with rmsd’s of 0.77 – 0.79 Å. The two tetramers in the asymmetric unit are very similar (rmsd for Cα’s of ABCD onto EFGH 0.15 Å). The asymmetry of the AB and CD dimers is manifested in the relative occupancy of APS in the B and D vs. A and C subunits (Figure 4(c)). The tetrameric arrangement would allow interaction with Trx near the active site of any given subunit.
The quaternary structure positions the nucleotides in subunits B and D ~35 Å apart. At the AB and CD interfaces, two loops are juxtaposed at Gly222 and Pro237, while at the AC and BD interfaces there are extensive contacts between β3 strands and α5 helices on opposing subunits (Figure 4(c)). The closest contacts at each interface involve conserved glycines: Gly222 (Cα-Cα distance 3.5 Å); and Gly88 in the LDTG motif (Cα-Cα distance 4.4 Å). Conservation of these glycines suggests that other bacterial APS reductases may also be tetramers (Supplementary Figure 2). In this respect, two of three prolines within β3 and α5 are not conserved in the M. tuberculosis, M. smegmatis, and R. meliloti enzymes, which function as monomers1.
State of the enzyme in crystals and mobility of C-terminal residues
In the absence of Trx, APS reductase reacts with APS, producing the thiosulfonated adduct of the conserved cysteine, Cys256 (Figure 2)6,18. Since formation of the covalent intermediate stabilizes the cluster toward oxidation and loss of activity18, we anticipated that its formation would constrain enzyme conformation and favor crystallization. However, the C-terminal residues beyond Glu249 are disordered in all eight subunits in the asymmetric unit while APS is observed in four of the active sites (subunits B, D, F, H) (Supplementary Figure 3). Because the crystals were grown with >60-fold excess of APS over enzyme, we hypothesized that the C-terminal segment carrying the thiosulfonate adduct is displaced from the active site by unreacted APS.
To test this hypothesis we carried out several experiments. First, the stability of the thiosulfonate intermediate was tested against small-molecule reductants of varying reduction potential. As previously observed, only thioredoxin is able to catalyze the second step of the reaction (Supplementary Table 1). In particular, neither dithiothreitol nor dithionite, which have lower reduction potentials, were able to release detectable sulfite during the incubation time of the assay and at 10 mM concentration. Hence, the presence of reductants in crystallization drops (2.3 mM DTT and 2.5 mM dithionite) would not be expected to cleave the thiosulfonate.
Second, washed and re-dissolved crystals were assayed by mass spectrometry, showing quantitative sulfonation (31359.8 Da, 31360.1 Da theoretical) consistent with the expectation that the enzyme should form the enzyme-thiosulfonate intermediate during crystallization (Supplementary Figure 4(a)). Consideration of the anaerobic conditions, concentrations of reagents present, and rates, assure that the thiosulfonate can only arise from enzymatic activity (Materials and Methods). In subunits B, D, F, and H, APS was sufficiently ordered to visualize difference electron density (Supplementary Figure 3), although the B-factors were high (Table 1); in subunits A, C, E, and G extra density in the active site was also observed, but could not be modeled with confidence. Apparently, sufficient APS was present to displace the sulfonated C-terminal residues in these subunits as well. This interpretation was supported by the mass spectrum of M. tuberculosis APS reductase, acquired under nondenaturing conditions and after exposure to 20 μM APS, which showed the presence of sulfonated enzyme with noncovalently bound AMP, as well as sulfonated enzyme with noncovalently bound APS (Supplementary Figures 4(b), 4(c)). Thus, the enzyme-thiosulfonate intermediate can still bind substrate. (Previously, at concentrations of 10 μM APS or less, sulfonated enzyme with only AMP bound was observed6.) Together, these data indicate that high concentrations of APS displace the Cys256Sγ-SO3− intermediate from the active site.
Table 1.
Crystallographic Statistics
| Crystals, Unit Cells, and Data Sets | |||||
| APSr SAD | APSr MAD | APSr Native | |||
| Space Group | P1 | P1 | P1 | ||
| a, b, c (Å) | 60.25 103.27 140.26 | 60.24 102.94 140.04 | 60.03 102.79 139.37 | ||
| α, β, γ(deg) | 90.36 102.38 89.95 | 90.44 102.45 89.96 | 90.09 102.57 89.95 | ||
| SSRL Beam Line | BL 9-2 | BL 9-2 | BL 9-1 | ||
| Peak | Peak | Inflection | Remote | ||
| Wavelength (Å) | 1.731 | 1.737 | 1.742 | 1.348 | 0.984 |
| Resolution (Å) | 3.01 | 3.13 | 3.13 | 2.99 | 2.70 |
| Observations | 391,272 | 247,396 | 223,050 | 280,915 | 168,061 |
| Reflections | 61,646 | 56,889 | 55,324 | 65,516 | 85,404 |
| Redundancy | 6.3 | 4.3 | 4.0 | 4.3 | 2.0 |
| Completeness (%) [1] | 94.0 (89.0) | 98.4 (97.2) | 95.6 (93.9) | 98.5 (97.8) | 95.6 (95.6) |
| <I>/<σI> | 4.5 (2.1) | 5.0 (2.1) | 5.3 (2.2) | 4.1 (2.2) | 5.9 (1.9) |
| Rsymm(I) | 0.129 (0.272) | 0.146 (0.325) | 0.137 (0.299) | 0.172 (0.307) | 0.105 (0.337) |
| MAD Phasing | |||||
| f ′ (e) | −5.51 | −8.45 | −0.29 | ||
| f ″ (e) | 4.37 | 2.12 | 2.57 | ||
| 8 site model at 4.5 Å | |||||
| Phasing Power | 1.65 | 2.05 | 0.66 | ||
| Figure of Merit | 0.63 | ||||
| 32 site model at 3.5 Å | |||||
| Phasing Power | 1.26 | 1.49 | 0.38 | ||
| Figure of Merit | 0.53 | ||||
| Refinement | |||||
| Resolution Range (Å) | 50.0 – 2.70 | ||||
| Reflections > 0.0 σF | 85,402 | ||||
| R-factor | 0.229 | ||||
| Rfree (% of data) | 0.265 (4.7) | ||||
| rmsd deviation bonds (Å) | 0.008 | ||||
| rmsd deviation angles (deg) | 1.46[2] | ||||
| Model | |||||
| No. of residues / <B-factor> (Å2) [3] | |||||
| Subunit A | 222 / 31.8 | FS4 / 21.6 | --- | ||
| Subunit B | 223 / 46.0 | FS4 / 33.5 | APS / 108.9 | ||
| Subunit C | 222 / 28.3 | FS4 / 22.9 | --- | ||
| Subunit D | 223 / 45.2 | FS4 / 33.7 | APS / 109.8 | ||
| Subunit E | 222 / 28.4 | FS4 / 20.2 | --- | ||
| Subunit F | 223 / 46.2 | FS4 / 32.6 | APS / 111.0 | ||
| Subunit G | 222 / 32.0 | FS4 / 20.2 | --- | ||
| Subunit H | 223 / 46.5 | FS4 / 33.1 | APS / 106.8 | ||
| H2O molecules | 151 / 22.1 | ||||
Values for highest resolution shell in parentheses.
Ramachandran plot: 87.9% of residues in most favored regions; 11.6% in allowed regions; 0.5% in generously allowed regions; 0.1% in disfavored regions.
The Wilson B value for 2.70 Å native data is 47.7 Å2.
Third, to probe the conformation of the enzyme in solution, M. tuberculosis APS reductase was subjected to limited trypsin proteolysis in the absence of APS, and in the presence of equimolar APS; the resultant peptides were purified by HPLC and analyzed by mass spectrometry (Figure 5, Supplementary Figure 5). In the absence of APS, essentially all M. tuberculosis APS reductase was proteolyzed to lower molecular weight fragments missing significant portions of the C-terminus (Figure 5, Supplementary Figure 5(a)). In the presence of stoichiometric APS, however, the major tryptic fragment comprised residues 55 to the end of the protein including an intact, sulfonated C-terminal tail. A similar effect was found with P. aeruginosa APS reductase (Figure 5, Supplementary Figure 5(b)). In this case proteolysis in the absence of APS occurred primarily at Arg171 in the Arg-loop, and at basic residues near the N- and C-termini (Arg12, Lys254, Lys266) (Figures 3, 5). In the presence of APS, the major fragment consisted of residues 13–266, which included the sulfonated catalytic cysteine. In contrast to the protection afforded by equimolar substrate, addition of a five-fold excess APS resulted in significant cleavage at Arg171 and Lys254 (data not shown). Considering the results for M. tuberculosis and P. aeruginosa APS reductase together, both the Arg-loop and the C-terminal tail are susceptible to proteolysis in the absence of substrate, but in the presence of equimolar substrate, thiosulfonate formation protects against proteolysis by ordering these mobile elements. However, in the presence of a large excess of substrate, as in crystals, or in solution, a third state exists with APS bound and both the Arg-loop and the sulfonated C-terminal tail expelled from the active site.
Figure 5.
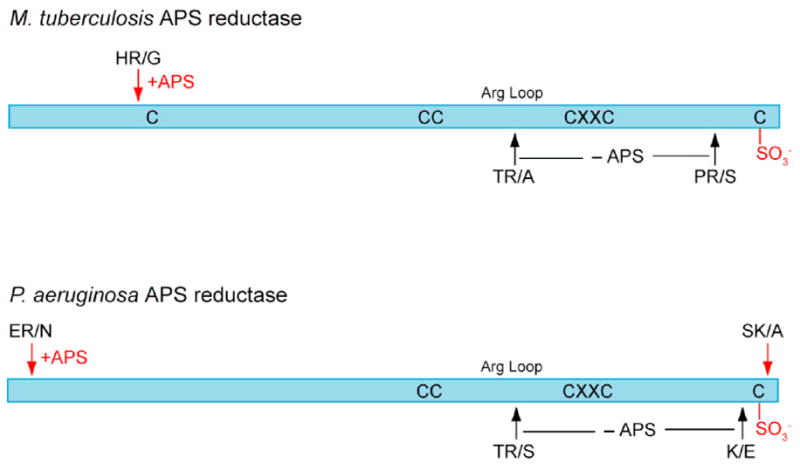
Sites of trypsin proteolysis in M. tuberculosis and P. aeruginosa APS reductase. In the absence of substrate (-APS, black arrows) both the Arg-loop and the C-terminal tail are cleaved; in the presence of equimolar substrate (+APS, red arrows), formation of the thiosulfonate intermediate protects these structural elements, and only sites at the extreme N- and C-termini are cleaved. See Supplementary Figure 5 for SDS-PAGE analysis and peptide masses.
Conformational states in steps of the reaction
We propose a model in which the C-terminal tail is mobile: in the “open” form, APS can bind; in the “closed” form with APS bound, the thiosulfonate intermediate is formed. The intermediate is stable with respect to small-molecule reductants, but is reducible by Trx, which can interact with the Cys256Sγ-SO3− to catalyze release of sulfite. At the same time, formation of the intermediate protects the C-terminal residues, as well as the Arg-loop, from proteolysis. In the presence of excess substrate, as during crystallization, APS binding displaces the sulfonated C-terminal residues, which in the absence of Trx become disordered. Hence, consideration of the crystal structure together with biochemical data provides a model for the conformational states of the C-terminal residues during the overall reaction.
The above observations predict that when Trx concentrations are limiting, elevated concentrations of APS should result in substrate inhibition. To test the model we assayed enzyme activity as a function of APS and Trx concentrations. The inhibitory effect of increasing APS concentration above its Km is clear (Figure 6(a)), consistent with APS acting as a competitive inhibitor, i.e. it prevents recognition of the sulfonated C-terminal residues by Trx. This interpretation is supported by kinetic analysis with 10-fold additional Trx (Figure 6(b)). In this case, a higher concentration of Trx favors sulfite release and regeneration of free enzyme. As a result, additional APS is required to observe the inhibitory effect (KiAPS increases from 7.4 μM to 81 μM). Thus, consistent with the crystallization conditions, elevated concentrations of APS displace the C-terminal thiosulfonated residues, which prevents productive association of Trx and inhibits the enzyme. This model is summarized in Figure 6(c).
Figure 6.
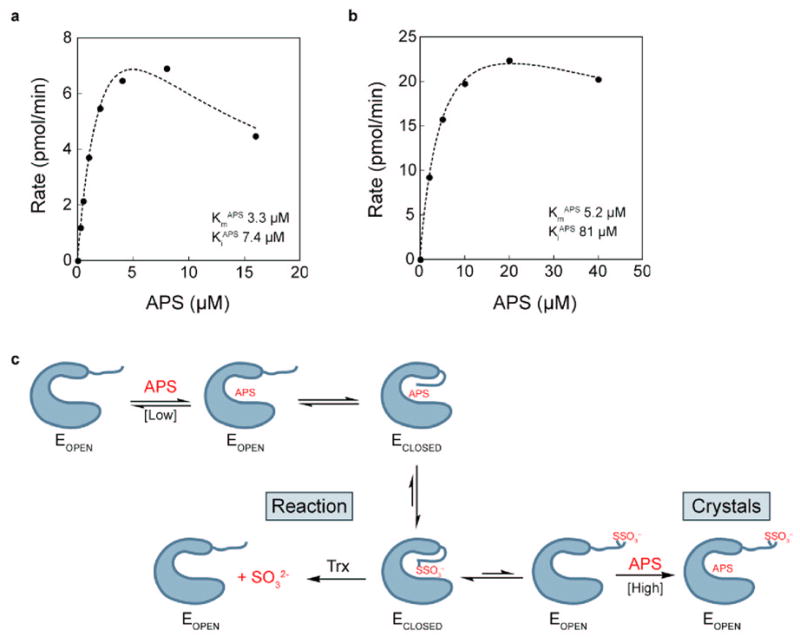
Dependence of APS reductase activity on APS concentration. (a) Reaction rate measured in the presence of 1 μM thioredoxin. (b) Reaction rate measured in the presence of 10 μM thioredoxin. Data were fit with Eq. 1b, derived from the inhibitory model in Eq. 1a (see Methods). (c) Model for mobility of C-terminal tail during APS reduction. The C-terminal peptide of APS reductase (E) can be in an open or closed conformation. Higher concentrations of Trx favor sulfite release and regeneration of free enzyme, resulting in a higher apparent KiAPS. In the presence of excess APS, but absence of Trx, the enzyme is trapped in the inhibitory open form, as observed in the crystals.
Iron-sulfur cluster
Based on the experimental phases, the starting model for the cluster was a center of mass only (Methods); hence, the derived cluster model is unbiased. The cluster is ligated by four cysteines, Cys228 and Cys231, positioned at the tip of a β-loop, and the tandem pair, Cys139 and Cys140, within a long, kinked helix, α6A (Figure 7(a)). Despite constraints imposed by the tandem coordination, the [4Fe-4S] cluster has tetrahedral symmetry and typical metric parameters. At the same time, residues 136–143 exhibit normal α-helical geometry, although helix α6 is kinked where Lys144 is oriented into the active site (Figure 4(a)). In the context of a regular α-helix, the peptide bond linking Cys139 and Cys140 straddles one face of the cluster (Figure 7(b), Supplementary Figure 6). Analysis of the cluster coordinates by the method of circumcenters22 shows that the Sγ atoms are only slightly displaced from ideal tetrahedral positions. The χ1,χ2 torsion angles about the Cα-Cβ and Cβ-Sγ bonds are typical for Cys139, Cys228, and Cys231, but the χ1,χ2 torsion angles for Cys140 are (-g, cis), i.e. the Cα and Fe atoms are virtually eclipsed. Consequently, accommodation of the CysCys motif to the [4Fe-4S] cluster results in distortion of the Cys140 side chain, and leads to steric clashes between both cysteines and the cluster (Figure 7(b), Supplementary Figure 6).
Figure 7.
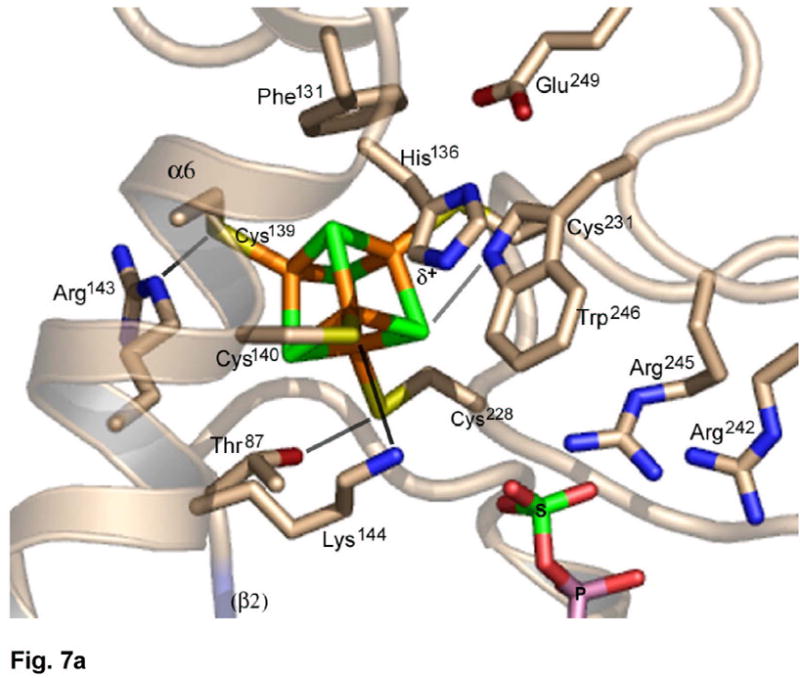
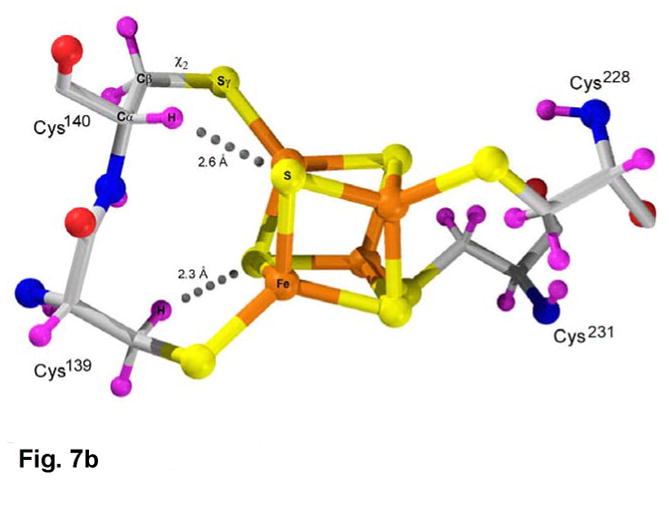
(a) The environment of the [4Fe-4S] cluster in P. aeruginosa APS reductase. The [4Fe-4S] cluster is ligated by four cysteines at residues 139, 140, 228 and 231. Four conserved residues participate in charged or polar NH…S or OH…S hydrogen bonds to inorganic S or cysteine Sγ atoms – Thr87, Arg143, Lys144 and Trp246. In addition, His136 may be hydrogen bonded to Cys140. (b) Molecular details of the [4Fe-4S] cluster and its cysteine ligands in P. aeruginosa APS reductase. The positions of H atoms, calculated based on C and N atomic coordinates, indicate steric clashes (dotted lines). Due to linkage of tandem cysteines in an α-helix, the χ2 torsion angle is cis (Supplementary Figure 6) and the Cys140 Cα-H atom is ~2.6 Å from inorganic S of the cluster. In addition, an H atom on Cβ of Cys139 is ~2.3 Å from a S atom. The sum of van der Waals radii for S and H is 3.00 Å.
[4Fe-4S](CysSγ)4 cluster sites, having a net charge of −2, often exhibit NH…S hydrogen bonds involving amide dipoles within ~3.5 Å of S or Sγ23. In P. aeruginosa APS reductase there are no such interactions; rather, there are five charged and/or polar NH…S or OH…S hydrogen bonds involving side chains, four representing strictly conserved residues (Figure 7(a), Supplementary Figure 2). In particular, the CysCys motif interacts with a pair of basic residues, Arg143 and Lys144, on the next turn of helix α6; interactions also involve Thr87 in the LDTG motif, and Trp246. A fifth interaction might involve His136, which is His or Arg in two-thirds of APS reductase sequences (Figure 7(a), Supplementary Figure 2). Lastly, Cys231, and the face of the cluster opposite the APS binding site, pack against Phe131 and Pro230, forming a hydrophobic interface. These residues participate in a network of generally conserved aromatic amino acids surrounding the opposite side of the cluster, which includes Phe129, Tyr132, Trp246, Trp247, and Trp248.
Substrate recognition
APS is bound to subunits B, D, F, and H in the two tetramers in the asymmetric unit (Figure 4(c)). In the absence of an ordered C-terminal segment the active site is accessible to solvent (Figure 8). APS fits into the deep active site cavity with the phosphosulfate moiety extending toward the protein surface, which is slightly concave where it surrounds the active site cleft. In addition to APS, the active site accommodates at least one H2O molecule adjacent to O3′ of ribose. Conserved and semi-conserved residues line the active site cavity. The adenosine moiety participates in four hydrogen bonds with main chain amides and carbonyls (Figure 9(a)). The 2′ hydroxyl of the ribose sugar wedges between strands β1 and β4, and the N6 and N1 atoms of adenine interact with Leu85, the first residue in the conserved LDTG motif on strand β2 (Figure 4(a)). Complementary hydrogen bonding between adenine and main chain atoms of a β-strand residue is a conserved feature of adenine nucleotide α hydrolases5,24. Hydrogen bonds within the conserved motifs cooperatively stabilize the β-strands for recognition (Figure 9(a)). The adenine ring is sandwiched between the side chains of Leu85 and Ser62; nearby residues not in van der Waals contact are Phe83, Lys144, Val148 and Trp158.
Figure 8.
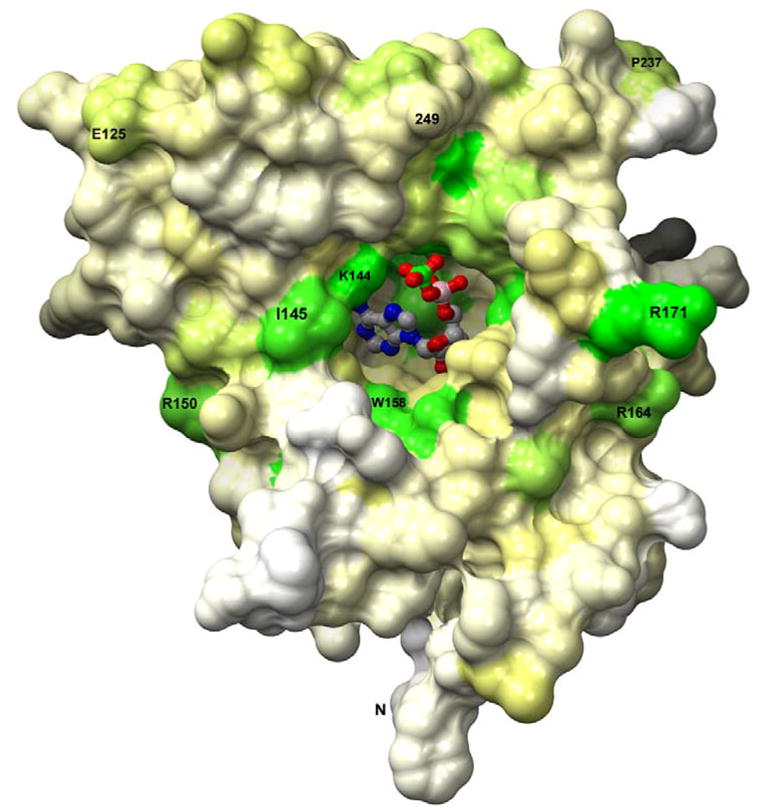
APS binds in a deep active site pocket of P. aeruginosa APS reductase such that only the sulfate group is exposed. The solvent accessible surface is depicted, and residues are colored by degree of sequence conservation (Supplementary Figure 2) among 72 APS and PAPS reductases; green – most conserved; yellow – partial conservation; white – variable). The orientation is similar to that in Figure 4(a).
Figure 9.

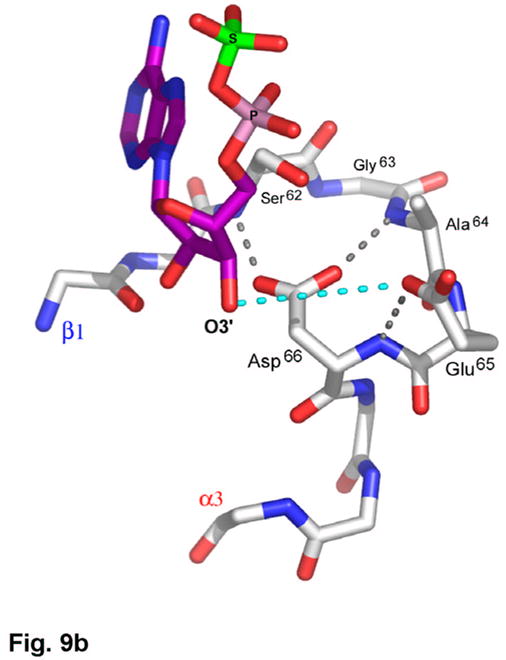
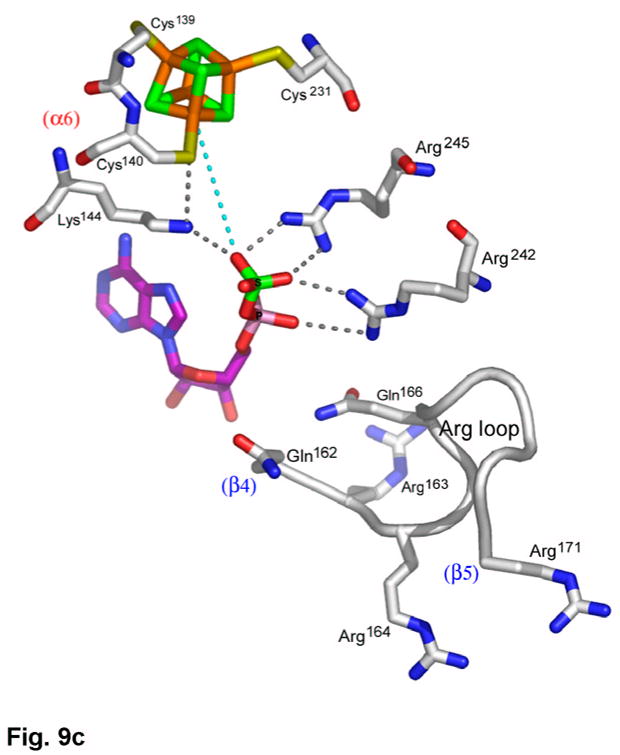
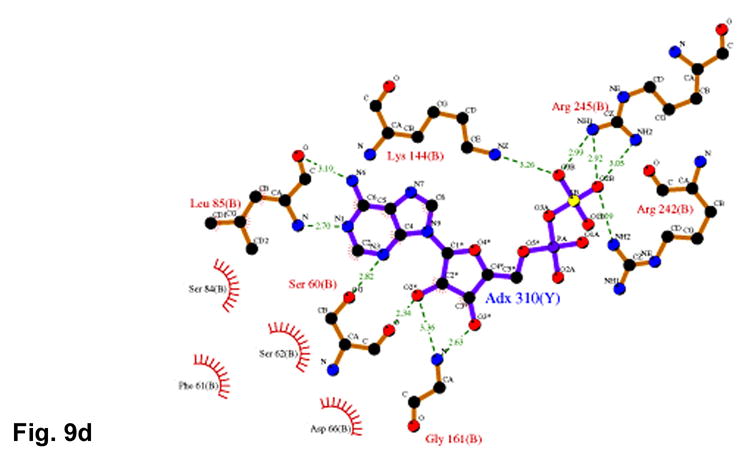
(a) Recognition of the adenosine moiety of APS by conserved residues on strands β1, β2, and β4, which participate in four main chain hydrogen bonds with adenine and the ribose O2′ hydroxyl group. In addition, these residues stabilize the conformation of the β-strands through hydrogen bonds within two conserved motifs (Leu85Asp86Thr87Gly88 and Thr160Gly161). (b) The P-loop comprising conserved residues 60–66 connects strand β1 and helix α3 in the P. aeruginosa APS reductase active site. Three amides hydrogen bond with Glu65 or Asp66, but in PAPS reductases these acidic residues are replaced by Gln and Ala or Ser, respectively. The distance between Glu65 and the O3′ of ribose is 5.3 Å (cyan dotted line). (c) Conserved basic residues in the vicinity of the active site interact with the phosphate and sulfate groups of APS, or reside on the Arg-loop, comprising residues 162–175 between strands β4 and β5. The shortest distance between a sulfate oxygen atom and Fe of the [4Fe-4S] cluster is ~7.0 Å (cyan dotted line). (d) Summary of all active site contacts to APS in subunit B of the asymmetric unit (PDB deposition 2GOY) plotted in two dimensions; hydrogen bond distances are indicated in Å.
A second aspect of the active site entails substrate recognition by the P-loop (Figure 9(b)). The P. aeruginosa APS reductase sequence, Ser62Gly63Ala64Glu65Asp66, is strongly conserved in APS reductases, and related to the P-loop motif in other ATPases5,24 and nucleotide binding enzymes25. In particular, the E. coli PAPS reductase sequence, SSSFGIQA, which contains the consensus motif SXG26, aligns with SFS-GAED in P. aeruginosa APS reductase (Figure 3). It appears that Glu65 and Asp66 in P. aeruginosa APS reductase, which interact with three amides of the P-loop, and are positioned above the dipole of the α3 helix, mimic the interaction of the negatively charged 3′-phosphate group in PAPS reductase (Figure 9(b)).
Interaction between the APS phosphosulfate moiety and APS reductase occurs via strictly conserved basic residues, Lys144, Arg242, and Arg245 (Figure 9(c)). Lys144 plays a role in cluster interactions as well, and along with Arg143 (Figure 7(a)), these four basic residues balance the combined negative charge of the phosphosulfate and [4Fe-4S](CysSγ)42− cluster. The phosphosulfate displays an extended conformation with respect to C5′ and O5′ of the ribose, and is positioned opposite the [4Fe-4S] cluster and Cys140. The sulfate group is somewhat distal from the cluster and, while no atoms intervene, the shortest distances between sulfate oxygen and Fe and Sγ of Cys140 are 7.03 Å and 6.09 Å, respectively (Figures 7(a), 9(c)). Two segments of the Arg-loop, Arg163Arg164Asp165Gln166Ser167 and Thr170Arg171, are not in contact with APS, although they represent consensus sequences for APS and PAPS reductases, and Arg171 is one of only six strictly conserved residues in both classes of enzymes (Supplementary Figure 2). Interactions with APS are summarized in Figure 9(d).
Discussion
By crystallography, we have captured P. aeruginosa APS reductase in the state following the first step of the catalytic cycle, in which the sulfite group of APS is transferred to Cys256. In the absence of Trx the second step (reduction of thiosulfonate) does not occur, but in the presence of excess substrate, APS binds, and the thiosulfonated C-terminal peptide is displaced from the active site. Consequently, the analysis reveals a dynamic role for the C-terminal tail in substrate binding and product release, such that APS binding is accompanied by closure of the C-terminal tail over the active site, bringing the catalytic cysteine into proximity with the substrate (Figure 6(c)). In the absence of a large excess of substrate, the C-terminus of the enzyme-thiosulfonate intermediate remains ordered, protecting it from proteolysis and preventing non-specific hydrolysis to sulfate. However, the second half of the reaction may require conformational rearrangement to make the enzyme-thiosulfonate intermediate accessible for reduction by Trx. Perhaps, the reductase activity represented in the first half of the reaction evolved from a protein ancestor that interacted with APS or a related metabolite. Since proteins that reduce disulfides are abundant in ancient bacterial species it may not have been necessary for an ancestral reductase to reduce the thiosulfonate bond in the second half of the reaction27. Instead, the glutathione-like GluCysGly motif at the C-terminus of APS reductase, which includes the catalytic cysteine, could have promoted recognition by Glutaredoxin or Trx.
Mössbauer analyses of the plant, P. aeruginosa and B. subtilis enzymes established the presence of a [4Fe-4S] cluster in APS reductase2,16,28. However, the unusual cysteine motif has spurred debate about the identity of the fourth ligand coordinating the [4Fe-4S] cluster2,18,20. This work establishes that the [4Fe-4S] cluster is ligated by four cysteines, Cys228 and Cys231 on one side, and the tandem pair, Cys139 and Cys140, within an α-helix on the other side (Figure 7(a)). Coordination by sequential cysteines to a [4Fe-4S] cluster has been observed in one other structure - the N2 cluster in the Nqo6 subunit of Thermus thermophilus NADH:ubiquinone oxidoreductase (Complex I)29. These tandem cysteines also reside within an α-helix. Further, Complex I exhibits substrate-induced conformational change in the Nqo6 subunit, suggesting that tandem cysteine coordination is associated with conformationally dynamic clusters30.
The [4Fe-4S] cluster and its four cysteine ligands crosslink and stabilize the protein fold. Cysteine mutagenesis and iron content analysis for M. tuberculosis18 and P. aeruginosa20 APS reductases demonstrate that the [4Fe-4S] cluster is required for catalytic activity. In addition, high resolution FT-ICR mass spectrometry data show that an intact [4Fe-4S] cluster is required for interaction with AMP or Trx, and that formation of the thiosulfonate intermediate stabilizes the cluster toward oxidation and loss of activity18. Furthermore, resonance Raman spectra of P. aeruginosa APS reductase show a marked change in Fe-Sγ stretching modes upon binding APS or AMP20,31. Taken together, these data imply an interaction between APS and the cluster and, in this context it has been previously proposed that the cofactor acts as a Lewis acid to facilitate nucleophilic attack on the sulfate moiety6,18. Yet in the current structure, the APS sulfate group is not in direct contact with the cluster (Figure 9(c)). Given the specific recognition of APS deep within the active site (Figures 9(a), (b)), interaction during the catalytic cycle would require movement of the cluster toward the sulfate group. This might entail motion of helices α5 and α6A (i.e. residues ~115–143; Figure 4(a)) translating the tandem CysCys ligands and the cluster. Conformational change involving the cluster would not be surprising, as the data indicate two other structural elements (Arg-loop and C-terminal tail) undergo substrate-associated conformational change. It would also be consistent with differential labeling of the cysteine ligands in cluster extrusion experiments, which are dependent upon the presence of ligand in the active site18.
In aconitase32, 4-hydroxybutyryl-CoA dehydratase33, and radical SAM enzymes34,35,36,37,38, substrates bind to one Fe site of a [4Fe-4S]2+ cluster23. In ferredoxin:thioredoxin reductase (FTR) one Fe of a [4Fe-4S]2+ cluster interacts with a fifth cysteine in a redox active disulfide39, and in heterodisulfide reductase (HDR) one Fe interacts with a substrate thiol40,41. In order for the APS reductase cluster to interact with APS, there must be rearrangement relative to the observed structure. One possibility, supported by biochemical, spectroscopic, and mass spectrometry experiments with M. tuberculosis APS reductase, proposes that a cysteine in the tandem pair (Cys140 in P. aeruginosa APS reductase) repositions itself while remaining ligated to iron6,18. Movement of Cys140, could be facilitated by the steric clashes that arise from CysCys ligation (Figure 7(b), Supplementary Figure 6). This model is supported by Mössbauer data for plant and P. aeruginosa APS reductases, which describe a diamagnetic [4Fe-4S]2+ cluster with Fe subsites in 3:1 ratio2,16, and changes in Fe-Sγ stretching modes upon addition of APS or AMP20,31.
PAPS reductases catalyze the same reaction as APS reductases (Figure 1). E. coli PAPS reductase, crystallized in the absence of substrate, has a fold that is similar to P. aeruginosa APS reductase with adenosine recognition and P-loop motifs, an Arg-rich loop, and a positively charged active site pocket26. The E. coli enzyme dimer corresponds to the AB pair in the P. aeruginosa APS reductase tetramer (Figure 4(c)). PAPS reductase also exhibits flexibility in the C-terminal tail as the last ordered residue observed is His21626 (Figure 3). In PAPS reductase the Arg-loop is folded over the active site (Figure 10) when it is unoccupied by substrate, rather than being displaced onto the enzyme surface (Figure 8).
Figure 10.
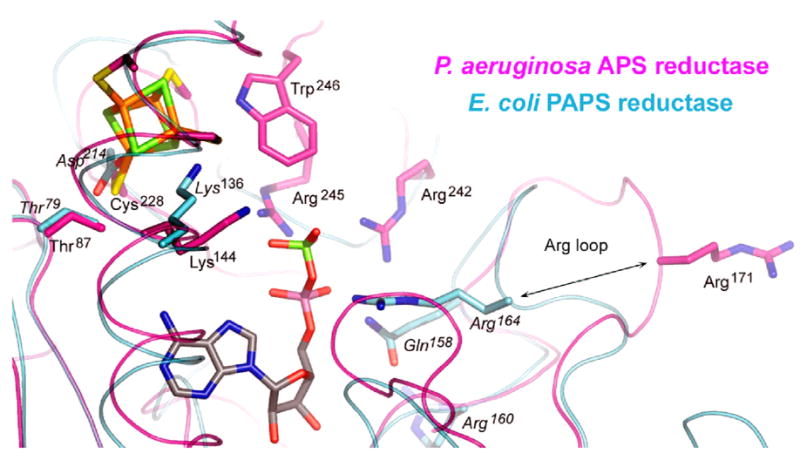
Superposition of the structures of P. aeruginosa APS reductase and E. coli PAPS reductase showing the positions of the [4Fe-4S] cluster and APS bound in APS reductase with respect to conserved active site residues in both enzymes. Distinct conformations of the Arg loop are indicated, showing that the loop folds into the active site of PAPS reductase in the absence of substrate. Arg164 (P. aeruginosa) and Arg171 (E. coli) are equivalent residues, conserved in 72 species of APS and PAPS reductases (Supplementary Figure 2).
Based on the structural similarity between P. aeruginosa APS and E. coli PAPS reductases, and the fact that the residues involved in adenosine recognition are largely conserved among sulfonucleotide reductases, it is likely that PAPS binds in a manner similar to that of APS. Consequently, the additional 3′-phosphate of PAPS may be accommodated in the P-loop, where Glu65 is predominantly replaced with Gln, and Asp66 is replaced with Ser or Ala (Figures 3, 9(b)). These substitutions would allow interaction of the amides with a 3′ phosphate and accommodate the bulkier moiety. It appears that Glu65 and Asp66 define substrate specificity in P. aeruginosa APS reductase, compensating for the charge, volume, and hydrogen bonding potential of a 3′ phosphate, as if the substrate were PAPS.
Sequence alignment highlights the evolutionary divergence of APS vs. PAPS reductases (Figure 3, Supplementary Figure 2). In APS reductases, 38 sequences indicate the presence of a [4Fe-4S] cluster, whereas in 34 PAPS reductases, an alternative constellation of residues is present. Superposition of the P. aeruginosa APS and E. coli PAPS reductase structures results in an rmsd of 6.67 Å for 188 pairs Cα atoms. Within this framework only three active site residues are invariant, Thr87/Thr79, Lys144/Lys136, and Arg171/Arg164 (Figure 10). The Arg appears to play a key role in alternate conformations of the Arg-loop. The lysine interacts with the substrate and appears capable of adopting multiple conformations. The Thr interacts with a cluster ligand (APS reductase), or with the conserved residue Asp214 (PAPS reductase). In light of the absence of a cofactor in PAPS reductase, perhaps the interaction between Thr79 and Asp214 is stabilizing in place of the cluster, like another involving Tyr131 with His216, which is conserved in 32 of 34 PAPS reductases (Supplementary Figure 2). In contrast, different residues are conserved in association with the [4Fe-4S] cluster, such as Arg143 and Trp246 (Figure 7(a)). An exception exists in the B. subtilis enzyme, which utilizes both APS and PAPS as substrates, and which contains an essential [4Fe-4S] cluster28. In this case, the sequence is APS reductase-like, except for the P-loop, which is more PAPS reductase-like (Figure 3).
In addition to E. coli PAPS reductase26, a search for homologous folds in the Protein Data Bank42 yields two significant alignments with P. aeruginosa APS reductase: Pseudomonas syringae ATP sulfurylase5, and E. coli GMP synthetase24. Both enzymes are ATP pyrophosphatases with adenine recognition and P-loop motifs. It is notable that ATP sulfurylase shares additional similarities to APS reductase, including an arginine-rich loop following the β4 strand, a lysine oriented like Lys144/Lys136 in APS/PAPS reductases, and mobility of C-terminal residues, which are positioned above the ATP site for sulfate binding5. Further, the involvement of a G protein in ATP sulfurylase4,5 reflects a requirement for conformational change during synthesis of APS, reflecting perhaps the mobility of the C-terminal tail, Arg-loop, and apparently the [Fe-S] cluster as well, in APS reductase.
Materials and Methods
Materials used, and details of protein expression and purification, which followed previously published procedures3,6,18, are described in the Supplemental Material.
General kinetic analysis
APS reduction reactions were carried out as described previously6,18. Reactions were performed at 30 °C and contained 5 nM APS reductase, 20 μM APS, 10 μM E. coli Trx, 5 mM DTT in 50 mM Bis-Tris Propane pH 7.0 and 100 mM NaCl. Reactions were followed to completion (≥ 5 half lives), except for very slow reactions. The initial linear portion of the reaction (≤ 20% reaction) was used to calculate the reaction rate. Kinetic data were measured in at least two independent experiments and the standard error was less than 15%.
Effect of small-molecule reductants on APS reductase activity
The ability of various small-molecule reductants to support APS reduction by M. tuberculosis APS reductase was assayed as described above, except that 10 mM reductant (DTT, GSH, reduced lipoic acid or dithionite) was used in place of 10 μM E. coli Trx and 5 mM DTT (Supplementary Table 1 online). Control reactions contained 10 μM E. coli Trx, supplemented with 10 mM DTT (required to regenerate reduced Trx)6.
Sodium dithionite (DT) alone is not able to reduce the APS reductase thiosulfonate intermediate (Supplementary Table 1), but it is acid labile, decomposing to sulfite and other products, and sulfite is known to react with protein disulfides to yield thiosulfonate. Under the conditions used for anaerobic crystallization (below) the relative concentrations of components at pH 6.5 were 40 uM protein, 2.5 mM APS, 2.3 mM DTT, and 2.5 mM DT. The data support the conclusion that the sulfate group attached to Cys256 during crystallization is derived from the substrate, APS, and not from sulfite via decomposition of dithionite (Supplementary Figure 4a). In particular, the reduced protein does not contain disulfides and the anaerobic conditions would prevent their formation. In addition, the enzyme reaction with APS is fast (Supplementary Figure 5), and a >60-fold excess of APS over enzyme was present. On the other hand, if DT decomposition products were to react with free sulfhydryls, 115-fold excess reduced DTT over Cys256 would quench this side reaction.
Dependence of APS reductase activity on APS concentration
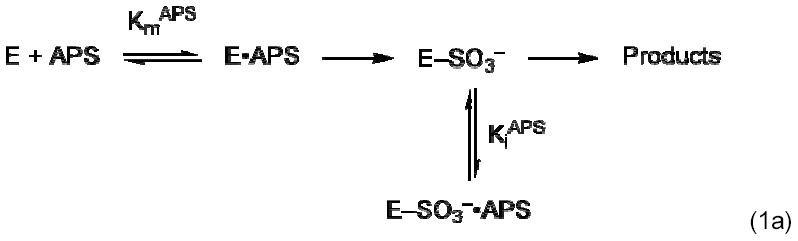
The apparent Km APS for M. tuberculosis APS reductase was determined at two E. coli Trx concentrations, 1 and 10 μM. Reaction rate was quantified as a function of APS concentration. APS concentration dependencies reproducibly gave inhibition above 5 μM (1 μM Trx) and 15 μM (10 μM Trx) APS. These concentration dependencies were therefore fit to a model in which a second APS that is inhibitory can bind to the E-Cys-Sγ–SO3− complex (Eq. 1b), derived from the reaction scheme in Eq. 1a. Using nonlinear regression analysis, fits of the data to this model gave values of R2 > 0.98 (KaleidaGraph, Synergy Software, Reading, PA).
| (1b) |
Limited proteolysis of APS reductase
P. aeruginosa or M. tuberculosis APS reductase (50 μM) was incubated with or without equimolar APS for 10 min at RT (Supplementary Figure 5). Subsequently, all samples were incubated on ice with 10 μg/ml trypsin. At the time points indicated, a 15-μl aliquot of the proteolysis reaction was quenched by the addition of SDS-load dye and heated at 100 °C for 2 min. Samples were analyzed by SDS-PAGE using 4–12% Criterion gradient gels (Bio-Rad, Hercules, CA). To map trypsin digest sites, reactions identical to those described above were allowed to proceed for a total of 60 min (P. aeruginosa APS reductase) or 90 min (M. tuberculosis APS reductase) and stopped by freezing in liquid nitrogen. Peptide fragments were separated by reversed-phase chromatography on a Vydac 218TP54 protein and peptide C18 column (The Separations Group, Hesperia, CA). The molecular weights of peptide fragments were determined by electrospray mass spectrometry and their identities determined by analysis using GPMAW43.
Mass spectrometric analysis
All mass spectrometry data were acquired on a Bruker Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with an actively shielded 7 Tesla superconducting magnet as previously described6. Details, including the preparation and analysis of dissolved crystal samples, are provided in the Supplemental Methods.
Crystallization
APS reductase enzymes from four bacterial species were expressed, purified, and screened for crystallization by vapor diffusion. Samples of M. tuberculosis, M. smegmatis, R. meliloti, and P. aeruginosa APS reductase were concentrated under N2 and used for crystallization trials in an anaerobic glove box (<1 ppm O2). Solutions were degassed, and freshly prepared sodium dithionite was added to buffers to a concentration of 5–10 mM. Protein solubility curves for each APS reductase were determined using a matrix of four protein concentrations vs. precipitant concentration (PEG3350 or ammonium sulfate) at four discrete pHs (5.6, 6.5, 7.5, and 8.5). The curves indicated a sharp transition in solubility for each protein at ~2 mg/ml and pH 7.5. These conditions were then used in sparse matrix screening, which yielded the most hits with P. aeruginosa APS reductase. Following refinement of the best conditions, clusters of thin, blade-shaded, brown crystals could be grown reproducibly within 1–2 weeks. Specifically, P. aeruginosa APS reductase at 2.7 mg/ml in 50 mM TrisHCl pH 8.0, 150 mM NaCl, 10% glycerol, 5 mM DTT, was mixed with aliquots of APS and sodium dithionite stock solutions in the glove box, and then mixed in 1:1 ratio with a reservoir solution of 1.5 M ammonium sulfate, 100 mM sodium cacodylate, pH 6.5. The resulting final concentrations in the crystallization drop (5 μl) were: 1.2 mg/ml APS reductase (40 μM), 22.5 mM TrisHCl pH 8.0, 67.5 mM NaCl, 4.5% glycerol, 2.3 mM DTT, 2.5 mM APS, 2.5 mM sodium dithionite, 0.75 M ammonium sulfate, and 50 mM sodium cacodylate pH 6.5. To improve the size and yield of single crystals, crystals were harvested and used to seed 2 μl drops of the same solution, except at 0.65 M ammonium sulfate. Seeded drops were incubated under paraffin oil at 24 °C for 1–2 weeks in the glove box. Larger single crystals are uniformly birefringent. For data collection, seeded drops under paraffin oil were removed from the glove box, and crystals were transferred to 200 μl of cryoprotectant solution (0.8 M ammonium sulfate, 100 mM sodium cacodylate pH 6.5, 5 mM sodium dithionite, 25% glycerol). Within ~10 min. the crystals were transferred to nylon loops and flash frozen in liquid N2. During this time, no discoloration of the crystals was observed.
Crystallographic Analysis
The structure was solved using Fe K-edge SAD and MAD data, combined with non-crystallographic symmetry averaging, solvent flattening, and phase extension, and refined at 2.7 Å resolution (Table 1).
For initial diffraction experiments, duplicate data sets were collected at BL 9-1. Data processing established that the space group was P1, and not P21, with two APS reductase tetramers in the unit cell (63% solvent) related by a pseudo-21 screw axis parallel with the b-axis. For all crystals, Rmerge was >0.25 for data indexed and scaled in monoclinic space groups, but <0.10 for space group P1. All data sets indicated normal intensity statistics without indication of twinning. A native set was collected to 2.70 Å resolution (Table 1).
The expected anomalous and dispersive diffraction ratios for 4 Fe atoms per 30 kD protein were ~0.037 and ~0.040, respectively. A SAD data set was collected near the Fe K-edge; due to the triclinic symmetry, frames collected over 720° achieved only ~6-fold redundancy (Table 1). The anomalous difference Patterson map at 5.0 Å resolution contained 6-8σ peaks for the [Fe-S] clusters; the coordinates for eight clusters were solved using both CNS44 and ShelxD45. The eight [Fe-S] clusters occur as two tetramers related by pseudo-21 symmetry; further, cluster sites within each tetramer are arranged as a trigonal antiprism with 2-fold symmetry only. This defines 4-fold non-crystallographic symmetry (NCS) for the entire unit cell, and accounts for the crystals being triclinic and not monoclinic.
A three wavelength MAD data set was collected (Table 1); due to decay, these data sets had lower redundancy than the SAD data sets (540° total rotation per wavelength). Using the 8 cluster positions as eight pseudo-atom sites at 4.5 Å resolution, MAD phases were calculated, and the electron density map was subjected to 4-fold NCS averaging and solvent flattening using CNS. Approximately 70% of the polypeptide chain in subunits A and B was modeled into this map as poly-Ala using Xfit46. The model was used for phase combination with the 4.5 Å MAD phases, the resulting phases were extended to 4.0 Å by NCS averaging and solvent flattening, and more of the polypeptide was modeled. This process was repeated at 3.7 Å and 3.5 Å. In the 3.5 Å map cysteine ligation to the [Fe-S] clusters in subunits A and B of the NCS averaged map was readily apparent, together with the density for a [4Fe-4S] cluster in each subunit. An idealized [4Fe-4S] cluster was used to fit the density with the directional restraints provided by the cysteine ligands, resulting in a 32-site model for the individual Fe positions. The individual Fe sites were refined and used to calculated MAD phases to 3.5 Å resolution using CNS (Table 1). The phase combination, phase extension, NCS-averaging, solvent flattening, and model building process was repeated as before at 3.5 Å, 3.2 Å and 3.0 Å, until the model for subunits A and B was essentially complete. The model was then expanded to all 8 subunits in the unit cell, the NCS restraints were relaxed, and the refinement proceeded normally to 2.70 Å using model based σA-weighted 2|Fo|-|Fc| and composite omit maps in CNS. A final, unbiased, σA-weighted |Fo|-|Fc| difference map was used to model APS into subunits B, D, F, and H (Supplementary Figure 3), and identify tightly bound H2O molecules. Statistics for the refinement and final model are summarized in (Table 1).
Protein Data Bank accession code
Coordinates have been deposited with the RCSB (accession code 2GOY).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants GM-48870 to CDS and AI-51622 to CRB. KSC was supported by a postdoctoral fellowship from the Damon Runyon Cancer Research Foundation (DRG-1783-03). We thank David S. King of the HHMI Mass Spectrometry Laboratory at the University of California, Berkeley for assistance with mass spectrometry and peptide mapping. We thank the generous assistance of staff personnel at the Stanford Synchrotron Radiation Laboratory. SSRL is a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.
References
- 1.Bick JA, Dennis JJ, Zylstra GJ, Nowack J, Leustek T. Identification of a new class of 5′-adenylylsulfate (APS) reductases from sulfate-assimilating bacteria. J Bacteriol. 2000;182:135–142. doi: 10.1128/jb.182.1.135-142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopriva S, Buchert T, Fritz G, Suter M, Weber M, Benda R, Schaller J, Feller U, Schurmann P, Schunemann V, Trautwein AX, Kroneck PM, Brunold C. Plant adenosine 5′-phosphosulfate reductase is a novel iron-sulfur protein. J Biol Chem. 2001;276:42881–42886. doi: 10.1074/jbc.M107424200. [DOI] [PubMed] [Google Scholar]
- 3.Williams SJ, Senaratne RH, Mougous JD, Riley LW, Bertozzi CR. 5′-Adenosinephosphosulfate lies at a metabolic branch point in mycobacteria. J Biol Chem. 2002;277:32606–32615. doi: 10.1074/jbc.M204613200. [DOI] [PubMed] [Google Scholar]
- 4.Leyh TS, Taylor JC, Markham GD. The sulfate activation locus of Escherichia coli K12: cloning, genetic, and enzymatic characterization. J Biol Chem. 1988;263:2409–2416. [PubMed] [Google Scholar]
- 5.Mougous JD, Lee DH, Hubbard SC, Schelle MW, Vocadlo DJ, Berger JM, Bertozzi CR. Molecular basis for G protein control of the prokaryotic ATP sulfurylase. Mol Cell. 2006;21:109–122. doi: 10.1016/j.molcel.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KS, Gao H, Chen H, Stout CD, Leary JA, Bertozzi CR. A conserved mechanism for sulfonucleotide reduction. PLoS Biology. 2005;3:e250. doi: 10.1371/journal.pbio.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampreia J, Pereira AS, Moura JJG. Adenylylsulfate reductases from sulfate-reducing bacteria. Methods in Enzymology. 1994;243:241–260. [Google Scholar]
- 8.Aleman M, Beigier-Bompadre M, Borghetti C, de la Barrera S, Abbate E, Isturiz M, Sasiain MC. Activation of peripheral blood neutrophils from patients with active advanced tuberculosis. Clin Immunol. 2001;100:87–95. doi: 10.1006/clim.2001.5044. [DOI] [PubMed] [Google Scholar]
- 9.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 12.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci USA. 2001;98:12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senaratne RH, De Silva AD, Williams SJ, Mougous JD, Reader JR, Zhang T, Chan S, Sidders B, Lee DH, Chan J, Bertozzi CR, Riley LW. 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol Microbiol. 2006;59:1744–1753. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 14.Berendt U, Haverkamp T, Prior A, Schwenn JD. Reaction mechanism of thioredoxin: 3′-phospho-adenylylsulfate reductase investigated by site-directed mutagenesis. Eur J Biochem. 1995;233:347–356. doi: 10.1111/j.1432-1033.1995.347_1.x. [DOI] [PubMed] [Google Scholar]
- 15.Harjes S, Bayer P, Scheidig AJ. The crystal structure of human PAPS synthetase 1 reveals asymmetry in substrate binding. J Mol Biol. 2005;347:623–635. doi: 10.1016/j.jmb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Kopriva S, Buchert T, Fritz G, Suter M, Benda R, Schunemann V, Koprivova A, Schurmann P, Trautwein AX, Kroneck PM, Brunold C. The presence of an iron-sulfur cluster in adenosine 5′-phosphosulfate reductase separates organisms utilizing adenosine 5′-phosphosulfate and phosphoadenosine 5′-phosphosulfate for sulfate assimilation. J Biol Chem. 2002;277:21786–21791. doi: 10.1074/jbc.M202152200. [DOI] [PubMed] [Google Scholar]
- 17.Lansdon EB, Segel IH, Fisher AJ. Ligand-induced structural changes in adenosine 5′-phosphosulfate kinase from Penicillium chrysogenum. Biochemistry. 2002;41:13672–13680. doi: 10.1021/bi026556b. [DOI] [PubMed] [Google Scholar]
- 18.Carroll KS, Gao H, Chen H, Leary JA, Bertozzi CR. Investigation of the iron-sulfur cluster in Mycobacterium tuberculosis APS reductase: implications for substrate binding and catalysis. Biochemistry. 2005;44:14647–14657. doi: 10.1021/bi051344a. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Suter M, Brunold C, Kopriva S. Sulfate assimilation in higher plants: Characterization of a stable intermediate in the adenosine 5′-phosphosulfate reductase reaction. Eur J Biochem. 2000;267:3647–3653. doi: 10.1046/j.1432-1327.2000.01394.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim SK, Rahman A, Bick JA, Conover RC, Johnson MK, Mason JT, Hirasawa M, Leustek T, Knaff DB. Properties of the cysteine residues and iron-sulfur cluster of the assimilatory 5′-adenylyl sulfate reductase from Pseudomonas aeruginosa. Biochemistry. 2004;43:13478–13486. doi: 10.1021/bi048811t. [DOI] [PubMed] [Google Scholar]
- 21.Kim SK, Rahman A, Conover RC, Johnson MK, Mason JT, Gomes V, Hirasawa M, Moore ML, Leustek T, Knaff DB. Properties of the cysteine residues and the iron-sulfur cluster of the assimilatory 5′-adenylyl sulfate reductase from Enteromorpha intestinalis. Biochemistry. 2006;45:5010–5018. doi: 10.1021/bi0519250. [DOI] [PubMed] [Google Scholar]
- 22.Fee JA, Castagnetto JM, Case DA, Noodleman L, Stout CD, Torres RA. The circumsphere as a tool to assess distortion in [4Fe-4S] atom clusters. J Biol Inorg Chem. 2003;8:519–526. doi: 10.1007/s00775-003-0445-8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 24.Tesmer JJ, Klem TJ, Deras ML, Davisson VJ, Smith JL. The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families. Nat Struct Biol. 1996;3:74–86. doi: 10.1038/nsb0196-74. [DOI] [PubMed] [Google Scholar]
- 25.Bork P, Koonin EV. A P-loop-like motif in a widespread ATP pyrophosphatase domain: implications for the evolution of sequence motifs and enzyme activity. Proteins. 1994;20:347–355. doi: 10.1002/prot.340200407. [DOI] [PubMed] [Google Scholar]
- 26.Savage H, Montoya G, Svensson C, Schwenn JD, Sinning I. Crystal structure of phosphoadenylyl sulphate (PAPS) reductase: a new family of adenine nucleotide alpha hydrolases. Structure. 1997;5:895–906. doi: 10.1016/s0969-2126(97)00244-x. [DOI] [PubMed] [Google Scholar]
- 27.Martin JL. Thioredoxin – a fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/s0969-2126(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 28.Berndt C, Lillig CH, Wollenberg M, Bill E, Mansilla MC, de Mendoza D, Seidler A, Schwenn JD. Characterization and reconstitution of a 4Fe-4S adenylyl sulfate/phosphoadenylyl sulfate reductase from Bacillus subtilis. J Biol Chem. 2004;279:7850–7855. doi: 10.1074/jbc.M309332200. [DOI] [PubMed] [Google Scholar]
- 29.Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science. 2006;311:1430–1436. doi: 10.1126/science.1123809. [DOI] [PubMed] [Google Scholar]
- 30.Mamedova AA, Holt PJ, Carroll J, Sazanov LA. Substrate-induced conformational change in bacterial complex I. J Biol Chem. 2004;279:23830–23836. doi: 10.1074/jbc.M401539200. [DOI] [PubMed] [Google Scholar]
- 31.Kim SK, Rahman A, Mason JT, Hirasawa M, Conover RC, Johnson MK, Miginiac-Maslow M, Keryer E, Knaff DB, Leustek T. The interaction of 5′-adenylylsulfate reductase from Pseudomonas aeruginosa with its substrates. Biochim Biophys Acta. 2005;1710:103–112. doi: 10.1016/j.bbabio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Beinert H, Kennedy MC, Stout CD. Aconitase as Iron-Sulfur Protein, Enzyme, and Iron-Regulatory Protein. Chem Rev. 1996;96:2335–2374. doi: 10.1021/cr950040z. [DOI] [PubMed] [Google Scholar]
- 33.Martins BM, Dobbek H, Cinkaya I, Buckel W, Messerschmidt A. Crystal structure of 4-hydroxybutyryl-CoA dehydratase: radical catalysis involving a [4Fe-4S] cluster and flavin. Proc Natl Acad Sci U S A. 2004;101:15645–9. doi: 10.1073/pnas.0403952101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkovitch F, Nicolet Y, Wan JT, Jarrett JT, Drennan CL. Crystal structure of biotin synthase, an S-adenosylmethionine-dependent radical enzyme. Science. 2004;303:76–79. doi: 10.1126/science.1088493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanzelmann P, Schindelin H. Crystal structure of the S-adenosylmethionine-dependent enzyme MoaA and its implications for molybdenum cofactor deficiency in humans. Proc Natl Acad Sci USA. 2004;101:12870–12875. doi: 10.1073/pnas.0404624101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Layer G, Grage K, Teschner T, Schunemann V, Breckau D, Masoumi A, Jahn M, Heathcote P, Trautwein AX, Jahn D. Radical S-adenosylmethionine enzyme coproporphyrinogen III oxidase HemN: functional features of the [4Fe-4S] cluster and the two bound S-adenosyl-L-methionines. J Biol Chem. 2005;280:29038–29046. doi: 10.1074/jbc.M501275200. [DOI] [PubMed] [Google Scholar]
- 37.Layer G, Kervio E, Morlock G, Heinz DW, Jahn D, Retey J, Schubert WD. Structural and functional comparison of HemN to other radical SAM enzymes. Biol Chem. 2005;386:971–980. doi: 10.1515/BC.2005.113. [DOI] [PubMed] [Google Scholar]
- 38.Lepore BW, Ruzicka FJ, Frey PA, Ringe D. The x-ray crystal structure of lysine-2,3-aminomutase from Clostridium subterminale. Proc Natl Acad Sci USA. 2005;102:13819–13824. doi: 10.1073/pnas.0505726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai S, Schwendtmayer C, Schurmann P, Ramaswamy S, Eklund H. Redox signaling in chloroplasts: cleavage of disulfides by an iron-sulfur cluster. Science. 2000;287:655–658. doi: 10.1126/science.287.5453.655. [DOI] [PubMed] [Google Scholar]
- 40.Hedderich R, Hamann N, Bennati M. Heterodisulfide reductase from methanogenic archaea: a new catalytic role for an iron-sulfur cluster. Biol Chem. 2005;386:961–970. doi: 10.1515/BC.2005.112. [DOI] [PubMed] [Google Scholar]
- 41.Shokes JE, Duin EC, Bauer C, Jaun B, Hedderich R, Koch J, Scott RA. Direct interaction of coenzyme M with the active-site Fe-S cluster of heterodisulfide reductase. FEBS Lett. 2005;579:1741–1744. doi: 10.1016/j.febslet.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Holm L, Sander C. Mapping the protein universe. Science. 1996;273:595–603. doi: 10.1126/science.273.5275.595. [DOI] [PubMed] [Google Scholar]
- 43.Peri S, Steen H, Pandey A. GPMAW--a software tool for analyzing proteins and peptides. Trends Biochem Sci. 2001;26:687–689. doi: 10.1016/s0968-0004(01)01954-5. [DOI] [PubMed] [Google Scholar]
- 44.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 45.Sheldrick GM. Macromolecular phasing with SHELXE. Z Kristallogr. 2002;217:644–650. [Google Scholar]
- 46.McRee DE. XtalView/Xfit--A versatile program for manipulating atomic coordinates and electron density. J Struct Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.