Abstract
Aim: Increased proliferation of tumour cells has prognostic value in human invasive breast carcinomas (IBCs), and high histology grade and cyclin A expression, which may reflect high proliferation rate, are associated with poor prognosis. Expression of HsMCM2 is related to cell proliferation. This study evaluates the correlation between the expression of cyclins A, D1, D3, and E, Ki-67, proliferating cell nuclear antigen (PCNA), histology grade, and HsMCM2 expression, in addition to the independent prognostic value of HsMCM2 expression in human IBCs.
Methods: Immunohistochemistry to evaluate HsMCM2, Ki-67, and PCNA expression in tumours from 147 patients with IBC.
Results: Nuclear staining for HsMCM2 was seen in 10–30% of the tumour cells in 30 samples, in 30–70% in 40 samples, in > 70% in 44 samples, and in < 10% in 33 samples. One way ANOVA showed a significant association between expression of HsMCM2 and cyclin A, D3, E, histology grade, and Ki-67. A borderline correlation was seen between HsMCM2 and PCNA. In multivariate analysis, the only association was with cyclin A, in addition to a borderline association with histology grade. In a Cox regression hazards model, expression of HsMCM2 was associated with poor patient survival, although it lost its independent prognostic value when cyclin A expression was included. Ki-67 and PCNA expression were not associated with patient survival.
Conclusion: Cyclin A expression is independently associated with HsMCM2 expression, histology grade, and Ki-67. HsMCM2 expression is associated with poor patient survival, although it loses prognostic value when adjusted for cyclin A.
Keywords: HsMCM2, breast carcinomas, immunohistochemistry, cyclin A, histology grade
The replication of genomic DNA is limited to a single round in each cell cycle by a licensing factor, which binds to origins of replication in M phase and is released after the origins have fired in S phase. One component of licensing factor is a complex of six minichromosome maintenance (MCM) proteins, which bind to the origin recognition complex.1, 2 As predicted by the licensing model, most MCM proteins are released from chromatin during the S phase and reassociate at the end of mitosis.3–5 In addition to promoting replication, MCMs may also aid replication fork movement. MCM2 is a human member of the family of MCM proteins. The MCM proteins are found in all eukaryotes and are believed to play a role in DNA replication, especially in the machinery that insures its once in each cycle regulation.6–8 In both yeast and mammalian cells, MCMs are far more abundant than replication origins.9, 10 The excess of MCMs over origins suggests that these proteins may also have other roles, such as in transcription.11 The amino acid sequence of MCM2 is most similar to that of the Saccharomyces cerevisiae protein, MCM2, suggesting a new designation of MCM2 as MCM2 from Homo sapiens or HsMCM2.
HsMCM2 mRNA and protein values remain constant during the cell cycle in human cell lines,12 but decrease greatly in cells with a lower proliferation rate. Amounts of HsMCM2 mRNA were found to decrease dramatically during in vitro differentiation of human myeloblastoid HL-60 cells. These findings, together with the role of the protein in DNA replication, suggest a close relation between the expression of the HsMCM2 protein and the rate of cell proliferation.
In the eukaryotic cell cycle there are two crucial transition steps; the onset of the replication of chromosomal DNA and the entry into cell division. These two steps, separated by the cell cycle phase termed G2, seem to be coupled.13 It is possible that the same proteins may take part in the regulation and/or the execution of both events. An example is the complex of protein kinase cdc2 with cyclins, which has been shown to play an important role in the cell cycle of both yeast and mammalian cells.14
“The minichromosome maintenance proteins are found in all eukaryotes and are believed to play a role in DNA replication, especially in the machinery that insures its once in each cycle regulation”
According to their sequence motif, pattern of expression, and activity, cyclins exert a regulatory function in the control of transition stages of the cell cycle, and are grouped as cyclins of the G1, S, and G2/M phases. The phase activity of cyclins contributes to phosphorylation of specific substrates, such as cyclin dependent kinases and retinoblastoma protein required for the progression through G1, the beginning of the different phases, and the completion of the cell cycle.15 Among the G1 cyclins, the ectopic expression of cyclin D1 and cyclin E in mammalian fibroblasts shortens G1 and reduces the serum dependency of these cells for S phase entry.16–19. Unlike G1 phase cyclins, A and B family cyclins achieve their maximum values later in the cycle. Cyclin A, which is synthesised during DNA replication and the G2/M transition, is involved in cellular activities that promote both replication and transcription,20, 21 and reflects the proliferative activity of the tumour. The activities of the B cyclins are essential for G2 transition and mitosis.18, 22 Cyclin A is a mitotic cyclin and is required for DNA replication in the S phase of the cell cycle. It has been suggested that cyclin A plays a role in cellular transformation because of its ability to form complexes with the adenovirus E1A protein23 and transcription factors DP-1, which is involved in the regulation and coordination of early cell cycle progression, and elongation factor 2 (E2F), in addition to retinoblastoma protein.24, 25
The histology grade of an invasive carcinoma of the breast is calculated according to the Nottingham modification of the Bloom and Ricardson method,26 and may reflect the proliferative capacity of the tumour cells. Tubule formation, nuclear pleomorphism, and mitotic frequency are evaluated and each variable is given a score between 1 and 3. The cumulative score is used to assign a numerical grade of 1, 2, or 3. The grade derived by this method has been shown in multivariate analysis to be an independent prognostic factor.26 It has been shown in many series to be reproducible when strict criteria are applied to well fixed specimens.27–29
We have previously shown that the overexpression of cyclin A is associated with reduced survival in patients with invasive breast carcinoma.30 Because histology grade, HsMCM2 expression, and cyclin A expression may reflect the proliferative activity of the tumour cells, we wanted to evaluate the relation between these three parameters and their independent prognostic value in a series of patients with breast cancer who have a long follow up time. We were also interested in the relation between HsMCM2 expression and the expression of other cyclins known to play an important role in breast cancer tumorigenesis (cyclins D1, D3, and E). Because HsMCM2 is thought to be a cell proliferation marker, the correlation between HsMCM2 and other known proliferation markers, Ki-67 and proliferating cell nuclear antigen (PCNA), was also investigated.
PATIENTS AND METHODS
Patients
The patients in our study have been described previously.30 In brief, there were 147 patients with primary breast carcinoma in whom 14 (9.5%) tumours were classified as invasive lobular, 114 as invasive ductal (77.6%), 12 (8.2%) as other types, and seven (4.8%) patients had no available tumour classification. Lymph node dissection was performed in 144 patients. Of these, 81 (56%) patients were lymph node negative and 63 (44%) were lymph node positive. Seven of the tumours (5%) were classified as histological grade 1, 90 (61%) as grade 2, and 52 (34%) as grade 3. Grading of the tumours was based on the recommendations made by Elston and Ellis.26 All samples included in our study were judged after histological evaluation to contain more than 50% tumour tissue. The follow up time was between 10 and 14 years. Eighty one of the patients developed distant metastases during the follow up time. Seventy three patients died during the follow up period. Of these, 49 (33.3%) patients died of breast cancer, 23 of other causes, and for one patient information about the cause of death could not be obtained.
Immunohistochemistry
The immunohistochemical methodology has been described previously.31 Briefly, 4–6 μm thick sections from formalin fixed, paraffin wax embedded tumour tissue obtained at the time of surgery were cut and mounted on to coated slides. After antigen retrieval by microwave, immunostaining was performed in an Optimax plus automated cell stainer (model 1.5; BioGenex, San Ramon, California, USA) according to the operating manual. Table 1 shows the antibodies used for the detection of HsMCM2, Ki-67, PCNA, and the cyclins, together with their sources. All series included positive and negative controls. Only cells with staining of the nuclei were scored as positive. The number of immunoreactive cells was estimated semiquantitatively, as follows: grade +, 10–30% positive cells; grade ++, 30–70% positive cells; and grade +++, > 70% positive cells. Tumour samples showing immunoreactivity in < 10% of tumour cells were scored grade 0. For every sample, at least 200 (usually more than 500) tumour cells were analysed. Tumour samples were analysed by two investigators (IRK Bukholm and JM Nesland) independently. In cases with discrepancies in immunohistochemistry grading, a consensus was achieved after re-examination.
Table 1.
Antibodies, their source, and working conditions
| Antibody/antigen | Dilution | Source | Pretreatment |
| HsMCM2 | 1/300 | Transduction Laboratories (Newington, New Hampshire, USA) | 2×5 minutes in microwave |
| Cyclin A | 1/50 | Novocastra (Newcastle upon Tyne, UK) | 2×5 minutes in microwave |
| Cyclin D1 | 1/200 | Oncogene Research (Manhasset, New York, USA) | 2×5 minutes in microwave |
| Cyclin D3 | 1/25 | Dako (Carpenteria, California, USA) | 4×5 minutes in microwave |
| Cyclin E | 1/100 | Santa Cruz Biotechnology (Santa Cruz, California, USA) | 4×5 minutes in microwave |
| Ki-67 | 1/25 | Dako | 5×5 minutes in microwave |
| PCNA | 1/500 | Novocastra | 2×5 minutes in microwave |
Double immunostaining methods
Double staining was performed on sections from five cases to evaluate whether the same cell expressed both cyclin A and HsMCM2.
In the double immunoenzymatic technique alkaline phosphatase was used as label for the first antibody and peroxidase for the second antibody. Dewaxed sections were treated with 1% hydrogen peroxidase for 10 minutes to block endogenous peroxidase. To unmask the cyclin A epitopes, the sections were microwaved in 1mM EDTA (pH 8.0) for 2 × 5 minutes. The sections were incubated for 30 minutes with monoclonal anticyclin A antibodies before sequentially incubating with goat antimouse IgG (Dako, Carpenteria, California, USA), diluted 1/100, for 30 minutes and alkaline phosphatase mouse anti-alkaline phosphate (Dako), diluted 1/50, for 20 minutes. The alkaline phosphatase was detected using a mixture of nitroblue tetrazolium (NBT) and 5-bromo 4-chloro-3indolyl-phosphatase (BCIP) (Boehringer Mannheim Biochemica, Tokyo, Japan) for 45 minutes in the dark. After staining with NBT/BCIP the sections were microwaved in 10mM citrate buffer (pH 6.0) for 2 × 5 minutes to exclude the possibility of a crossreaction. The sections were treated with monoclonal anti-HsMCM2 antibodies for 30 minutes before sequentially incubating with biotin labelled secondary antibody (BioGenex), diluted 1/30 for 20 minutes, streptavidin–peroxidase (BioGenex), diluted 1/30 for 20 minutes, and diaminobenzidine for five minutes. Tissue sections were mounted in glycerin jelly. Controls included omission of the first primary antibody and omission of the second primary antibody.
Statistical methods
Follow up data were taken from the time of the last clinical appointment or the date of death. All statistical calculations were performed using the SPSS (Chicago, Illinois, USA) Data Analysis Program (SPSS for Windows version 11). For analysis of survival, the Cox regression hazard model was used. The proportional hazard criteria for use of the model were fulfilled. Pearson’s correlation was used to assess initial correlations between HsMCM2 and other covariates. The association between HsMCM2 and the cyclins was tested in univariate models with one way ANOVA and in multivariate models with linear regression. Significance was set at p = 0.05.
RESULTS
When HsMCM2 immunoreactivity was evaluated, we detected strong (+++) staining of the tumour cell nuclei in 44 (30.0%) breast carcinomas. Forty (27.2%) samples showed staining of 30–70% of the tumour cells, and were scored as ++. In 30 (20.4%) samples, staining was detected in only 10–30% of the tumour cell nuclei; these samples were scored as +. Thirty three (22.4%) samples showed staining in less than 10% of the tumour cell nuclei; these samples were scored as 0. The cut off value was set at 10% because positive immunoreactivity to HsMCM2 could be detected in up to 10% of the cells in some cases of normal tissue. However, most of the sections of normal tissue did not show immunoreactivity to HsMCM2.
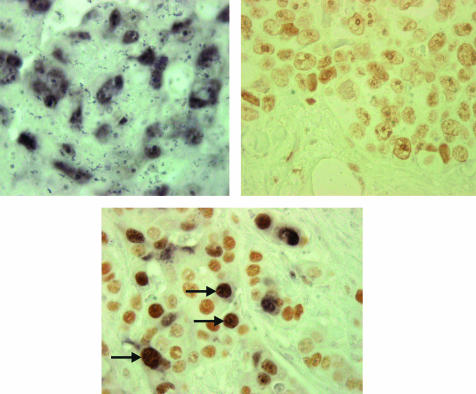
Of the 30 tumour samples scored as 0, only four samples showed immunoreactivity in 1–10% of the cells; the remainder showed no immunoreactivity. Whenever present, normal tissue showed little or no immunoreactivity to HsMCM2. Figure 1B,C shows examples of HsMCM2 immunoreactivity and double staining.
Figure 1.
Tumour cell nuclei showing immunoreactivity to (A) cyclin A and (B) HsMCM2. (C) Double staining for cyclin A and HsMCM2 is seen in tumour cells (arrows). Immunoreactivity was detected only in the tumour cell nuclei.
Tissue samples from 72 patients were available for Ki-67 immunostaining. Of these, 50 showed immunoreactivity (23 scored as +, 18 as ++, and nine as +++), whereas 22 were scored as 0. When immunoreactivity to PCNA was evaluated in tumour tissue from 92 patients, 87 of these showed positive immunoreactivity (10 scored as +, 30 as ++, and 47 as +++). Only five samples were scored as 0 (table 2).
Table 2.
Distribution of immunoreactivity scores for the different proteins
| Immunostaining score | ||||
| Protein | 0 | + | ++ | +++ |
| HsMCM2 | 33 | 30 | 40 | 44 |
| Ki-67 | 22 | 23 | 18 | 9 |
| PCNA | 5 | 10 | 30 | 47 |
| Cyclin A | 30 | 62 | 37 | 18 |
| Cyclin D1 | 87 | 38 | 12 | 10 |
| Cyclin D3 | 90 | 19 | 19 | 19 |
| Cyclin E | 82 | 28 | 22 | 15 |
0, <10% of the tumour cell positive; +, 10–30% of the tumour cells positive; ++, 30–70% of the tumour cells positive; +++, >70% of the tumour cells positive.
The immunoreactivity results of cyclins (A, D1, D3, and E) have been reported previously.30 In summary, when immunoreactivity to cyclins (A, D1, D3, E) was evaluated, we detected immunoreactivity to cyclin A in 117 samples (62 scored as +, 37 as ++, and 18 as +++). For cyclin D1, immunoreactivity was detected in 60 samples (38 scored as +, 12 as ++, and 10 as +++). Cyclin D3 immunoreactivity was detected in 57 samples (19 scored as +, 19 as ++, and 19 as +++), whereas immunoreactivity to cyclin E was detected in 65 samples (28 scored as +, 22 as ++, and 15 as +++) (table 2). Only the overexpression of cyclin A showed a significant association with relative survival (cyclin A, p = <0.00001; cyclin D1, p = 1.0; cyclin D3, p = 0.139; and cyclin E, p = 0.40).
HsMCM2 expression, adjusted for age, was associated with significantly shorter patient survival with a hazards ratio (HR) of cancer specific death of 1.32 (95% confidence interval (CI), 1.01 to 1.73; p = 0.041). However, the prognostic value of HsMCM2 was lost when the expression of cyclin A was added to the multivariate analysis of survival function (Cox regression; p = 0.79; HR, 0.96; 95% CI, 0.72 to 1.28) (table 3). Because the very strong prognostic value of cyclin A with regard to cancer specific death (HR, 2.45; p = 2.25 × 10−8) was not altered when HsMCM2 and cyclin A were examined simultaneously in the model, the correlation between cyclin A and HsMCM2 was examined further. A highly significant correlation between HsMCM2 expression and cyclin A was found (Pearson correlation, 0.41; p = 1.8 × 10−7).
Table 3.
The p values for association between expression of HsMCM2 and different clinicopathological parameters
| p Values | ||
| Parameter | ANOVA | Multivariate* |
| Cyclin A | 0.000001 | 8.5×10−6 |
| Cyclin D1 | 0.59 | 0.41 |
| Cyclin D3 | 0.001 | 0.68 |
| Cyclin E | 0.038 | 0.29 |
| Tumour grade | 0.003 | 0.09 |
| Ki-67 | <0.01 | <0.05 |
| PCNA | 0.074 | 0.075 |
| Survival** | 0.041 | 0.79 |
*Linear regression; †univariate analysis: Kaplan Meier, multivariate analysis: Cox regression hazard model.
The results of double staining showed that most of the cells expressing cyclin A also expressed HsMCM2, which explains the highly significant association between HsMCM2 and cyclin A overexpression. However, there were many tumour cells that only expressed HsMCM2. In general, immunoreactivity to HsMCM2 was much more frequent than to cyclin A (fig 1C).
There was also a significant association between the expression of cyclin A and histology grade (one way ANOVA; p = 1.2 × 10−6).
When the associations between HsMCM2 expression and other clinicopathological parameters, in addition to Ki-67, PCNA, and other cyclins (D1, D3, E), were examined in one way ANOVA, we found a significant correlation between HsMCM2 and cyclin D3 (p = 0.001), cyclin E (p = 0.038), histology grade (p = 0.003), and Ki-67 (p < 0.01). No association was seen between HsMCM2 and the expression of cyclin D1, whereas a borderline correlation was seen between HsMCM2 and PCNA (p = 0.074). In multivariate analysis (linear regression model) including cyclins A, D1, and D3 and the histology grade, the only significant association was seen between HsMCM2 protein expression and the expression of cyclin A (p = 8.5 × 10−6). However, there was also a borderline association between the expression of HsMCM2 and the histology grade in the linear regression (p = 0.09) (table 3).
No significant association was found between the expression of Ki-67 and relative patient survival (p = 0.58), and PCNA expression also showed no significant association with patient survival (p = 0.22).
DISCUSSION
We have shown an association between HsMCM2 protein expression and the expression of cyclin A, and an association between HsMCM2 and Ki-67; in addition we found a borderline association between HsMCM2 expression and histology grade and between HsMCM2 and PCNA. A highly significant association was also seen between cyclin A expression and histology grade. We also showed that the strong negative prognostic value of cyclin A overexpression was maintained after adjusting for HsMCM2 expression.
Cell proliferation has been shown to be a valuable prognostic factor for breast carcinomas, as measured by various methods,32 and is of possible use in other neoplasms.33 The prognostic value of staining for the proliferation antigens PCNA and Ki-67 is controversial.34 In agreement with our findings, previous studies have shown that Ki-67 and PCNA immunostaining may not be a valuable prognostic factor in patients with breast cancer.34 HsMCM2/BM28, which is also a cell proliferation marker, has been shown to be associated with cell proliferation independently of PCNA and Ki-67, and therefore may provide a measure of cell proliferation distinct from those offered by PCNA and Ki-67.35 However, its prognostic value is yet to be established.
“HsMCM2 showed a significant association with survival only if cyclin A expression was excluded from the statistical analysis”
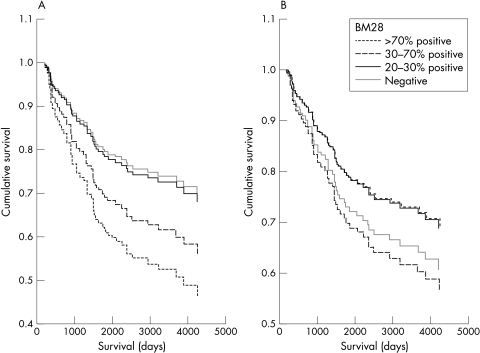
We have previously demonstrated an association between cyclin A expression and patient survival, and because cyclin A is thought to be a proliferation marker, we wanted to evaluate the relation between cyclin A overexpression and the expression of HsMCM2. We found a strong association between HsMCM2 and cyclin A expression. Both of these proteins seem to indicate cell proliferation. However, the independent prognostic value of these two proteins was very different in our series of patients. Cyclin A expression was highly associated with a poor prognosis,30 whereas HsMCM2 showed a significant association with survival only if cyclin A expression was excluded from the statistical analysis. This may reflect the biological differences between cyclin A overexpression and the overexpression of HsMCM2. The cyclin A–cyclin dependent kinase (cdk2) complex forms stable complexes with E2F-1, and this complex phosphorylates E2F-1 bound DP-1, leading to suppression of E2F-1 DNA binding activity. If E2F–DP-1 is not inactivated by cyclin A–cdk2, then E2F-1 activity persists inappropriately during S phase. It has been shown that p53 dependent apoptosis is regulated, at least in part, by binding to E2F. Cyclin A overexpression may interfere with the apoptotic capacity of p53, by competing with p53 at the E2F binding site.36 We think that this mechanism may explain the main differences between the prognostic value of HsMCM2 and cyclin A, because HsMCM2 has not been found to be involved in such a pathway, but reflects only the proliferation status of the tumour cells and, depending on the effect of adjuvant treatment, the impact of cell proliferation on patient prognosis may vary. It is possible that metastatic tumour cells with a high proliferation rate and with intact apoptotic machinery may respond better to adjuvant treatment, whereas metastatic tumour cells without an intact apoptotic machinery and high proliferation rate will lead to poor patient prognosis. This mechanism may explain, in part, the phenomena seen in fig 2.
Figure 2.
Patient survival according to HsMCM2 expression (A) adjusted for age and (B) adjusted for both age and cyclin A. In (A) there is a clear association between the degree of HsMCM2 protein expression and survival. In (B) such an association does not exist.
It is notable that the p value and HR of HsMCM2 in relation to patient survival changed significantly when cyclin A overexpression was included in the survival analysis (p = 0.041; HR, 1.32 when cyclin A overexpression was not included; p = 0.79; HR = 0.96 when cyclin A overexpression was included). This indicates that the lack of an independent significant association between HsMCM2 expression and patient survival cannot be explained simply by the sample size of the patients included in our study, and that HsMCM2 expression is probably not associated with cancer specific death.
We also found that HsMCM2 expression was highly associated with cyclin D3, cyclin E, and histology grade when these parameters were analysed one by one (one way ANOVA). However, all the parameters, except the expression of cyclin A, lost their significant association with HsMCM2 when they were analysed in a multivariate statistical model (linear regression), indicating that cyclin D3, cyclin E, and histology grade affect the association of each other with the expression of HsMCM2, whereas the association between HsMCM2 expression and cyclin A may be independent of the other parameters included in the analyses. Nevertheless, the expression of HsMCM2 also showed a borderline association with histology grade in a mulitivariate model (p = 0.09). This may indicate that there is an association between HsMCM2 expression and histology grade, but the patient material included in this study is insufficient to explore this association.
Immunoreactivity for HsMCM2 was seen more frequently than for cyclin A. This may result from different sensitivities of the two antibodies, but we believe that it reflects the biological differences between these two proteins. HsMCM2 is expressed in high amounts in all replicating cells, whereas this may not be the case for cyclin A.
In summary, in our study we demonstrate an independent significant association between the expression of HsMCM2 and the expression of cyclin A in breast carcinoma tissue. HsMCM2 expression showed a tendency towards poor prognosis in this series of patients, but failed to reach a significant association with patient survival.
Take home messages .
The expression of cyclin A is independently associated with HsMCM2 expression, histology grade, and Ki-67
The expression of HsMCM2 is associated with poor patient survival, although it loses its prognostic value when adjusted for cyclin A
Acknowledgments
The excellent technical assistance of GB Pedersen and E Hellesylt is greatly acknowledged.
Abbreviations
BCIP, 5-bromo 4-chloro-3indolyl-phosphatase
cdk, cyclin dependent kinase
CI, confidence interval
EF, elongation factor
HR, hazards ratio
MCM, minichromosome maintenance
NBT, nitroblue tetrazolium
PCNA, proliferating cell nuclear antigen
REFERENCES
- 1.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 1988;332:546–8. [DOI] [PubMed] [Google Scholar]
- 2.Yan H, Gibson S, Tye BK. Mcm2 and Mcm3, two proteins important for ARS activity, are related in structure and function. Genes Dev 1991;5:944–57. [DOI] [PubMed] [Google Scholar]
- 3.Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 1997;91:59–69. [DOI] [PubMed] [Google Scholar]
- 4.Liang C, Stillman B. Persistent initiation of DNA replication and chromatin-bound MCM proteins during the cell cycle in cdc6 mutants. Genes Dev 1997;11:3375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coue M, Kearsey SE, Mechali M. Chromatin binding, nuclear localization and phosphorylation of xenopus cdc21 are cell-cycle dependent and associated with the control of initiation of DNA replication. EMBO J 1996;15:1085–97. [PMC free article] [PubMed] [Google Scholar]
- 6.Chong JP, Mahbubani HM, Khoo CY, et al. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature 1995;375:418–21. [DOI] [PubMed] [Google Scholar]
- 7.Kubota Y, Mimura S, Nishimoto S, et al. Identification of the yeast MCM3-related protein as a component of xenopus DNA replication licensing factor. Cell 1995;81:601–9. [DOI] [PubMed] [Google Scholar]
- 8.Madine MA, Khoo CY, Mills AD, et al. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature 1995;375:421–4. [DOI] [PubMed] [Google Scholar]
- 9.Donovan S, Harwood J, Drury LS, et al. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci U S A 1997;94:5611–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young MR, Tye BK. Mcm2 and Mcm3 are constitutive nuclear proteins that exhibit distinct isoforms and bind chromatin during specific cell cycle stages of Saccharomyces cerevisiae. Mol Biol Cell 1997;8:1587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yankulov K, Todorov I, Romanowski P, et al. MCM proteins are associated with RNA polymerase II holoenzyme. Mol Cell Biol 1999;19:6154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todorov IT, Lavigne J, Sakr F, et al. Nuclear matrix protein mitotin messenger RNA is expressed at constant levels during the cell cycle. Biochem Biophys Res Commun 1991;177:395–400. [DOI] [PubMed] [Google Scholar]
- 13.Enoch T, Nurse P. Coupling M phase and S phase: controls maintaining the dependence of mitosis on chromosome replication. Cell 1991;65:921–3. [DOI] [PubMed] [Google Scholar]
- 14.Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci 1990;15:378–83. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995;81:323–30. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 1993;259:1908–12. [DOI] [PubMed] [Google Scholar]
- 17.Musgrove EA, Lee CS, Buckley MF, et al. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci U S A 1994;91:8022–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnitzky D, Gossen M, Bujard H, et al. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 1994;14:1669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldin V, Lukas J, Marcote MJ, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 1993;7:812–21. [DOI] [PubMed] [Google Scholar]
- 20.Zindy F, Lamas E, Chenivesse X, et al. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun 1992;182:1144–54. [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Bertino JR. Functional roles of E2F in cell cycle regulation. Oncogene 1997;14:1191–200. [DOI] [PubMed] [Google Scholar]
- 22.Hunter T, Pines J. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell 1994;79:573–82. [DOI] [PubMed] [Google Scholar]
- 23.Faha B, Ewen ME, Tsai LH, et al. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science 1992;255:87–90. [DOI] [PubMed] [Google Scholar]
- 24.Bandara LR, Adamczewski JP, Hunt T, et al. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature 1991;352:249–51. [DOI] [PubMed] [Google Scholar]
- 25.Mudryj M, Devoto SH, Hiebert SW, et al. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell 1991;65:1243–53. [DOI] [PubMed] [Google Scholar]
- 26.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- 27.Dalton LW, Page DL, Dupont WD. Histologic grading of breast carcinoma. A reproducibility study. Cancer 1994;73:2765–70. [DOI] [PubMed] [Google Scholar]
- 28.Frierson HF, Jr, Wolber RA, Berean KW, et al. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol 1995;103:195–8. [DOI] [PubMed] [Google Scholar]
- 29.Robbins P, Pinder S, de Klerk N, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol 1995;26:873–9. [DOI] [PubMed] [Google Scholar]
- 30.Bukholm IR, Bukholm G, Nesland JM. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer 2001;93:283–7. [DOI] [PubMed] [Google Scholar]
- 31.Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch 2000;436:224–8. [DOI] [PubMed] [Google Scholar]
- 32.Keshgegian AA, Cnaan A. Proliferation markers in breast carcinoma. Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol 1995;104:42–9. [DOI] [PubMed] [Google Scholar]
- 33.Kruger S, Muller H. Correlation of morphometry, nucleolar organizer regions, proliferating cell nuclear antigen and Ki67 antigen expression with grading and staging in urinary bladder carcinomas. Br J Urol 1995;75:480–4. [DOI] [PubMed] [Google Scholar]
- 34.Hall PA, Woods AL. Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet 1990;23:505–22. [DOI] [PubMed] [Google Scholar]
- 35.Todorov IT, Werness BA, Wang HQ, et al. HsMCM2/BM28: a novel proliferation marker for human tumors and normal tissues. Lab Invest 1998;78:73–8. [PubMed] [Google Scholar]
- 36.Hsieh JK, Yap D, O’Connor DJ, et al. Novel function of the cyclin A binding site of E2F in regulating p53-induced apoptosis in response to DNA damage. Mol Cell Biol 2002;22:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]