Abstract
Aims: Folate receptors (FRs) mediate cellular uptake of folates in many cancer cells and in folate deficiency heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) mediates translational upregulation of FR in cultured cervical cancer cells. hnRNP-E1 can also interfere with human papillomavirus 16 (HPV-16) viral capsid protein synthesis (and thereby HPV proliferation) in vitro. This study aimed to evaluate prospectively the relevance of FR and hnRNP-E1 expression in the normal cervix, cervical dysplasia, and cancer.
Methods: Cervical tissues from 12 women with normal histology and 69 consecutive women with varying grades of cervical dysplasia and cancer were prospectively evaluated for immunohistochemical expression of FR, hnRNP-E1, proliferating cell nuclear antigen (PCNA), and HPV. There were 22 women with low grade squamous intraepithelial lesions (LGSIL), 22 with high grade squamous intraepithelial lesions (HGSIL), and 25 with invasive cervical carcinoma.
Results: Among normal subjects, 100% and 92% expressed hnRNP-E1 and FR, respectively. FR expression decreased from 91% in LGSIL to 68% and 64% in women with HGSIL and cancer, respectively. Similarly, hnRNP-E1 expression decreased from 86% in LGSIL to 68% and 40% in HGSIL and cancer, respectively. There was a highly significant positive correlation between the extent of FR and hnRNP-E1 expression, and an inverse correlation between HPV infection and hnRNP-E1 expression during progression of cervical dysplasia to cancer.
Conclusion: These results are consistent with a hypothesis that reduced hnRNP-E1 expression may be permissive for HPV proliferation and progression to cervical cancer, and support the need for prospective longitudinal studies of hnRNP-E1 expression in HPV-16 infected women.
Keywords: folate receptors, heterogenous nuclear ribonucleoprotein E1, cervical cancer, human papillomavirus
The incidence of cervical cancer varies from about 10/100 000 women in many Western nations to 40/100 000 in developing countries.1 Of the more than half million cases of cervical cancer reported worldwide each year, at least 80% occur in developing countries.1,2 Cervical cancer is the most common female gynaecological malignancy in India, accounting for 26% of female cancers, with 90 000 women developing the disease each year.3 It is one of the leading causes of death from cancer among women, with an overall five year survival rate of 40%.4
“Information on heterogeneous ribonucleoprotein E1 expression in the cervix will be valuable in assessing its possible functional role in the regulation of folate receptor synthesis in vivo”
Human papillomavirus (HPV) is causally associated with cervical neoplasia.5–8 The prevalence of latent HPV infection is about 40%, and whereas 5–10% of these patients will develop squamous intraepithelial lesions, only 1% or less will develop cancer6; thus, most young women with a positive HPV test will become negative within 24 months. However, a persistent high viral load of high risk HPV types (especially HPV-16) for many years is predictive for the development of cervical dysplasia and cancer.9–11 Furthermore, by the time high grade squamous intraepithelial lesions (HGSILs) are established, it is unlikely that HPV will be cleared. Therefore, any approach12–14 that focuses on control of viral proliferation is a potential cancer prevention strategy, especially for women in whom viral persistence is well documented. Moreover, knowledge of the determinants of viral proliferation within cervical tissue can expand the scope of non-invasive local control strategies.
The acquisition of folates in many cells is mediated primarily through folate receptors (FRs) that bind to physiological folates with high affinity in the nanomolar range.15,16 FRs are expressed in rapidly proliferating cells, including cervical cancer cell lines,16,17 but there has been no systematic assessment of the expression of FRs in women with cervical dysplasia and cervical cancer. In a small subset of samples of cervical cancer evaluated for the expression of FR mRNA, very few showed expression of FR mRNA.18 However, because there was no assessment of FR protein, this assessment was incomplete. Analysis of FR protein expression is particularly important because, despite their constitutive overexpression,17 FRs can be further upregulated (up to 20 times) in cultured cervical cancer cells, primarily at the translational level in folate deficiency (AC Antony, Y-S Tang, RA Khan, et al, unpublished data, 2003; AC Antony, Y-S Tang, RA Khan, et al. Presented at 20th Annual Convention of the Indian Association for Cancer Research, January 19–21, Ahmedabad, Gujerat State, India, 2001).
Our work on the regulation of FR in cervical cancer cells has led us to an intimate study of heterogeneous ribonucleoprotein E1 (hnRNP-E1), which can specifically bind to the sense strand of the HPV-16 L2 viral capsid protein mRNA to inhibit its synthesis in vitro.8 We have accumulated substantial experimental evidence to support a model for translational upregulation of the FR in folate deficiency. This is based on a crucial role for accumulated intracellular homocysteine, which promotes the interaction of a trans-factor with an 18 base cis-element located in the 5′ untranslated region of FR mRNA (AC Antony, Y-S Tang, RA Khan, et al, unpublished data, 2003; AC Antony, Y-S Tang, X Xiao, et al. Presented at 20th Annual Convention of the Indian Association for Cancer Research, January 19–21, Ahmedabad, Gujerat State, India, 2001),19 leading to increased biosynthesis of FR at the translational level. This trans-factor is identical to hnRNP-E1.20 Therefore, information on hnRNP-E1 expression in the cervix will be valuable in assessing its possible functional role in the regulation of FR synthesis in vivo. In addition, because hnRNP-E1 expression can potentially modulate HPV viral proliferation in the cervix, knowledge of hnRNP-E1 expression patterns could also provide insights into the possible biological constraint(s) exerted by these proteins on HPV proliferation. Moreover, this would be particularly informative in women with HPV mediated transformation of cervical tissue to cancer. Accordingly, we initiated this prospective study in women to characterise the expression of FR and hnRNP-E1 in relation to HPV infection at various stages of tumour progression in the uterine cervix.
PATIENTS AND METHODS
Patient selection
Tissue samples for our study were obtained from patients attending the gynaecological clinics of the Sree Avittom Thirunal Hospital Medical College, and the Regional Cancer Centre, both in Thiruvananthapuram, Kerala State, India. Our study was approved by the research advisory committee of the Regional Cancer Centre, Thiruvananthapuram. In total, 81 consecutive cervical tissue samples were analysed prospectively. There were 12 samples from women with apparently normal cervical epithelium, 22 samples from women with low grade squamous intraepithelial lesions (LGSILs), 22 samples from women with HGSIL, and 25 samples from women with invasive cervical carcinoma (comprising both large cell non-keratinising carcinoma and small cell non-keratinising types). Diagnostic tissue samples were obtained as punch biopsies. Apparently normal cervical epithelium was obtained from patients undergoing hysterectomy for various non-malignant reasons. Paraffin wax embedded sections, 4 μm thick, were placed on poly L lysine coated glass slides. One section from all samples was stained with haematoxylin and eosin for routine histopathological evaluation, and duplicate serial sections were used for immunohistochemistry and the determination of HPV infection.
Preparation of antiserum to FR and hnRNP-E1
Polyclonal rabbit anti-hnRNP-E1 (oligopeptide) IgG antibodies were directed against a 19 amino acid stretch of sequences (SLAQYLINARLSSEKGMGC) of hnRNP-E1, as described previously.20 The specificity of these IgG antibodies has been validated.20 Polyclonal antiserum to purified placental FR was generated in rabbits and validated as described previously.21–23 This antiserum recognises full length and truncated forms of both human FR-α17,24 and FR-β.25
Antisera directed against the HPV E6 protein and proliferating nuclear antigen (PCNA) were obtained commercially from Oncogene Science (Cambridge, Massachusetts, USA) and Dako, (PCNA-PC 10; Dako A/S, Glostrup, Denmark), respectively, and used as described previously.26,27
Immunohistochemical detection of FR, hnRNP-E1, HPV E6, and PCNA
Sections were processed for antigen retrieval as described by our laboratory previously.28 Briefly, sections were incubated with primary antibody overnight at 4°C. The dilutions used for HPV E6 antisera, FR antisera, and hnRNP-E1 antisera were 1/50, 1/200, and 1/200, respectively. Negative controls were run by substituting primary antibody with phosphate buffered saline. (Immunohistochemistry with phosphate buffered saline and non-immune serum consistently gave identical results for normal, LGSIL, HGSIL and invasive cancer specimens.) The reaction was visualised using a streptavidin–biotin immunoperoxidase system (Dako) with diaminobenzidine as chromogen. All sections were counterstained with haematoxylin.
Evaluation of immunoreactivity
A total of 1000 cells was evaluated in all sections. The expression of PCNA was considered significant when characteristic nuclear immunoreactivity was present in at least 10% of tumour cells. For FR and hnRNP-E1, immunoreactivity was graded as negative, mild, moderate, and intense. In normal and premalignant lesions, immunoreactivity was recorded layer wise as basal, parabasal, and spinal. In carcinomas, immunoreactivity was predominant in basaloid cells and was recorded as the percentage of positive cells. In addition, an expression index was generated by classifying protein expression into four categories: grade 1, where less than 10% of cells showed positivity (insignificant); grade 2, with 11–30% expression (mild); grade 3, with 31–50% expression (moderate); and grade 4, with greater than 51% expression (intense). For HPV-E6 protein expression, only the presence or absence of immunoreactivity was considered.26
Determination of HPV infection
HPV infection in cervical tissues was analysed by the polymerase chain reaction (PCR) as described previously,29 using custom designed primers specific for HPV-6, HPV-11, HPV-16, and HPV-18 (Sigma Genosys, Pampisford, Cambridgeshire, UK).
Data analysis
The expression of FR, hnRNP-E1, PCNA, and HPV E6, and the presence of HPV infection in different tissue samples were analysed by means of χ2, Mann Whitney, and the Spearman correlation tests. Results with a confidence interval above 95% (p < 0.05) were considered significant.
RESULTS
In general, FRs were expressed uniformly on the cell membranes of normal cells, but in dysplastic (LGSIL and HGSIL) lesions and invasive cancer the immunoreactivity also showed a “beaded” pattern. However, with very intense staining, there was blurring of the distinction between membrane and “cytoplasmic” immunoreactivity. The hnRNP-E1 protein, which was predominantly localised to the cytoplasm, was also found in the nucleus. With respect to cell specific expression of FR and hnRNP-E1 in invasive cancer, FRs were expressed in the basaloid cells of large cell non-keratinising carcinomas, whereas hnRNP-E1 was found in most cells of the small cell non-keratinising carcinomas.
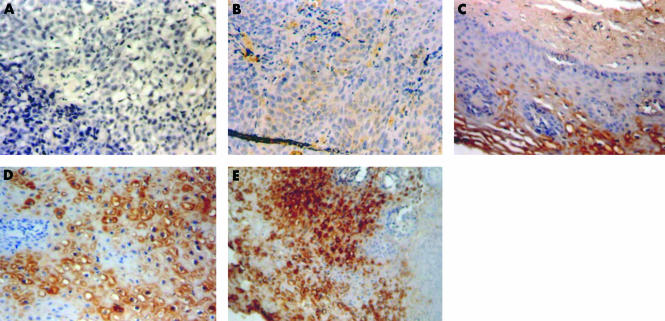
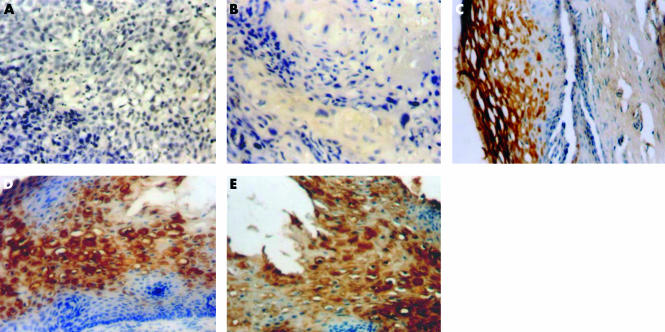
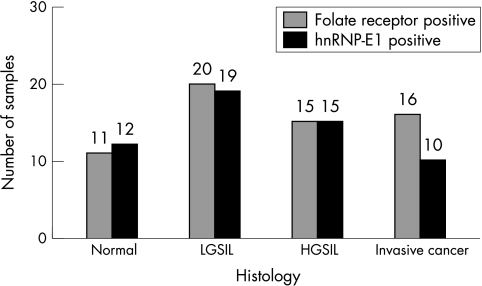
Figures 1 and 2 demonstrate the spectrum of negative and positive immunohistochemical staining characteristics found for FR and hnRNP-E1, respectively. FR and hnRNP-E1 expression was assessed semiquantitatively, based on four grades (1–4) of immunoreactivity. Tables 1 and 2 summarise the grades of expression of FR and hnRNP-E1 seen among 12 women with benign cervical histology, 22 women with LGSIL, 22 women with HGSIL, and 25 women with invasive cancer. For both hnRNP-E1 and FR, most of the positive cases were either grade 3+ or 4+. All 12 patients with benign histology exhibited hnRNP-E1 expression, whereas most (11 of 12 patients; 92%) of the benign lesions also expressed FRs, ranging from mild in three patients, moderate in three, to intense expression in five (table 1). With the development of progressive dysplasia (grade 2+ to 4+), FR expression appeared to decrease from 91% (20 of 22) in women with LGSIL to 68% (15 of 22) and 64% (16 of 25) in women with HGSIL and invasive cancer, respectively. Similarly, with progression of dysplasia to invasive cancer, the percentage of specimens that were (grade 2+ to 4+) positive for hnRNP-E1 progressively decreased from 86% (19 of 22) in LGSIL to 68% (15 of 22) and 40% (10 of 25) in HGSIL and invasive cancer, respectively. When the expression of FR and hnRNP-E1 was assessed as a function of the grade of dysplasia and cancer, there was a positive correlation between the extent of FR expression and hnRNP-E1 immunoreactivity (r = 0.38701; p = 0.00036; fig 3; table 3).
Figure 1.
Folate receptor (FR) expression in different histological types. (A) Negative control (original magnification, ×20); (B) invasive cancer showing grade 1 immunoreactivity (original magnification, ×20); (C) low grade squamous intraepithelial lesion showing grade 2 immunoreactivity (original magnification, ×40); (D) high grade squamous intraepithelial lesion (HGSIL) showing grade 3 immunoreactivity (original magnification, ×40); (E) HGSIL showing grade 4 immunoreactivity (original magnification, ×20).
Figure 2.
Heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) expression in different histological types. (A) Negative control (original magnification, ×20); (B) invasive cancer showing grade 1 immunoreactivity (original magnification, ×40); (C) low grade squamous intraepithelial lesion showing grade 2 immunoreactivity (original magnification, ×40); (D) high grade squamous intraepithelial lesion (HGSIL) showing grade 3 immunoreactivity (original magnification, ×40); (E) HGSIL showing grade 4 immunoreactivity (original magnification, ×40).
Table 1.
FR expression in different histological types
| Negative FR expression | Positive FR expression | ||||
| Histology | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total cases |
| Benign | 1 | 3 | 3 | 5 | 12 |
| LGSIL | 2 | 4 | 4 | 12 | 22 |
| HGSIL | 7 | 4 | 4 | 7 | 22 |
| Invasive cancer | 9 | 4 | 4 | 8 | 25 |
FR, folate receptor; LGSIL, low grade squamous intraepithelial lesion; HGSIL, high grade grade squamous intraepithelial lesion.
Table 2.
hnRNP-E1 expression in different histological types
| Negative hnRNP-E1 expression | Positive hnRNP-E1 expression | ||||
| Histology | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total cases |
| Benign | 0 | 4 | 5 | 3 | 12 |
| LGSIL | 3 | 6 | 5 | 8 | 22 |
| HGSIL | 7 | 3 | 6 | 6 | 22 |
| Invasive cancer | 15 | 2 | 2 | 6 | 25 |
hnRNP-E1, heterogenous nuclear ribonucleoprotein E1; LGSIL, low grade squamous intraepithelial lesion; HGSIL, high grade grade squamous intraepithelial lesion.
Figure 3.
Concomitant expression of folate receptor (FR) and heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) in various phases of tumour progression in the uterine cervix. HGSIL, high grade squamous intraepithelial lesion; LGSIL, low grade squamous intraepithelial lesion.
Table 3.
Distribution of study subjects in relation to hnRNP-E1 and FR expression with histology
| Benign | LGSIL | HGSIL | Invasive cancer | Significance | |
| hnRNP-E1 negative | – | 3 | 7 | 15 | r=−0.46975 |
| hnRNP-E1 positive (percent hnRNP-E1 positive) | 12 (100%) | 19 (86%) | 15 (68%) | 10 (40%) | p=0.00001 |
| FR negative | 1 | 2 | 7 | 9 | r=−0.27504 |
| FR positive (percent FR positive) | 11 (92%) | 20 (91%) | 15 (68%) | 16 (64%) | p=0.01295 |
FR, folate receptor; hnRNP-E1, heterogenous nuclear ribonucleoprotein E1; LGSIL, low grade squamous intraepithelial lesion; HGSIL, high grade grade squamous intraepithelial lesion.
Among all cases analysed, FR and hnRNP-E1 were absent in 12 cases, whereas 49 cases showed both FR and hnRNP-E1 positivity. Of the 12 cases with negative FR and hnRNP-E1 expression, nine were invasive cancer, whereas one was LGSIL and two were HGSIL. Thirteen cases were positive for FR but negative for hnRNP-E1; of these, six were invasive cancers and the rest were either LGSIL (n = 2) or HGSIL (n = 5). In addition, seven cases were positive for hnRNP-E1 but negative for FR; among these, one was normal, and three each were HGSIL and invasive cancer.
HPV subtyping of cervical tissues revealed that only 3% of the study population (invasive cancer group) showed multiple HPV infection (HPV types 6, 11, and 16). None of the samples in our study was positive for HPV-18. HPV-16 was detected by PCR using type specific primers, where the 152 base pair amplified PCR product indicated the presence of HPV-16 infection. The HPV E6 protein was mostly found in the cytoplasm, although a few cells showed nuclear immunoreactivity. Moderate expression of HPV E6 protein was seen in HGSIL, whereas intense expression was seen in most of the specimens of invasive cervical carcinoma. There was a highly significant inverse association between HPV-16 infection and hnRNP-E1 expression (r = −0.39267; p = 0.00029), and most of the HPV negative tumours had intense hnRNP-E1 expression (table 4). This association was also evident in the case of the HPV E6 protein (r = −0.48375; p < 0.0001). However, both HPV DNA detection and HPV E6 immunoreactivity showed no apparent association with the expression of FR.
Table 4.
HPV infection and its association with hnRNP-E1 and FR expression
| HPV-16 | HPV E6 | |||
| Negative | Positive | Negative | Positive | |
| hnRNP-E1 negative | 5 | 20 | 7 | 18 |
| hnRNP-E1 positive | 35 | 21 | 44 | 12 |
| FR negative | 7 | 12 | 11 | 8 |
| FR positive | 33 | 29 | 40 | 22 |
FR, folate receptor; hnRNP-E1, heterogenous nuclear ribonucleoprotein E1; HPV, human papillomavirus.
Most HPV negative biopsies (table 4) exhibited either normal or LGSIL histology. Specifically, six of 12 normal biopsies and 21 of 22 LGSIL biopsies were HPV negative; in contrast, only five of 22 HGSIL and three of 25 invasive cancer biopsies were HPV negative. With respect to these HPV negative specimens, all DNA samples were first analysed using wide spectrum HPV primers and then analysed for HPV types 16, 11, and 18. Although this reduces the possibility of missing HPV DNA (so that a negative result indicates a true negative), misclassification bias, which has been documented when PCR is used to detect HPV DNA in biopsy specimens,30 might also explain HPV negativity in some of our cases. All cases were also analysed for PCNA immunoreactivity, which was used as a surrogate marker for cell proliferation.27 Of the 12 normal samples, five were PCNA negative, but all 22 LGSIL and HGSIL samples and 24 of 25 invasive cancers were PCNA positive (r = 0.6238; p < 0.0001). There was a significant positive correlation between PCNA immunoreactivity and the histological type of the tumours (r = 0. 62384; p < 0.0001). There was also a significant inverse correlation between PCNA expression and hnRNP-E1 immunoreactivity (r = −0.30296; p = 0.005). Similarly, FR also had an inverse association with proliferation as assessed by PCNA (r = −0. 32030; p = 0015).
DISCUSSION
Genetic factors, immune response, viral load, and HPV-16 variants are among the factors that modulate the risk of HPV induced progression of squamous intraepithelial neoplasia to cancer.6 Among these, high titre viral persistence of HPV appears to be a common finding in the natural history and evolution of progressive dysplasia to cervical neoplasia.9–11 Therefore, any approach to interfere with viral replication would be a rational cancer prevention strategy. HPV-16 is one of the most common HPV subtypes associated with cervical cancer.6,9–11,31 HPV-16 late genes encoding major and minor viral capsid proteins L1 and L2, respectively, are expressed in terminally differentiated epithelial cells in the superficial layers of the squamous epithelium. Recently, it was shown that hnRNP-E1 binds to HPV-16 L2 mRNA in a sequence specific manner and efficiently inhibits translation of HPV-16 L2 mRNA in vitro.8 Therefore, hnRNP-E1 may also have a role in the regulation of HPV-16 late gene expression and virus production in vivo. As a first step in the discovery of such a possible role, it was important to define the extent of expression of this protein in normal, dysplastic, and cancerous cervical tissues. Our study has defined the relation between the coexpression of hnRNP-E1 and FR in the cervix of women with various stages of HPV mediated transformation to neoplasia.
The polyclonal antiserum to FR has been well validated and is specific for FR. In addition, the antipeptide IgG antibodies to hnRNP-E1 had been carefully chosen for specificity, and we have previously shown that the effects of the antiserum can be quenched with purified recombinant hnRNP-E1.20 hnRNP-E1 belongs to a family of hnRNPs that share a common K homology domain.32 Because some members of the hnRNP family with common K homology domains (such as hnRNP-K) can also interact with the L2 mRNA of HPV,8 we generated antipeptide antibodies against a unique peptide fragment in hnRNP-E1 that is not found in other members of the hnRNP family. A BLAST search showed that the only other species with over 80% homology was a closely related isoform of hnRNP-E1, known as hnRNP-E2.32 Although we have not directly tested anti-hnRNP-E1 antibody reactivity with hnRNP-E2, a protein database search (known as Gapped BLAST and PSI-BLAST)33 also indicated that homology at the amino acid level was 84% (with 16 of 19 amino acids in hnRNP-E2 present in sequence with hnRNP-E1). Thus, it is highly likely that the immunoreactivity seen in our study includes both hnRNP-E1 and hnRNP-E2 proteins. This is not a trivial issue because hnRNP-E2 also binds HPV-16 L2 sense mRNA and probably exerts a similar inhibitory effect on HPV-16 L2 protein synthesis.8
“Our data are consistent with the hypothesis that the loss of heterogenous nuclear ribonucleoprotein E1 expression may allow human papillomavirus infected cervical tissues to undergo virus mediated transformation from cervical dysplasia to cancer”
As predicted from studies on single cells in culture, FRs are primarily located on the plasma membrane of cells. However, when intense membrane immunoreactivity with the anti-FR antiserum was seen, “cytoplasmic” staining was also noted in some of the specimens. This might be explained by increased intensity of staining, with “overflow” from membrane to cytoplasmic staining. Another possibility relates to the observations from an earlier cell fractionation study in cultured human cells; this study documented that (in addition to their plasma membrane location) FRs are also located on membranes surrounding organelles, often at very high concentrations.34 With respect to the patterns of expression of hnRNP-E1 in cervical tissue, although hnRNP-E1 was predominantly found in the cytoplasm, some hnRNP-E1 related staining was also seen in the nucleus of cells. This is quite consistent with our northwestern blot analysis of cytoplasmic and nuclear fractions (from human placental tissue),20 and similar data have also been generated in cultured cervical cancer cells.8
Whereas the extent of hnRNP-E1 expression remained high in normal cervical tissue and in LGSIL, there was a consistently lower expression of hnRNP-E1 with transformation to HGSIL and cancer. Thus, our data are consistent with the hypothesis that the loss of hnRNP-E1 expression may allow HPV infected cervical tissues to undergo virus mediated transformation from cervical dysplasia to cancer. However, whether the reduction of hnRNP-E1 is permissive for further viral proliferation remains unanswered, and will require both prospective clinical and preclinical studies to clarify this possibility.
Our study provided evidence that FRs are expressed in normal, dysplastic, and cancerous cervical tissues. Earlier, we identified an inverse relation between FRs and cell proliferation in cultured cervical cancer cells that were transduced with various amounts of FR cDNA, which were encapsidated in recombinant adenoassociated virus 2.17 The significant inverse association between FRs and proliferation, as assessed by PCNA (a surrogate marker of proliferation that reflects the total proliferative compartment in a tissue) has now confirmed this association in women for the first time.
Our data have limitations in that we have not determined whether the abundant expression of the FR protein observed is accompanied by a proportionate increase in FR mRNA expression. Nonetheless, the concordant expression of hnRNP-E1 and FR in women with various grades of transformation from cervical dysplasia to cancer was striking, and supports a possible role for hnRNP-E1 in the regulation of FR synthesis in cervical tissue. However, because hnRNP-E1 is a multifunctional protein,20,32 there is probably an abundance of this protein—far beyond that which is necessary for the translation of FR—as has been noted previously.19 This may explain why a more precise stoichiometric relation between FR and hnRNP-E1 was not seen during the transformation of cervical tissue to cancer (fig 3). In addition, although the extent of PCNA immunoreactivity correlated with the presence of HPV DNA, we have no data on the actual HPV viral load, which is another limitation of our study.
FRs have become a focus of interest since Low’s laboratory demonstrated the preferential and specific uptake of folate conjugated toxins and chemotherapeutic agents by the FRs, which are overexpressed on cancer cells.35,36 Additional advances in the use of folate tethered liposomes containing a variety of toxins and biological molecules that are intended to perturb cell proliferation have expanded the potential clinical uses of this approach.37,38 The identification of FRs in cervical cancer should provide new incentives for the use of these innovative approaches in women with this malignancy.
Take home messages.
Reduced expression of heterogenous nuclear ribonucleoprotein E1 (hnRNP-E1) may be permissive for human papillomavirus (HPV) viral proliferation and progression towards cervical cancer
Prospective longitudinal studies of hnRNP-E1 expression in the cervix of women infected with HPV-16 are needed to confirm this hypothesis
hnRNP-E1 may play a role in the regulation of folate receptor synthesis in cervical tissue
Inhibiting the synthesis of HPV viral capsid proteins by modulating hnRNP-E1 expression through gene therapy has the potential to be a non-invasive alternative to prevent persistence of HPV proliferation and progression to cervical cancer
In conclusion, our results are consistent with a hypothesis that a reduction of hnRNP-E1 expression may be permissive for HPV viral proliferation and progression towards cervical cancer. These results also support the need for prospective longitudinal studies of hnRNP-E1 expression in the cervix of women infected with HPV-16 to confirm this hypothesis. Finally, a strategy that focuses on inhibiting the synthesis of HPV viral capsid proteins by modulating hnRNP-E1 expression through gene therapy has the potential to be a non-invasive alternative to prevent persistence of HPV proliferation and progression to cervical cancer.
Acknowledgments
This work was supported in part by National Institute of Health Grants R01CA58919, R01HD39295, and a Veterans Affairs Merit Review Award.
Abbreviations
FR, folate receptors
hnRNP-E1, heterogenous nuclear ribonucleoprotein E1
LGSIL, low grade squamous intraepithelial lesion
HGSIL, high grade squamous intraepithelial lesion
PCNA, proliferating nuclear antigen
PCR, polymerase chain reaction
REFERENCES
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 1999;80:827–41. [DOI] [PubMed] [Google Scholar]
- 2.Berumen J, Ordonez RM, Lazcano E, et al. Asian–American variants of human papillomavirus 16 and risk for cervical cancer: a case–control study. J Natl Cancer Inst 2001;93:1325–30. [DOI] [PubMed] [Google Scholar]
- 3.Shanta V, Krishnamurthi S, Gajalakshmi CK, et al. Epidemiology of cancer of the cervix: global and national perspective. J Indian Med Assoc 2000;98:49–52. [PubMed] [Google Scholar]
- 4.Suris JC, Dexeus S. Survival in cervical cancer. Eur J Gynaecol Oncol 1998;19:11–13. [PubMed] [Google Scholar]
- 5.Bosch FX, Munoz N, Meijer CJ M, et al. The causal relation between human papillomavirus and cervical cancer [review]. J Clin Pathol 2002;55:244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston C. Quantitative tests for human papillomavirus [editorial]. Lancet 2000;355:2179–80. [DOI] [PubMed] [Google Scholar]
- 7.Burk RD. Pernicious papillomavirus infection [editorial]. N Engl J Med 1999;341:1687–8. [DOI] [PubMed] [Google Scholar]
- 8.Collier B, Goobar-Larsson L, Sokolowski M, et al. Translational inhibition in vitro of human papillomavirus type 16 L2 mRNA mediated through interaction with heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1 and 2. J Biol Chem 1998;273:22648–56. [DOI] [PubMed] [Google Scholar]
- 9.Ylitalo N, Sorensen P, Josefsson AM, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case–control study. Lancet 2000;355:2194–8. [DOI] [PubMed] [Google Scholar]
- 10.Josefsson AM, Magnusson PKE, Ylitalo N, et al. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case–control study. Lancet 2000;355:2189–93. [DOI] [PubMed] [Google Scholar]
- 11.Wallin K-L, Wiklund F, Angstrom T, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med 1999;341:1633–8. [DOI] [PubMed] [Google Scholar]
- 12.Cox JT. Management of cervical intraepithelial neoplasia [editorial]. Lancet 1999;353:857–9. [DOI] [PubMed] [Google Scholar]
- 13.Galloway DA. Is vaccination against human papillomavirus a possibility? Lancet 1998;351(suppl 3):22SIII–4SIII. [DOI] [PubMed] [Google Scholar]
- 14.Bonn D, Bradbury J. The warts and all approach to tackling cervical cancer. Lancet 1998;351:810. [DOI] [PubMed] [Google Scholar]
- 15.Antony AC. The biological chemistry of folate receptors. Blood 1992;79:2807–20. [PubMed] [Google Scholar]
- 16.16. Antony AC. Folate receptors. Annu Rev Nutr 1996;16:501–21. [DOI] [PubMed] [Google Scholar]
- 17.Sun XL, Murphy BR, Li QJ, et al. Transduction of folate receptor cDNA into cervical carcinoma cells using recombinant adeno-associated virions delays cell proliferation in vitro and in vivo. J Clin Invest 1995;96:1535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev 1999;8:775–82. [PubMed] [Google Scholar]
- 19.Sun XL, Antony AC. Evidence that a specific interaction between an 18-base cis-element in the 5′-untranslated region of human folate receptor-alpha mRNA and a 46-kDa cytosolic trans-factor is critical for translation. J Biol Chem 1996;271:25539–47. [PubMed] [Google Scholar]
- 20.Xiao X, Tang Y-S, Mackins JY, et al. Isolation and characterization of a folate receptor mRNA-binding trans-factor from human placenta: evidence favoring identity with heterogeneous nuclear ribonucleoprotein E1. J Biol Chem 2001;276:41510–17. [DOI] [PubMed] [Google Scholar]
- 21.Antony AC, Utley C, Van Horne KC, et al. Isolation and characterization of a folate receptor from human placenta. J Biol Chem 1981;256:9684–92. [PubMed] [Google Scholar]
- 22.Antony AC, Utley CS, Marcell PD, et al. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem 1982;257:10081–9. [PubMed] [Google Scholar]
- 23.Antony AC, Bruno E, Briddell RA, et al. Effect of perturbation of specific folate receptors during in vitro erythropoiesis. J Clin Invest 1987;80:1618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma RS, Gullapalli S, Antony AC. Evidence that the hydrophobicity of isolated, in situ, and de novo-synthesized native human placental folate receptors is a function of glycosyl-phosphatidylinositol anchoring to membranes. J Biol Chem 1992;267:4119–27. [PubMed] [Google Scholar]
- 25.Reddy J, Haneline L, Srour E, et al. Expression and functional characterization of the beta-isoform of the folate receptor on CD34+ cells. Blood 1999;93:3940–8. [PubMed] [Google Scholar]
- 26.Nair P, Jayaprakash PG, Nair MK, et al. Telomerase, p53 and human papillomavirus infection in the uterine cervix. Acta Oncol 2000;39:65–70. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmi S, Nair MB, Rajalakshmi TN, et al. Proliferating cell nuclear antigen expression and the progression of cervical intra epithelial neoplasia. Oncol Rep 1996;3:1195–8. [DOI] [PubMed] [Google Scholar]
- 28.Pillai MR, Kesari AL, Chellam VG, et al. Spontaneous programmed cell death in infiltrating duct carcinoma: association with p53, BCL-2, hormone receptors and tumor proliferation. Pathol Res Pract 1998;194:549–57. [DOI] [PubMed] [Google Scholar]
- 29.Nair P, Nair KM, Jayaprakash PG, Pillai MR. Decreased programmed cell death in the uterine cervix associated with high risk human papillomavirus infection. Pathol Oncol Res 1999;5:95–103. [DOI] [PubMed] [Google Scholar]
- 30.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia [see comments]. J Natl Cancer Inst 1993;85:958–64. [DOI] [PubMed] [Google Scholar]
- 31.Koutsky LP. Epidemiology of genital human papillomavirus infection. Am J Med 1997;102(suppl 5A):3–8. [DOI] [PubMed] [Google Scholar]
- 32.Ostareck-Lederer A, Ostareck DH, Hentze MW. Cytoplasmic regulatory functions of the KH-domain proteins hnRNPs K and E1/E2 [review]. Trends Biochem Sci 1998;23:409–11. [DOI] [PubMed] [Google Scholar]
- 33.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs [review]. Nucleic Acids Res 1997;25:3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHugh M, Cheng YC. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells. J Biol Chem 1979;254:11312–18. [PubMed] [Google Scholar]
- 35.Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A 1991;88:5572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leamon CP, Low PS. Membrane folate-binding proteins are responsible for folate–protein conjugate endocytosis into cultured cells. Biochem J 1993;291(Pt 3):855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother 2002;51:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers [review]. Crit Rev Ther Drug Carrier Syst 1998;15:587–627. [PubMed] [Google Scholar]