Abstract
CAR (constitutive androstane receptor) is a nuclear receptor that regulates the transcription of target genes, including CYP (cytochrome P450) 2B and 3A. The transactivation by CAR is regulated by its subcellular localization; however, the mechanism that governs nuclear translocation has yet to be clarified. It has been reported recently that AMPK (AMP-activated protein kinase) is involved in phenobarbital-mediated CYP2B induction in a particular culture system. We therefore investigated in vivo whether AMPK is involved in the activation of CAR-dependent gene expression. Immunoblot analysis using an antibody which recognizes Thr-172-phosphorylated AMPKα1/2 revealed phenobarbital-induced AMPK activation in rat and mouse livers as well. Phenobarbital, however, failed to increase the liver phospho-AMPK level of tumour-bearing rats in which CAR nuclear translocation had been impaired. In in vivo reporter gene assays employing PBREM (phenobarbital-responsive enhancer module) from CYP2B1, an AMPK inhibitor 8-bromo-AMP abolished phenobarbital-induced transactivation. In addition, Cyp2b10 gene expression was attenuated by 8-bromo-AMP. Forced expression of a dominant-negative mutant and the wild-type of AMPKα2 in the mouse liver suppressed and further enhanced phenobarbital-induced PBREM-reporter activity respectively. Moreover, the AMPK activator AICAR (5-amino-4-imidazolecarboxamide riboside) induced PBREM transactivation and an accumulation of CAR in the nuclear fraction of the mouse liver. However, AICAR and metformin, another AMPK activator, failed to induce hepatic CYP2B in mice and rats. These observations suggest that AMPK is at least partly involved in phenobarbital-originated signalling, but the kinase activation by itself is not sufficient for CYP2B induction in vivo.
Keywords: AMP-activated protein kinase (AMPK), constitutive androstane receptor (CAR), cytochrome P450 (CYP), gene expression, nuclear receptor, nuclear translocation
Abbreviations: AICAR, 5-amino-4-imidazolecarboxamide riboside; AMPK, AMP-activated protein kinase; CaMK, Ca2+/calmodulin-dependent protein kinase; CAR, constitutive androstane receptor; CREB, cAMP-response-element-binding protein; CYP, cytochrome P450; ERK, extracellular-signal-regulated kinase; FoxO1, forkhead box transcription factor O1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MRP, multidrug-resistance protein; mTOR, mammalian target of rapamycin; PB, phenobarbital; PBREM, PB-responsive enhancer module; PKA, cAMP-activated protein kinase; PROD, 7-pentoxyresorufin O-dealkylase; PXR, pregnane X receptor; SIK, salt-inducible kinase; STK11/LKB1, serine/threonine kinase 11; TORC2, transducer of regulated CREB activity 2; TSC, tuberous sclerosis complex
INTRODUCTION
Nuclear receptors play a pivotal role in the expression of a battery of genes involved in the detoxification of xenobiotics. CAR (constitutive androstane receptor) is a nuclear receptor originally found to regulate the transcription of the genes encoding the CYP (cytochrome P450) 2B subfamily [1,2], which is induced most significantly by PB (phenobarbital) in the rat liver. It has come to be understood that CAR governs not only CYP2B but also other drug-metabolizing enzymes and transporters as well, including CYP3A, CYP2C, UGT1A1 (UDP glucuronosyltransferase 1 A1), GSTA1 (glutathione S-transferase Alpha 1), OATP2 (organic ion transporter polypeptide 2), MRP2 (multidrug-resistance protein 2) and MRP3 (reviewed in [3]), suggesting a multi-functionality for this receptor. Therefore changes in the regulation of CAR function may affect the entire range of pharmacokinetic processes. CAR translocates from the cytoplasm to the nuclear compartment in a PB-dependent manner [4], although to date there is no evidence that PB acts as a direct ligand for this nuclear receptor. On the other hand, TCPOBOP {1,4-bis[2-(3,5-dichloropyridyloxy)]benzene}, the most powerful activator of murine CAR, has been shown to bind and induce nuclear translocation of the transcription factor CAR [5]. Nuclear CAR heterodimerizes to the RXR (retinoid X receptor) and positively regulates gene expression by binding to the PBREM (PB-responsive enhancer module) located in the upstream region of the target genes [2]. Therefore nuclear translocation of this transcription factor is a key event in its transactivating function. On the other hand, it has been reported that phosphorylation is involved in activation processes of CAR [4,6]. Although these results are still far from accounting for the entire machinery governing the PB-mediated CAR activation, it is possible that the functionality of the nuclear receptor is regulated in multiple steps. The reason the regulation of CAR function has not been understood well thus far, compared with that of other nuclear receptors involving CYP gene expression such as the aryl hydrocarbon receptor and PXR (pregnane X receptor), is that the in vivo function of this transcription factor has not been reproducible in experimental conditions using in vitro cell systems. Endogenous CAR expression in primary hepatocytes and hepatoma cell lines is very low and exogenously introduced CAR expresses in the nuclear compartment without the stimulus. These experimental hurdles have delayed the clarification of the entire machinery for nuclear translocation of CAR.
AMPK (AMP-activated protein kinase) is a cellular energy sensor and is activated by an elevated AMP/ATP ratio due to cellular and environmental stress such as heat shock, hypoxia and ischaemia [7]. The energy depletion-induced AMPK activation causes an increase in compensatory catabolism and inactivation of anabolic pathways. In addition, a decrease in cellular energy increases glucose uptake, glycolysis and fatty acid oxidation via AMPK-dependent pathways (reviewed in [8]). Recently, AMPK has been reported to be involved in the pathway leading to the induction of CYP2B by PB [9]. Previous findings had indicated that the induction of CYP2B is attenuated in diabetic Zucker rats [10] and ketone bodies such as 3-hydroxybutyrate induce CYP2B and CYP3A in hepatocytes [11]. In addition, PB-induced CYP2B gene expression is enhanced in streptozotocin-induced diabetic rats [12]. These observations suggest that the energy status of the hepatocyte is important in the control of CYP2B induction. On the basis of these findings, AMPK was identified as a candidate for the PB-regulated signalling molecule in the induction of CYP2B gene expression [9]. Most of these experiments have, however, been conducted by using hepatoma cells selected under fructose pressure. Therefore the physiological significance of AMPK-mediated signalling in the PB-induction of drug-metabolizing enzymes has yet to be elucidated. Moreover, direct evidence that AMPK activates CAR function is lacking. The present study verifies in vivo that AMPK transduces signal(s) originating from PB-like inducers to the nuclear translocation of CAR and transactivation of CYP2B genes. However, AMPK activation itself is insufficient for CYP2B induction. The results of the present study suggest that PB uses AMPK signalling to enhance CAR activation under physiological conditions.
EXPERIMENTAL
Materials
Anti-(human CAR) antibody was purchased from Perseus Proteomics. Anti-phospho-AMPKα (Thr-172), anti-phospho-AMPKα1 (Ser-485)/phospho-AMPKα2 (Ser-491) and anti-AMPKα antibodies were purchased from Cell Signaling Technology.
Animals
All experiments were conducted under NIH (National Institutes of Health) guidelines for the care and use of laboratory animals and were approved by the Showa University Institutional Animal Care and Use Committee. Male F344 rats (4 weeks of age for experiments of chemical carcinogenesis and 7 weeks of age for all other experiments), male Wistar rats (7 weeks of age) and male C3H/HeN mice (7 weeks of age) were purchased from Japan SLC. The modified Solt–Farber model of hepatocarcinogenesis in F344 rats has been described elsewhere [13]. Animals were administered with PB intraperitoneally (80 mg/kg of body mass for rats and 100 mg/kg for mice) 24 h before being killed unless indicated otherwise. PB and AICAR (5-amino-4-imidazolecarboxamide riboside) were dissolved in saline.
Construction of plasmids
An expression plasmid for rat CAR (CAR/pcDNA3.1) and a rat CYP2B1 PBREM reporter plasmid (PBREM/pTAL-Luc) were described previously [13]. cDNA fragments encoding a full-length rat AMPKα2 and its truncated version (amino acids 1–312) were PCR-amplified with the high-fidelity enzyme Advantage-HF 2 (Clontech) using a rat liver cDNA library. The rat AMPKα2(1–312)T172A/pcDNA3.1 plasmid encoding a dominant-negative mutant of the enzyme [14] was generated by site-directed mutagenesis using the GeneEditor system (Promega) to change Thr-172 to Ala in the truncated mutant. The mutation primer was 5′-GTGAATTTCTACGAGCTAGCTGTGGATCGC-3′ (the mutation site is underlined). These constructs were confirmed by sequence analysis.
Enzyme assays and blotting analyses
CYP contents in liver microsomes were determined using an analysis of the dithionite-reduced CO difference spectra [15] using the Shimadzu MPS-2450 spectrophotometer. PROD (7-pentoxyresorufin O-dealkylase) activity in the microsomal preparations was measured in terms of conversion of 7-pentoxyresorufin into resorufin as described previously [13]. In brief, the microsomal proteins (60 μg) were incubated with 2 μM 7-pentoxyresorufin and the NADPH-generating system for 10 min. The reaction was stopped by adding 2 ml of ice-cold methanol and centrifuging at 1800 g for 10 min. The resulting supernatant was used for measuring the fluorescence (λex 530 nm, λem 585 nm) using the Shimadzu RF-5000 DR-15 fluorescence spectrometer. Liver nuclear extracts were prepared using NE-PER® nuclear extraction reagents (Pierce Biotechnology) essentially according to the manufacturer's instructions. Immunoblot analysis was carried out as described in [16] using 20 μg of proteins. Total RNA (20 μg) isolated from frozen liver samples was subjected to Northern blot analysis as described previously [16]. The probes used were the oligoDNA (5′-TTCCTTGAAGGTTGGCTCAACGACAGCAACT-3′) for mouse Cyp2b10 and the 0.5 kb insert of a GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA purified from a GD5 plasmid [17]. Cyp2b10 mRNA levels were normalized to the respective GAPDH expressions.
Reporter assay
Mice were intravenously injected with 8 μg of PBREM/pTAL-Luc along with 2 μg of a Renilla luciferase expression vector pRL-TK (Promega) using the TransIT in vivo gene delivery system (Mirus Bio). After 24 h, mice were killed and livers were homogenized in 20 vol. of a passive lysis buffer (Promega). The homogenate was centrifuged at 20000 g for 15 min, and the resulting supernatant was used for the dual-luciferase assay system (Promega). Firefly luciferase activity was normalized to the respective Renilla luciferase activity.
Statistical analysis
All data are given as the means±S.E.M. Significance of the difference between the control and treated group(s) was assessed by Welch's t test or Steel's test for data from two or multiple groups respectively. The Steel–Dwass test was performed to compare data from multiple groups with each other. Values of P<0.05 were taken to be significant.
RESULTS
AMPK exists in heterotrimeric complexes composed of a catalytic α-subunit and regulatory β- and γ-subunits. AMPKα is phosphorylated on the activation loop threonine residue (Thr-172) within the catalytic subunit by upstream kinases such as STK11/LKB1 (serine/threonine kinase 11), a product of the tumour-suppressor gene responsible for Peutz–Jeghers syndrome [18,19]. Therefore the activation states of AMPK were examined by using the anti-phospho-AMPKα (Thr-172) antibody to determine whether PB activates AMPK in the rat liver. Although saline showed no effect at least by 24 h (results not shown), PB at a dose of 80 mg/kg, which causes the activation of CAR function and the induction of CYP2B1/2 transcription, increased phosphorylation of AMPKα at Thr-172 in the liver of rats in a time-dependent manner (Figure 1A). The increased phosphorylation lasted for at least 24 h post-PB treatment. On the other hand, the levels of AMPKα1/α2 phosphorylation at Ser-485/491, which negatively regulates activation of AMPK [20], did not change significantly during the observed period after PB treatment (Figure 1A). Midazolam, a PB-like CYP2B inducer [21], increased the Thr-172 phosphorylation of AMPKα (Figure 1B) at doses which induced PROD activity (Figure 1C), a typical marker for CYP2B activity. These results reveal for the first time that CYP2B inducers activate AMPK in the animal liver.
Figure 1. PB-like inducers activate AMPK in rat liver.
(A) Wistar rats were administered intraperitoneally with PB (80 mg/kg) for the times indicated. Liver post-mitochondrial fractions were subjected to immunoblot analysis. The blots were probed sequentially with anti-phospho-AMPKα (Thr-172), anti-phospho-AMPKα1 (Ser-485)/phospho-AMPKα2 (Ser-491) and anti-AMPKα antibodies. The upper panel demonstrates typical band patterns from the same blot. Band intensities obtained from phospho-specific antibodies were normalized with those of the respective AMPKα1 and are illustrated in the lower panel (*P<0.05 compared with control; n=3). (B) F344 rats were treated intraperitoneally with midazoram (MDZ, 50 mg/kg) for 24 h. Liver cytosolic fractions were subjected to immunoblot analysis. Band intensities of phospho-AMPKα (Thr-172) were normalized with those respective to AMPKα (*P<0.05; n=3). (C) PROD activity was measured in microsomal fractions obtained from F344 rats treated intraperitoneally with MDZ (50 mg/kg) or PB (80 mg/kg) for 24 h (**P<0.01, n=3).
We have reported previously that the attenuation of the CYP2B induction by PB in hepatic pre-neoplastic lesions is evidently a consequence of impaired activation of CAR [13]. The hepatic-tumour-bearing animals thus might be a useful model to elucidate the activation machinery of CAR. Therefore we examined whether PB activates AMPK in the rat model of hepatocarcinogenesis. The augmented phosphorylation of AMPKα at Thr-172 by PB observed in the normal rats disappeared in the tumour-bearing rats during the monitored period (Figure 2). These results imply a link between the functions of CAR and AMPK.
Figure 2. PB failed to activate AMPK in the hepatic tumour.
F344 rats were subjected to the modified Solt–Farber model of hepatocarcinogenesis. The normal and the adenocarcinoma group of rats were treated intraperitoneally with PB (80 mg/kg) 24 h before being killed. The liver cytosolic fractions were subjected to immunoblot analysis using anti-phospho-AMPKα (Thr-172) and anti-AMPKα antibodies. The upper panel demonstrates typical band patterns from the same blot. Band intensities obtained from the phospho-specific antibody were normalized with those of the respective AMPKα1 and are illustrated in the lower panel (*P<0.05 compared with control; n=3).
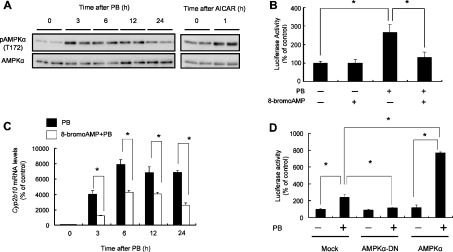
To address the possibility that the AMPK pathway lies upstream of CAR-dependent CYP2B transactivation, we used mice transiently transfected with the CYP2B1 PBREM reporter plasmid. Although the vehicle showed no effect by 24 h (results not shown), PB at a dose of 100 mg/kg induced hepatic AMPKα phosphorylation at Thr-172 in mice as well (Figure 3A). The activation peaked 3–6 h after the treatment (Figure 3A). The PBREM reporter activity increased by PB was inhibited almost completely by the pre-treatment of the mice with 8-bromo-AMP (Figure 3B), an AMPK inhibitor [22]. In addition, 8-bromo-AMP significantly attenuated PB induction of Cyp2b gene expression (Figure 3C). It has been shown that an AMPKα mutant generated by substitution of alanine for Thr-172 (T172A) inhibits the AMPK signalling in a dominant-negative fashion [14]. Forced expression of the dominant-negative mutant of AMPKα2 in mouse liver cancelled the PBREM transactivation caused by PB treatment (Figure 3D). Moreover, the ectopic expression of wild-type AMPKα2 accelerated PB-induced PBREM transactivation (Figure 3D). On the other hand, AICAR, a cell-permeable adenosine analogue that can be phosphorylated to the AMP analogue ZMP (AICAR monophosphate) and activates AMPK [23] (Figure 3A), induced the PBREM transactivation (Figure 4A) in a time-dependent manner (results not shown). Furthermore, AICAR significantly induced, although to a lesser extent than PB, nuclear accumulation of CAR in a time-dependent fashion (Figure 4B). All of these experimental data indicate that AMPK plays an important role in PB-induced CAR activation and PBREM transactivation in vivo.
Figure 3. AMPK inhibitors attenuate PB-mediated PBREM transactivation and Cyp2b10 gene expression in C3H mice.
(A) Mice were either untreated (0 h) or treated intraperitoneally with PB (100 mg/kg) or AICAR (100 mg/kg) for the times indicated. The liver cytosolic fractions were subjected to immunoblot analysis using anti-phospho-AMPKα (Thr-172) and anti-AMPKα antibodies. (B) Mice were injected intravenously with the PBREM-Luc and pRL-TK vectors and were simultaneously treated intraperitoneally with PB (100 mg/kg) and/or 8-bromo-AMP (20 mg/kg). After 24 h, the liver homogenate was subjected to dual-luciferase assay (*P<0.05; n=3). (C) Mice were treated intraperitoneally with PB (100 mg/kg) in conjunction with saline or 8-bromo-AMP (20 mg/kg) and were killed 3, 6, 12 or 24 h after injection. Total RNA (20 μg) obtained from the liver sample was subjected to Northern blot analysis using a mouse Cyp2b10 oligonucleotide probe. Level of Cyp2b10 mRNA was normalized with the respective GAPDH mRNA (*P<0.05; n=3–4). (D) Mice were injected intravenously with PBREM-Luc (4 μg) and pRL-TK (1 μg) in conjunction with empty pcDNA3.1 (Mock), AMPKα2(1–312)T172A/pcDNA3.1 (AMPKα-DN; dominant-negative) or AMPKα2/pcDNA3.1 (AMPKα) vectors (5 μg) and were simultaneously treated intraperitoneally with PB (100 mg/kg). After 24 h, the liver was subjected to dual-luciferase assay.
Figure 4. AMPK activators induce PBREM transactivation and nuclear translocation of CAR.
(A) Mice were injected intravenously with the PBREM-Luc and pRL-TK vectors and were simultaneously treated intraperitoneally with AICAR (100 mg/kg) or PB (100 mg/kg). After 24 h, the liver homogenate was subjected to dual-luciferase assay (*P<0.05; n=3). (B) Mice were either untreated (C) or treated intraperitoneally with AICAR (500 mg/kg; A) or PB (100 mg/kg; P) and were killed at the times indicated. Hepatic nuclear extract (20 μg) was subjected to Western blot analysis using CAR-specific antibody. The blot was sequentially reprobed with β-actin protein. The upper panel demonstrates typical band patterns from the same blot. Band intensities obtained from the anti-CAR antibody were normalized with those of the respective anti-β-actin antibody and are illustrated in the lower panel (*P<0.05 compared with control; n=3).
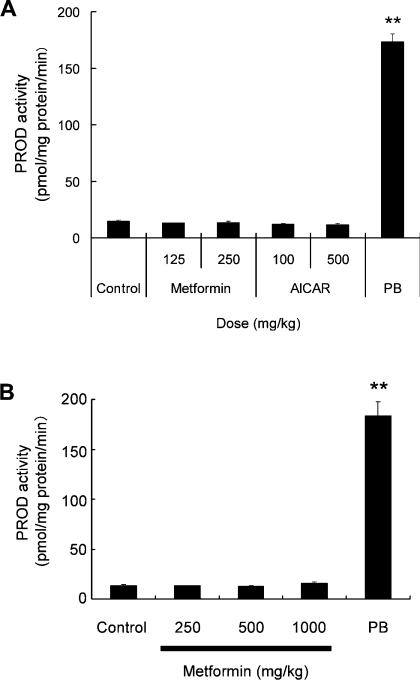
To examine whether AMPK activation causes Cyp2b induction, mice were treated with AICAR or metformin, another AMPK activator [24], at three consecutive doses. The AMPK activators, however, showed virtually no effect on microsomal PROD activity (Figure 5A) or Cyp2b10 levels (results not shown) as determined by immunoblot analysis of the liver of mice. Moreover, metformin did not induce either CYP2B1/2 mRNA (results not shown) or PROD activity (Figure 5B) in rat liver. These observations indicate that AMPK activation itself is not sufficient for CYP2B induction in vivo.
Figure 5. AMPK activators do not induce CYP2B in rats and mice.
(A) Mice were treated intraperitoneally with saline (Control), metformin (125 or 250 mg/kg), AICAR (100 or 250 mg/kg) or PB (100 mg/kg) once a day for 3 days. (B) Wistar rats were treated intraperitoneally with saline (Control), by mouth with metformin (250, 500 or 1000 mg/kg) or intraperitoneally with PB (80 mg/kg) once a day for 3 days. All animals were killed 24 h after the final injection. PROD activity was measured in liver microsomal fractions (**P<0.01 compared with control; n=3).
DISCUSSION
It is well known that the steady-state levels and inducibility of CYPs vary with diverse nutrients and different energetic and pathological conditions. Altered patterns of induction of CYP2B and CYP3A, which play a major role in the metabolism of the bulk of the medications on the market, may result in divergent pharmacokinetics. Therefore understanding the molecular machinery driving the modification of the inducibility of these drug-metabolizing enzymes under pathophysiological situations is a crucial step to predicting the individual patient and drug pharmacokinetics.
Increased expression of CYP2B and CYP3A has been shown in patients with diabetes mellitus, as well as in animal models, and insulin treatment reverses the increased expression. It is also known that the PB-mediated effects on drug-metabolizing enzymes are enhanced under fasting conditions. On the basis of these findings, two approaches from different laboratories have recently proposed the novel idea of a relationship between metabolic syndrome including diabetes and transcriptional regulation of the CYP genes. One has demonstrated that FoxO1 (forkhead box transcription factor O1), a key transcriptional regulator in gluconeogenesis, has cross-talk with CAR and PXR in terms of transactivation of their target genes [25]. FoxO1, which is modulated by insulin signalling, functions as a co-activatior in the CAR- and PXR-mediated transactivation. On the other hand, the nuclear receptors act as the co-repressor of FoxO1 in a ligand-dependent manner. Another approach has demonstrated that AMPK signalling plays a role in PB-mediated CYP2B induction [9]. These studies are particularly relevant to not only understand the molecular basis of the alteration of CYP induction under diverse energy status conditions, but also to gain insight into the reciprocal regulation of gluconeogenetic enzymes by AMPK and FoxO1. However, most of the experiments in these studies have been performed using particular cell cultures and forced gene expressions and therefore lack the verification of the in vivo activity of CYP inducers. The present study demonstrates that PB utilizes the AMPK pathway for induction of CYP2B transactivation in the murine liver. We have also disclosed for the first time that AMPK activates CAR nuclear translocation. Moreover, the PB-like inducer midazolam induced AMPK activation as well, suggesting that this enzyme is commonly utilized by the pathway of CYP2B induction by PB-like drugs.
Several lines of evidence have revealed that nuclear translocation and transactivation function of CAR are regulated by phosphorylation [4,26,27]. It is possible that AMPK phosphorylates CAR directly; however, no evidence showing CAR as a substrate for AMPK has been obtained to date. Recently, important target molecules responsible for the downstream signalling of AMPK have become successively clarified. Among these downstream molecules, TORC2 [transducer of regulated CREB (cAMP-response-element-binding protein) activity 2], a CREB co-activator, has recently been shown to be involved in AMPK-modulated gluconeogenesis [28,29]. TORC2, in accordance with CREB, positively transactivates genes encoding gluconeogenic proteins such as PPARγ (peroxisome-proliferator-activated receptor γ) co-activator-1α and PEPCK (phosphoenolpyruvate carboxykinase) [30]. AMPK has been shown to phosphorylate TORC2 at Ser-171 and modulate its function by blocking nuclear accumulation [28]. In addition, PKA (cAMP-activated protein kinase A) signalling has been shown to antagonize AMPK in terms of the nuclear translocation of TORC2 by inhibiting SIK-1 (salt-inducible kinase 1) [30], an AMPK-related serine threonine kinase [31]. Previous findings [27,32,33] indicate that the cAMP–PKA signalling modulates the induction of CYP2B and CYP3A gene expression. These observations would be consistent with the action of PKA signalling in the co-operative regulation of SIK and AMPK on TORC2 function. Study of the molecular machinery that connects AMPK and CAR activation is currently underway from the point of view of these aspects.
It has been postulated that Ca2+-mediated signalling, particularly CaMK (Ca2+/calmodulin-dependent protein kinase), is involved in PB-induced CYP2B and CYP3A gene expression [27,33]. It appears that CaMK does not act on CAR nuclear translocation, but rather on its transactivation function in the nucleus [34]. Interestingly, CaMKs have recently been shown to function as the alternative upstream molecules responsible for AMPK activation [35–37]. It is therefore possible that the Ca2+/calmodulin-mediated signals, including the CaMKs, promote CAR transactivation in the nucleus in addition to its nuclear translocation through the AMPK pathway.
The present study demonstrates that PB failed to activate AMPK in hepatic-tumour-bearing animals, which possess impaired CAR functionality and thereby reduced CYP2B inducibility [13]. These findings suggest that the impaired AMPK pathway during hepatocarcinogenesis causes disruption of the machinery of CAR nuclear translocation and results in CYP2B transactivation. The up- and down-stream molecules of AMPK-mediated signalling include products of tumour-suppressor genes, such as STK11/LKB1, TSC (tuberous sclerosis complex) 1/2 and p53, the mutation and altered product function of which are often observed in tumours, including liver carcinoma [38,39]. Therefore it can be postulated on the one hand that impaired function of the tumour-suppressor gene products in the AMPK pathway causes attenuated CAR activation in response to PB. On the other hand, it is postulated that high levels of glycogen present in premalignant lesions suppress AMPK activity [40,41]. The molecular machinery that governs attenuated AMPK and CAR activations in response to the PB-like drugs in the preneoplastic lesions will need to be clarified further with these findings in mind.
PB accelerates protein synthesis and cell growth in the animal liver. PB activation of ERK (extracellular-signal-regulated kinase) is involved in this process [42]. Conversely, AMPK inhibits protein synthesis in the heart and muscle [43] by modulating the function of a downstream effector mTOR (mammalian target of rapamycin) [44]. It has been demonstrated that TSC1/2, the direct substrates of AMPK in this pathway which modulate protein synthesis through mTOR down-regulation, may be inhibited by ERK [45]. Therefore the ERK pathway could be the upstream signalling that causes the AMPK pathway breakdown regarding protein synthesis. It is interesting to note that the ERK pathway antagonizes PBREM transactivation [42]. These observations, in conjunction with findings that indicate that Akt (or protein kinase B), an insulin-responsive kinase, interferes with the co-activator function of FoxO1 in the CAR- and PXR-dependent transactivation machinery [25], are consistent with those which indicate that insulin signalling, including the ERK and Akt pathways, antagonizes CYP2B and CYP3A induction.
The present study has demonstrated that the AMPK activators AICAR and metformin do not induce CYP2B in this model. These observations indicate that AMPK activation itself is not the sufficient obligatory signal required for CYP2B gene expression. On the other hand, observations indicating that AICAR induced PBREM transactivation and CAR nuclear translocation, but to a lesser degree than PB, seem to be inconsistent with those indicating that the AMPK activator had no effect on CYP2B gene expression. One possibility is that the AMPK signalling which activates CAR simultaneously exerts a negative effect on the chromatin remodelling necessary for endogenous CYP2B gene expression. Alternatively, the AMPK signalling could simultaneously produce negative effects, which were undetectable in the reporter assay used in the present study, on CYP2B gene expression. Further insight into the transactivation mechanism of CAR might greatly aid the effort to answer these questions.
In conclusion, the present study confirmed and extended previous findings suggesting that PB utilizes the AMPK pathway as signalling molecules leading to CYP2B gene expression [9]. Although the AMPK pathway is not the sole signal that directs CYP2B gene expression induced by drugs in vivo, the present observations in conjunction with previous data [9,25] may contribute to an improved understanding of the individual inducibility of CYPs in diverse pathophysiological and nutrient status conditions, including the metabolic syndrome, obesity and fasting.
Acknowledgments
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Forman B. M., Tzameli I., Choi H. S., Chen J., Simha D., Seol W., Evans R. M., Moore D. D. Androstane metabolites bind to and deactivate the nuclear receptor CAR-β. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 2.Honkakoski P., Zelko I., Sueyoshi T., Negishi M. The nuclear orphan receptor CAR–retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascussi J. M., Dvorak Z., Gerbal-Chaloin S., Assenat E., Maurel P., Vilarem M. J. Pathophysiological factors affecting CAR gene expression. Drug Metab. Rev. 2003;35:255–268. doi: 10.1081/dmr-120026394. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto T., Sueyoshi T., Zelko I., Moore R., Washburn K., Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzameli I., Pissios P., Schuetz E. G., Moore D. D. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol. Cell. Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamura A., Yoshida Y., Kimura N., Oda H., Kakinuma A. Biochem. Biophys. Res. Commun. 1999;264:530–536. doi: 10.1006/bbrc.1999.1544. [DOI] [PubMed] [Google Scholar]
- 7.Rutter G. A., da Silva Xavier G., Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 2003;375:1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Rencurel F., Stenhouse A., Hawley S. A., Friedberg T., Hardie D. G., Sutherland C., Wolf C. R. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J. Biol. Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- 10.Blouin R. A., Bandyopadhyay A. M., Chaudhary I., Robertson L. W., Gemzik B., Parkinson A. Cytochrome P450 2B enzyme (CYP2B) induction defect following phenobarbital treatment in the fa/fa Zucker rat: molecular characterization. Arch. Biochem. Biophys. 1993;303:313–320. doi: 10.1006/abbi.1993.1289. [DOI] [PubMed] [Google Scholar]
- 11.Zangar R. C., Novak R. F. Effects of fatty acids and ketone bodies on cytochromes P450 2B, 4A, and 2E1 expression in primary cultured rat hepatocytes. Arch. Biochem. Biophys. 1997;337:217–224. doi: 10.1006/abbi.1996.9785. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida Y., Kimura N., Oda H., Kakinuma A. Insulin suppresses the induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem. Biophys. Res. Commun. 1996;229:182–188. doi: 10.1006/bbrc.1996.1777. [DOI] [PubMed] [Google Scholar]
- 13.Numazawa S., Shindo S., Maruyama K., Chibana F., Kawahara Y., Ashino T., Tanaka S., Yoshida T. Impaired nuclear translocation of CAR in hepatic preneoplastic lesions: association with an attenuated CYP2B induction by phenobarbital. FEBS Lett. 2005;579:3560–3564. doi: 10.1016/j.febslet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Sakoda H., Ogihara T., Anai M., Fujishiro M., Ono H., Onishi Y., Katagiri H., Abe M., Fukushima Y., Shojima N., et al. Activation of AMPK is essential for AICAR-induced glucose uptake by skeletal muscle but not adipocytes. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1239–E1244. doi: 10.1152/ajpendo.00455.2001. [DOI] [PubMed] [Google Scholar]
- 15.Omura T., Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 16.Oguro T., Kaneko E., Numazawa S., Imaoka S., Funae Y., Yoshida T. Induction of hepatic heme oxygenase and changes in cytochrome P-450s in response to oxidative stress produced by stilbenes and stilbene oxides in rats. J. Pharmacol. Exp. Ther. 1997;280:1455–1462. [PubMed] [Google Scholar]
- 17.Maehara Y., Fujiyoshi T., Takahashi K., Yamamoto M., Endo H. 1.5 kb mRNA abundantly expressed in rat tumors encodes a 37 kilodalton protein in vitro. Biochem. Biophys. Res. Commun. 1985;131:800–805. doi: 10.1016/0006-291x(85)91310-5. [DOI] [PubMed] [Google Scholar]
- 18.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., Schlattner U., Wallimann T., Carling D., Hue L., Rider M. H. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J. Biol. Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 21.Hoen P. A., Bijsterbosch M. K., van Berkel T. J., Vermeulen N. P., Commandeur J. N. Midazolam is a phenobarbital-like cytochrome P450 inducer in rats. J. Pharmacol. Exp. Ther. 2001;299:921–927. [PubMed] [Google Scholar]
- 22.Musi N., Hayashi T., Fujii N., Hirshman M. F., Witters L. A., Goodyear L. J. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- 23.Hardie D. G., Carling D. The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur. J. Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama S., Koike C., Negishi M., Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidhu J. S., Omiecinski C. J. An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J. Pharmacol. Exp. Ther. 1997;282:1122–1129. [PubMed] [Google Scholar]
- 27.Marc N., Galisteo M., Lagadic-Gossmann D., Fautrel A., Joannard F., Guillouzo A., Corcos L. Regulation of phenobarbital induction of the cytochrome P450 2b9/10 genes in primary mouse hepatocyte culture: involvement of calcium- and cAMP-dependent pathways. Eur. J. Biochem. 2000;267:963–970. doi: 10.1046/j.1432-1327.2000.01083.x. [DOI] [PubMed] [Google Scholar]
- 28.Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 29.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., 3rd, Takemori H., et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Katoh Y., Takemori H., Horike N., Doi J., Muraoka M., Min L., Okamoto M. Salt-inducible kinase (SIK) isoforms: their involvement in steroidogenesis and adipogenesis. Mol. Cell. Endocrinol. 2004;217:109–112. doi: 10.1016/j.mce.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu J. S., Omiecinski C. J. cAMP-associated inhibition of phenobarbital-inducible cytochrome P450 gene expression in primary rat hepatocyte cultures. J. Biol. Chem. 1995;270:12762–12773. doi: 10.1074/jbc.270.21.12762. [DOI] [PubMed] [Google Scholar]
- 33.Galisteo M., Marc N., Fautrel A., Guillouzo A., Corcos L., Lagadic-Gossmann D. Involvement of cyclic nucleotide- and calcium-regulated pathways in phenobarbital-induced cytochrome P-450 3A expression in mouse primary hepatocytes. J. Pharmacol. Exp. Ther. 1999;290:1270–1277. [PubMed] [Google Scholar]
- 34.Yamamoto Y., Kawamoto T., Negishi M. The role of the nuclear receptor CAR as a coordinate regulator of hepatic gene expression in defense against chemical toxicity. Arch. Biochem. Biophys. 2003;409:207–211. doi: 10.1016/s0003-9861(02)00456-3. [DOI] [PubMed] [Google Scholar]
- 35.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 37.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Lasky T., Magder L. Hepatocellular carcinoma p53 G>T transversions at codon 249: the fingerprint of aflatoxin exposure? Environ. Health Perspect. 1997;105:392–397. doi: 10.1289/ehp.97105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoki K., Corradetti M. N., Guan K. L. Dysregulation of the TSC–mTOR pathway in human disease. Nat. Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 40.Ashrafian H. Cancer's sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet. 2006;367:618–621. doi: 10.1016/S0140-6736(06)68228-7. [DOI] [PubMed] [Google Scholar]
- 41.Sakoda H., Fujishiro M., Fujio J., Shojima N., Ogihara T., Kushiyama A., Fukushima Y., Anai M., Ono H., Kikuchi M., et al. Glycogen debranching enzyme association with β-subunit regulates AMP-activated protein kinase activity. Am. J. Physiol. Endocrinol. Metab. 2005;289:E474–E481. doi: 10.1152/ajpendo.00003.2005. [DOI] [PubMed] [Google Scholar]
- 42.Joannard F., Rissel M., Gilot D., Anderson A., Orfila-Lefeuvre L., Guillouzo A., Atfi A., Lagadic-Gossmann D. Role for mitogen-activated protein kinases in phenobarbital-induced expression of cytochrome P450 2B in primary cultures of rat hepatocytes. Toxicol. Lett. 2006;161:61–72. doi: 10.1016/j.toxlet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Chan A. Y., Dyck J. R. Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy. Can. J. Physiol. Pharmacol. 2005;83:24–28. doi: 10.1139/y04-107. [DOI] [PubMed] [Google Scholar]
- 44.Sarbassov D. D., Ali S. M., Sabatini D. M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]