Abstract
Aims: To investigate retrobulbar circulatory parameters in type 2 diabetic patients with and without diabetic retinopathy (DR) progression.
Methods: This was a prospective cohort study. One eye of 35 diabetic patients with background DR (BDR) were included in the study. Eyes without DR, with proliferative DR, photocoagulation, past surgical procedures, or other ophthalmic disease except BDR and cataract were excluded. The study was masked. Colour Doppler imaging (CDI) was used to measure the retrobulbar circulation at the beginning of the study and after a mean follow up interval of 21 months. Peak systolic velocity (PSV), end diastolic velocity (EDV), and resistivity index (RI) in the central retinal artery and vein and the posterior ciliary artery were measured.
Results: 18 patients who developed DR progression showed significantly increased central retinal vein PSV ( 5.6 (3.5–9.1) p = 0.003), EDV ( 3.4 (2.3–4.4) p = 0.04), and RI ( 0.43 (0.20–0.56) p = 0.02) at the final measurement compared to the initial measurement (PSV = 4.6 (3.2–7.0); EDV = 3.0 (2.3–3.7); RI = 0.40 (0.17–0.52)). Circulatory parameters in the central retinal artery and the posterior ciliary artery did not alter significantly after progression of DR. 17 patients were without DR progression and they did not show any significant differences in the measured circulatory parameters on entry compared to the final measurement.
Conclusion: The authors suggest that the initial changes in the retrobulbar circulation during DR progression occur in the central retinal vein.
Keywords: diabetic retinopathy, pathogenesis, circulation
Diabetic retinopathy (DR), the leading cause of blindness in the developed countries, is still a disease with a pathogenesis that has not been understood completely. Disturbances have been detected in many aspects of ocular circulation in diabetes; however, the role of the haemodynamics in DR has not been clearly defined.
Only a few follow up studies have been done concerning ocular circulation in DR. Rimmer et al,1 using blue light entoptic phenomenon, reported that foveal capillary blood flow decreased with the duration of diabetes. Konno et al,2 using laser Doppler flowmetry and monochromatic photography, found that as duration of diabetes increases and the disease becomes more severe, there is a transition from negative to positive retinal blood flow slopes. By evaluating the fundus of diabetic children and adolescents using fundus photography, Falck and Laatikainen3 reported that the blood vessel diameter significantly increased with the increase in the duration of DR. They also found that signs of early retinopathy developed more often in eyes with venous dilatation than in those without.
On the other hand, a number of studies have found a decrease in IOP in patients with progression of DR4–6 and pressure phenomena have been suggested to be involved in the pathogenesis of DR.6,7
In a cross sectional study using the same method as in the present study, we have reported significantly decreased blood velocity and resistivity index in the retrobulbar arteries and increased blood velocity and resistivity index (RI) in the central retinal vein in patients with DR compared to diabetic patients without DR and control subjects.8 Despite a number of cross sectional studies, there has been no cohort study of retrobulbar circulation in DR. In the present study we analyse the circulatory status in the retrobulbar blood vessels in patients with progression of DR compared to patients without progression of DR.
PATIENTS AND METHODS
Setting
The study was performed at the outpatient clinic of the Tokyo University Hospital. It was approved by the ethics committee of the University of Tokyo, School of Medicine. Informed consent was obtained from each patient. The research followed the tenets of the Declaration of Helsinki.
Study population
Thirty five type 2 diabetic patients were randomly chosen from incoming patients who visited the DR outpatient clinic of the University of Tokyo Hospital for their regular check ups. Patients with other ocular diseases that may affect the retrobulbar circulation, such as glaucoma, age related maculopathy, high degree myopia and those with a history of laser treatment or intraocular surgery were excluded. Inclusion in the study also depended on whether the patients were available for follow up. Patients were accumulated prospectively.
Data from one eye were included in the analysis. In the case of patients with progression of DR the eye with progression was included in the study. In the cases where progression was bilateral and in the non-progression group, data from the right eye were included in the analysis, unless there was laser photocoagulation, surgery, or other conditions that could affect the ocular circulation. There were only two subjects with unilateral progression of DR that was not enough to make a comparison of the retrobulbar circulation between the eyes. Patients were selected in the non-progression group if both of their eyes were without progression of DR.
Patients were enrolled in the study consecutively. The follow up period between the first and the last measurement varied between patients, ranging from 5 to 38 months with a mean of 21 months. Evaluation of the subjective and the main outcome measures was done before determination of patients’ DR. Patients were followed up according to their outpatient schedule, which did not differ from the schedule of patients who were not enrolled in the study and depended on their stage of DR. No interim values are presented because nine patients were absent after their second follow up and interim data were incomplete.
Fukuda’s classification of DR was used to determine the DR stage for each patient in this study. Patients with background DR in stages A1 (mild to moderate background retinopathy comprising microaneurysms and dot haemorrhages) and A2 (severe background retinopathy comprising blot haemorrhages and soft exudate in addition to pathological signs of A1 stage) were included in the study.
The criterion of progression of DR was an increase of at least one stage up the scale of Fukuda’s classification of DR.9 Stages of DR were determined by fundus examination and fluorescein angiography. The patients underwent assessment of best corrected visual acuity after standardised refraction. Intraocular pressure (IOP) was measured by Goldmann tonometry. Systolic (BPs) and diastolic (BPd) blood pressures were determined by an automatic sphygmomanometer. The mean blood pressure (BPm) in the brachial artery was calculated as follows
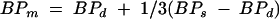 |
Observational procedures
All measurements were performed using a CDI set: Powervision SSA-380 A (Toshiba, Tokyo, Japan) using a 7 MHZ transducer. One observer (GD), blinded to the subjects’ characteristics, performed all the measurements.
The method of measurement has been described in our previous cross sectional study.8 We measured the following retrobulbar blood vessels: central retinal artery, central retinal vein, and posterior ciliary artery. We were unable to locate the posterior ciliary artery on follow up in three patients with progression of DR and in one patient without progression of DR.
The CDI method allows for accurate RI measurement because its values do not depend on the Doppler angle. We attempted to maintain the Doppler angle as parallel as possible to the measured blood vessel in order to obtain more accurate blood velocity data.
Main outcome measures
The haemodynamic parameters measured in the aforementioned blood vessels were peak systolic velocity (PSV) and end diastolic velocity (EDV). The resistivity index (RI) was calculated as follows:
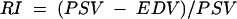 |
(1) |
During repeated measurements in the follow up study, care was taken to obtain the measurement always at the same location of the blood vessel. This was done in reference to the previously taken photographs and by adjusting the ultrasound gate at the same location.
Statistical analysis
Data were extracted from prospectively completed data forms. ANOVA was used to compare the clinical characteristics and the retrobulbar circulatory parameters of the patients with and without progression of DR. Because all the data did not follow a normal distribution, the non-parametric Wilcoxon signed rank test was used to determine significant differences between the first and the last measurement in both patient groups. p Values less than 0.05 were regarded as statistically significant. The statview program (Microsoft) was used for data analysis.
RESULTS
All 35 patients enrolled in the study were available for follow up. Eighteen patients (51%) had progression of DR. Ten of those patients progressed from mild/moderate BDR to severe BDR, while eight patients progressed from severe BDR to pre-proliferative DR. Seventeen patients (49%) were without progression of DR. Six of them had mild/moderate BDR and 11 had severe BDR.
There were no significant differences in the clinical characteristics among patients with and without progression of DR (Table 1) There were also no significant differences in the clinical characteristics of each patient group during the first and the last measurement of retrobulbar circulation (Table 2).
Table 1.
Systemic characteristics of patients with and without diabetic retinopathy (DR) progression, mean (SD)
| DR non-progression | DR progression | p Value | |
| Number | 17 | 18 | |
| Age | 63.8 (7.6) | 61.4 (9.4) | 0.41 |
| Sex | F : 8 M : 9 | F : 5 M : 13 | 0.26 |
| DM duration (years) | 15.6 (8.5) | 15.2 (7.9) | 0.87 |
| BMI (kg/m2) | 23.6 (4.9) | 23.7 (3.7) | 0.87 |
| Follow up period (months) | 21 (11) | 21 (10) | 0.89 |
Table 2.
Characteristics of patients with and without diabetic retinopathy progression during their first and last colour Doppler imaging measurement
| DR non-progression | DR progression | |||||
| 1st measurement | Last measurement | p Value | 1st measurement | Last measurement | p Value | |
| BP mean (mm Hg) | 99.5 (13.9) | 94.8 (11.3) | 0.12 | 96.0 (11.2) | 92.8 (9.9) | 0.14 |
| Pulse rate (beats/min) | 78.6 (14.3) | 78.9 (13.3) | 0.90 | 73.5 (11.5) | 75.7 (9.7) | 0.42 |
| IOP (mm Hg) | 16.8 (2.2) | 16.9 (1.8) | 0.82 | 16.5 (0.8) | 15.9 (1.4) | 0.24 |
| FBS (mg/dl) | 159.3 (51.6) | 175.7 (48.6) | 0.11 | 159.6 (26.5) | 173.2 (63.3) | 0.37 |
| HgA1c (%) | 7.1 (0.8) | 7.4 (1.4) | 0.20 | 7.5 (1.3) | 7.3 (1.5) | 0.75 |
| DR status | M/Mo BDR: 6 | M/Mo BDR: 10 | M/Mo BDR: 0 | |||
| S BDR: 11 | S BDR: 8 | S BDR: 10 | ||||
| PPDR: 8 | ||||||
BP mean = mean blood pressure; IOP = intraocular pressure; FBS = fasting blood sugar; NDR = no diabetic retinopathy; M/Mo BDR = mild to moderate background diabetic retinopathy; S BDR = severe background diabetic retinopathy; PPDR = preproliferative diabetic retinopathy.
During the initial CDI measurements circulatory parameters did not differ significantly between the progression and the non-progression groups (Table 3). In the DR progression group the PSV, EDV, and RI in the central retinal vein were significantly increased after progression of DR (p = 0.003; p = 0.04; p = 0.02) (Table 3). The central retinal artery and posterior ciliary artery did not show significant differences between the initial and the final measurement.
Table 3.
Retrobulbar circulatory parameters of patients with and without diabetic retinopathy progression during their first and last colour Doppler imaging measurement
| DR non-progression | DR progression | ||||||
| (cm/s) | 1st measurement | Last measurement | p Value | 1st measurement | Last measurement | p Value | |
| CRA | PSV | 7.2 (4.2∼9.6) | 7.2 (4.4∼10.3) | 0.29 | 6.7 (3.9∼9.8) | 7.0 (4.4∼10.3) | 0.62 |
| EDV | 1.9 (0.7∼2.6) | 1.8 (0.9∼3.1) | 0.99 | 1.8 (0.9∼3.2) | 1.9 (0.9∼2.6) | 0.77 | |
| RI | 0.75 (0.60∼0.89) | 0.74 (0.66∼0.88) | 0.61 | 0.72 (0.53∼0.82) | 0.73 (0.55∼0.79) | 0.95 | |
| CRV | PSV | 5.1 (2.6∼9.6) | 5.5 (3.0∼12.3) | 0.28 | 4.6 (3.2∼7.0) | 5.6 (3.5∼9.1) | 0.003* |
| EDV | 3.0 (1.8∼4.0) | 3.2 (1.9∼4.7) | 0.51 | 3.0 (2.3∼3.7) | 3.4 (2.3∼4.4) | 0.04* | |
| RI | 0.38 (0.23∼0.58) | 0.42 (0.28∼0.70) | 0.50 | 0.40 (0.17∼0.52) | 0.43 (0.20∼0.56) | 0.02* | |
| PCA | PSV | 8.6 (4.9∼17.2) | 7.9 (4.7∼19.4) | 0.73 | 7.9 (5.4∼15.5) | 7.9 (4.0∼14.5) | 0.65 |
| EDV | 2.5 (1.8∼5.6) | 2.1 (1.9∼4.4) | 0.57 | 2.3 (0.7∼4.4) | 2.3 (1.8∼4.2) | 0.89 | |
| RI | 0.70 (0.56∼0.82) | 0.68 (0.56∼0.89) | 0.69 | 0.70 (0.48∼0.91) | 0.71 (0.50∼0.86) | 0.88 | |
CRA = central retinal artery; CRV = central retinal vein; PCA = posterior ciliary artery; PSV = peak systolic velocity; EDV = end diastolic velocity; MV = mean velocity; *statistically significant difference.
The circulatory parameters of patients without progression of DR did not show significant differences during the first and last measurement.
DISCUSSION
The circulatory status of patients with type 2 diabetes who develop progression of DR has not been reported yet. In the present study of retrobulbar circulation in patients with type 2 diabetes, we detected a significant increase in blood velocity and RI in the central retinal vein in patients with progression of DR, while patients without progression of DR did not exhibit any change in the retrobulbar circulation during a similar follow up period.
In previous prospective follow up studies in diabetic subjects, the methods of circulatory measurement differed from those of the present study. Rimmer et al,1 using the blue light entoptic method, found that retinal capillary blood flow decreased in patients with background DR and pre-proliferative DR during a follow up period of 31 months. They evaluated blood flow through leucocyte velocity in the foveal capillaries.
Konno et al2 took measurements of the blood flow in the temporal retinal artery, using laser Doppler and monochromatic photography during a follow up period of 42 months. They reported a transition from decreasing to increasing retinal blood flow in patients with type 1 diabetes that depends on the duration of diabetes and the severity of the retinopathy.
The present study differs from the follow up studies of Rimmer et al1 and Konno et al,2 not only in the method and aspect of blood flow measurement, but also in the study design. Unlike the aforementioned two studies where patients with and without progression were included in the same group, such patients were assigned to separate groups in the present study.
Our results could be associated with the findings of Falck and Laatikainen3 who reported that retinal venous diameters in patients with type 1 diabetes increased significantly during a follow up period of 28 months and signs of early retinopathy developed more often in eyes with venous dilatation. Similar findings were reported by Skovborg et al,10 where larger average vein diameters were detected in patients with retinopathy, compared to diabetic patients without retinopathy, or control subjects. Other studies of ocular circulation have also found retinal venous blood flow alterations in patients having DR.11
In a previous cross sectional study8 using the same method (CDI), we detected a significant increase of central retinal vein velocity and RI in patients with DR compared with control subjects and diabetic patients without DR. We have confirmed these results by the present prospective study. To the best of our knowledge, this is the first report to show the correlation between the ocular circulation evaluation by CDI and the diabetic retinopathy progression by a prospective study. Significantly increased retrobulbar central retinal vein velocity was also reported by Mendevil12 in patients with proliferative DR (PDR) and by Li-Ping13 in patients with BDR and PDR. However, a study by Guven et al14 using CDI, did not find similar results.
Retinal venous abnormalities that occur in DR, such as microaneurysms and haemorrhages from the venous part of the capillaries, venous beading, loops, reduplication, and neovascularisation could be related to the reported circulatory alterations in the retinal veins in the above mentioned studies.
The net blood outflow from the eye must equal the inflow. In the present study, we did not detect any significant increase in the circulatory parameters of the retrobulbar central retinal artery of patients with progression of DR as it would have been suspected regarding the increased circulatory parameters in the central retinal vein. This led us to speculate that there may be a local retrobulbar increase of blood velocity in the central retinal vein. Klein et al15 reported an increase of the neuroretinal rim area in direct proportion to the duration of diabetes and there was a significant positive correlation between the increased neuroretinal rim area and the severity of diabetic retinopathy. They suggested that this was a result of nerve swelling, which is known to occur in diabetes.16 This finding led us to consider the possibility of an incomplete venous occlusion of the central retinal vein at the level of its passage through the optic nerve possibly because of nerve tissue swelling. The anatomy of the optic disc is known to affect the retinal venous pulsation.17 A local constriction of the lumen of the central retinal vein would tend to locally increase the blood velocity and the resistance to venous outflow, as was detected in the present study. A partial venous stenosis would increase the venous outflow pressure18 resulting in an increased retinal venous hydrostatic pressure that can be associated with some of the clinical signs of DR.
In favour of such a hypothesis is the fact that diabetic papillopathy, a rare condition that affects diabetic patients, has been reported to aggravate DR.19–21 In this condition there is marked venous engorgement probably due to a pressure exerted on the central retinal vein from swelling of the optic nerve tissue. On the other hand, myopia and glaucoma, which demonstrate a wider optic disc cup are known to be protective for progression of DR.5,7 Furthermore, the clinical signs of incomplete central retinal venous occlusion, such as haemorrhages, microaneurysms, engorged veins, and neovascularisation are similar to those in DR. However, the distribution of the pathological signs throughout the retina in DR differs from that in incomplete central retinal vein obstruction, which conflicts with this hypothesis.
Other mechanisms, such as metabolic factors or the effect of IOP pulsatility on the venous outflow could also be responsible for our results. A number of follow up studies have found decreasing IOP in patients with progression of DR4–6 and some authors suggest pressure phenomena to be involved in the pathogenesis of DR.6,7 This may be supported by the fact that conditions associated with altered IOP such as myopia, glaucoma, and carotid stenosis are preventive of DR.
Although some authors have shown a relation between IOP pulsation and venous outflow,22 we could not address this mechanism because we did not evaluate IOP pulsation in this study. Some cross sectional studies that evaluated IOP pulsation have found increased,23 decreased,24 and unchanged25 pulsatile blood flow in BDR.
The clinical characteristics of our patients were not significantly different between the first and the final measurement; however, we could not exclude the possibility that systemic or local factors had influenced the results. For example, the IOP was decreased in the progression group (p = 0.24) and this was suggested by some authors to occur in patients with progression of DR.4–6
It should also be noted that the CDI method evaluates blood velocity and not total blood flow. Furthermore, the follow up period in this study might not have been long enough for the arterial blood circulatory changes to become apparent.
In conclusion, in this study we report increased retrobulbar blood velocity and RI in the central retinal vein of patients with progression of DR, while patients without DR did not show any circulatory change in the retrobulbar circulation. These results suggest an involvement of altered venous blood outflow in the pathogenesis of DR. Further studies are needed to define the exact role of the blood circulation abnormalities in the progression of DR.
REFERENCES
- 1.Rimmer T, Fallon TJ, Kohner EM. Long-term follow up of retinal blood flow in diabetes using the blue light entoptic phenomenon. Br J Ophthalmol 1989;73:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konno S, Feke GT, Yoshida A, et al. Retinal blood flow changes in type 1 diabetes. Invest Opthalmol Vis Sci 1996;37:1140–8. [PubMed] [Google Scholar]
- 3.Falck A, Laatikainen L. Retinal vasodilation and hyperglycaemia in diabetic children and adolescents. Acta Ophthalmol Scand 1995;73:119–24. [DOI] [PubMed] [Google Scholar]
- 4.Armaly MF, Baloglou PJ. Diabetes mellitus and the eye. II Intraocular pressure and aqueous outflow facility. Arch Opthalmol 1967;77:493–502. [DOI] [PubMed] [Google Scholar]
- 5.Jain IS, Luthra CL. Diabetic retinopathy. Its relationship with intraocular pressure. Arch Ophthalmol 1967;78:198–200. [DOI] [PubMed] [Google Scholar]
- 6.Verma D. Pathogenesis of diabetic retinopathy-the missing link? Medical Hypotheses 1993;41:205–10. [DOI] [PubMed] [Google Scholar]
- 7.Moss SE, Klein R, Klein BEK. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology 1994;101:77–83. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrova G, Kato S, Tamaki Y, et al. Choroidal circulation in diabetic patients. Eye 2001;15:602–7. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M. Classification and treatment of diabetic retinopathy. Diabet Res Clin Pract 1994;24 (Suppl) S171–6. [DOI] [PubMed] [Google Scholar]
- 10.Skovborg F, Nielsen AV, Lauritzen E, et al. Diameters of the reinal vessels in diabetic and normal subjects. Diabetes 1969;18:292–8. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald JE, Brucker AJ, Schwartz SS, et al. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol 1986;104:991–6. [DOI] [PubMed] [Google Scholar]
- 12.Mendevil A. Ocular blood flow velocities in patients with proliferative diabetic retinopathy after panretinal photocoagulation. Surv Ophthalmol 1997;42:S89–5. [DOI] [PubMed] [Google Scholar]
- 13.Li Ping S. The clinical study of retinal hemodynamics in patients with diabetic retinopathy. Chinese Journal of Ocular Fundus Disease 1997;13:210–12. [Google Scholar]
- 14.Guven D, Ozdemir H, Hasanreisoglu B. Hemodynamic alterations in diabetic retinopathy. Opthalmology 1996;103:1245–9. [DOI] [PubMed] [Google Scholar]
- 15.Klein BEK, Moss SE, Klein R, et al. Neuroretinal rim area in diabetes mellitus. Invest Ophthalmol Vis Sci 1990;31:805–9. [PubMed] [Google Scholar]
- 16.Eaton RP, Qualls C, Bicknell J, et al. Structure-function relationships within peripheral nerves in diabetic neuropathy: the hydration hypothesis. Diabetologia 1996;39:439–46. [DOI] [PubMed] [Google Scholar]
- 17.Hedges TR, Baron EM, Hedges TR III, et al. The retinal venous pulse. Its relation to optic disk characteristics and choroidal pulse. Ophthalmology 1994;101:542–7. [PubMed] [Google Scholar]
- 18.Meyer-Schwickerath R, Kleinwachter T, Firsching R, et al. Central retinal venous outflow pressure. Graefes Arch Clin Exp Ophthalmol 1995;233:783–8. [DOI] [PubMed] [Google Scholar]
- 19.Regillo CD, Brown GC, Savino PJ, et al. Diabetic papillopathy-patient characteristics and fundus findings. Arch Ophthalmol 1995;113:889–95. [DOI] [PubMed] [Google Scholar]
- 20.Stransky TJ. Diabetic papillopathy and proliferative retinopathy. Graefes Arch Clin Exp Ophthalmol 1986;224:46–50. [DOI] [PubMed] [Google Scholar]
- 21.Katz B. Disc swelling in an adult diabetic patient. Surv Ophthalmol 1990;35:158–63. [DOI] [PubMed] [Google Scholar]
- 22.Michelson G, Harazny J. Relationship between ocular pulse pressures and retinal vessel velocities. Ophthalmology 1997;104:664–71. [DOI] [PubMed] [Google Scholar]
- 23.MacKinnon JR, O’Brien C, Swa K, et al. Pulsatile ocular blood flow in untreated diabetic retinopathy. Acta Ophthalmol Scand 1997;75:661–4. [DOI] [PubMed] [Google Scholar]
- 24.Langham ME, Grebe R, Hopkins S, et al. Choroidal blood flow in diabetic retinopathy. Exp Eye Res 1991;52:167–73. [DOI] [PubMed] [Google Scholar]
- 25.Smidt KG, von Ruckmann A, Kemkes-Metthes B, et al. Ocular pulse amplitude in diabetes mellitus. Br J Ophthalmol 2000;84:1282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


