Abstract
Aim: To determine the transcriptional proximity of retinal pigment epithelium (RPE) cells grown under different culture conditions and native RPE.
Methods: ARPE-19 cells were grown under five conditions in 10% CO2: “subconfluent” in DMEM/F12 + 10% FBS, “confluent” in serum and serum withdrawn, and “differentiated” for 2.5 months in serum and serum withdrawn medium. Native RPE was laser microdissected. Total RNA was extracted, reverse transcribed, and radiolabelled probes were hybridised to an array containing 5353 genes. Arrays were evaluated by hierarchical cluster analysis and significance analysis of microarrays.
Results: 78% of genes were expressed by native RPE while 45.3–47.7% were expressed by ARPE-19 cells, depending on culture condition. While the most abundant genes were expressed by native and cultured cells, significant differences in low abundance genes were seen. Hierarchical cluster analysis showed that confluent and differentiated, serum withdrawn cultures clustered closest to native RPE, and that serum segregated cultured cells from native RPE. The number of differentially expressed genes and their function, and profile of expressed and unexpressed genes, demonstrate differences between native and cultured cells.
Conclusions: While ARPE-19 cells have significant value for studying RPE behaviour, investigators must be aware of how culture conditions can influence the mRNA phenotype of the cell.
Keywords: cell culture density, laser capture microdissection, microarray analysis, retinal pigment epithelium, serum
The retinal pigment epithelial (RPE) cell line ARPE-19, displays significant functional differentiation that mimics native RPE.1 It provides a dependable source of cultured RPE cells for study. At the time of writing, 121 PubMed publications have utilised this cell line. The transcriptional profile of RPE cultures including ARPE-19 cells, and its proximity to native RPE, is however, unknown.
Recently, we found transcriptional differences among ARPE-19 cells grown on different matrices.2 Unexpectedly, ARPE-19 cells grown on plastic displayed the closest phenotype to native RPE. While obvious that the transcriptome will be influenced by culture conditions, few studies are available to articulate these differences. We hypothesised that culture conditions could be optimised so ARPE-19 cells could simulate the native RPE mRNA phenotype. To accomplish this goal, we laser microdissected native RPE and compared its global transcriptional profile with ARPE-19 cells grown under different culture conditions.
METHODS
Cell culture
ARPE-19 cells were seeded at 10 000/cm2 (“subconfluent” or “SS”) for 3 days or 100 000/cm2 in T-75 cm2 flasks and grown in Dulbecco’s Modified Eagle medium/nutrient mixture F12 (DMEM/F12; BioWhittaker Inc, Walkersville, MD, USA) + 10% fetal bovine serum (FBS; UBI Upstate, Lake Placid, NY, USA) at 37°C in 10% CO2. “Confluent” (CS) and “confluent, serum withdrawn” (CSW) cultures were grown for 7 days, and replaced with fresh medium containing serum or 1% bovine serum albumin (BSA), respectively for 3 more days. “Differentiated” (DS) and “differentiated serum withdrawn” (DSW) cells were grown for 2.5 months, and then in serum or 1%BSA for 3 days.
Tissue preparation
Ten eyes (45–95 years old) with a death enucleation time within 6 hours and from donors on life support systems for less than 24 hours were used since premorbid conditions have the greatest influence on RNA degradation3 (table 1). Donors were free of ocular disease, systemic inflammatory disease, and diabetes mellitus. A macular calotte was dissected and cryoprotected as previously described.4,5 Cryosections (7 μm) were stained with haematoxylin and eosin Y (Fisher Scientific, Inc) before microdis`section.
Table 1.
Donor eyes used for transcriptional analysis
| Donor | Age (years) | Sex | Race | D-E* (hours) | Cause of death |
| Microarray analysis | |||||
| 1 | 60 | M | W | 4:00 | Myocardial infarction |
| 2 | 80 | M | W | 3:35 | Respiratory failure |
| 3 | 45 | M | W | 4:45 | CVA† |
| 4 | 74 | F | W | 3:35 | Lung cancer |
| 5 | 95 | F | W | 3:05 | Leukaemia |
| Real time RT-PCR | |||||
| 6 | 83 | M | W | 3:10 | Myasthenia gravis |
| 7 | 82 | M | W | 3:00 | Melanoma |
| 8 | 84 | M | W | 5:00 | CHF‡ |
| 9 | 51 | F | W | 4:45 | Lung cancer |
| 10 | 57 | F | W | 5:45 | Ovarian cancer |
*D-E, death to enucleation time; † CVA, cerebral vascular attack; ‡ CHF, congestive heart failure.
Laser capture microdissection
RPE cells were removed with an Arcturus PixCell II (Arcturus Engineering, Inc Mountain View, CA, USA) as previously described.5 After dissection, the transfer cap was inspected for contaminating tissue before being placed in denaturing buffer.
RNA extraction
Total RNA was extracted using the RNeasy Mini-kit (Qiagen Inc, Valencia, CA, USA) and treated with DNase I (Qiagen, Inc) according to the manufacturer’s recommendations. RNA quality was assessed by GAPDH expression using RT-qPCR with primers designed at the 5′ end of gene, and intact 28S and 18S ribosomal RNA bands of an RPE sample by gel electrophoresis.
Probe synthesis
Total RNA from 5000 cells was reverse transcribed with 50 μCi[33P]dCTP and 50 μCi[33P]dATP with 0.5 μg oligo-dT according to our modified method of Sgroi et al.2,6 A second strand was synthesised with 50 μCi[33P]dCTP, 50 μCi[33P]dATP, 500 ng random hexamers, and 20U Klenow fragment (Gibco BRL). Probes were purified with a Bio-Spin 6 column (BioRad Laboratories, Hercules, CA, USA).
Microarray analysis
Labelled, double stranded cDNA was hybridised to the cDNA GeneFilter Human Microarray Release I (5353 genes; Invitrogen, Inc, Huntsville, AL, USA) using the manufacturer’s protocol. This array contains an insert DNA from a sequence verified IMAGE/LLNL clone using the 3′ end of the gene. Arrays were exposed for 3 days to a high density phosphorimager screen (BioRad Laboratories) and scanned at 50 μm resolution in a phosphorimager (FX Pro-Plus, BioRad Laboratories). The data appear on Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) using platform number GPL1488, and accession numbers GSM32029-GSM32048. Since Lee et al demonstrated that at least three replicate experiments are necessary for reliable microarray results,7 for each condition, at least three independent experiments were performed.
Image and statistical analysis
Signal intensity for each gene was quantified and normalised to 75% of the average signal intensity of the entire array by Pathways 3 software (Invitrogen, Inc). An individual gene was “expressed” if the signal intensity was ⩾1.4-fold above background8 in at least two of three, and three of five experiments for cultured and native RPE, respectively.
Gene expression signals were scaled according to the method of Tusher et al.2,9 A reference set was generated using the average expression from all arrays. Each hybridisation signal was compared with the reference signal in a cube root scatter plot, and intensity values for each gene were corrected with a “scaling factor.”
Hierarchical cluster analysis was performed with average linkage clustering by Cluster, and visualised with TreeView.10 Significance analysis of microarrays (SAM; version 1.12) determined differential gene expression between conditions.11
Real time RT-PCR
Total RNA (100 ng) was reverse transcribed with Sensiscript (Qiagen, Inc) as previously described.2 Primer sequences were designed to span consecutive exons using Primer 3 (Whitehead Institute/MIT, Cambridge, MA, USA). Sequences were verified using NCBI Unigene (table 2). First strand cDNA was amplified using the LightCycler (Roche Diagnostics, Nutley, NJ, USA) in a final volume of 20 μl containing SYBR Green PCR Master Mix (10 μl; Qiagen, Inc, USA), primers (0.5 μM each), and 2 μl DNA in 2.5 mM MgCl2 The standard curve for the gene of interest consisted of PCR products (1–10−6 pg). PCR products were checked by melting point analysis and quantified using the second derivate maximum values calculated by the Light-Cycler analysis software. Expression was normalised to acidic ribosomal phosphoprotein expression.12 The Student’s t test was used to compare the differential gene expression between conditions.
Table 2.
Real time RT-PCR primers and conditions
| Gene name | GenBankAcc No | Sequence | Location | Size (BP) | Cycles | Tm (°C) | |
| SEC13-like 1 (S cerevisiae) | AA496784 | F | CGTGTGTTCATTTGGACCTG | 868–1103 | 236 | 45 | 55 |
| R | CCCTCTGTCACTGATGCTGA | ||||||
| Topoisomerase (DNA) II α170 kDa | AA504348 | F | TCCTGCCAAAACCAAGAATC | 4494–4666 | 173 | 45 | 55 |
| R | GTACAGATTTTGCCCGAGGA | ||||||
| ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | AA427472 | F | TGCCCTGCACTACATAGCAC | 3326–3514 | 189 | 45 | 55 |
| R | GGGGAAGATCTCAGGGTCTC | ||||||
| Oxidase (cytochrome c) assembly 1-like | AA598582 | F | GTCGAATCAGAGAGGCCAAG | 722–948 | 227 | 45 | 55 |
| R | GAGATCCTGGAACCACCAGA | ||||||
| START domain containing 4, sterol regulated | H11369 | F | ACCGCTCAAGGGGTTATTCT | 979–1158 | 180 | 45 | 55 |
| R | CCAACACTTTGGGAGGCTAA | ||||||
| Acidic ribosomal phosphoprotein PO | M17885 | F | CGACCTGGAAGTCCAACTAC | 93–201 | 109 | 45 | 53 |
| R | ATCTGCTGCATCTGCTTG |
RESULTS
Expression profile of native and cultured RPE cells
Figure 1A shows healthy macular RPE cells with cuboidal columnar epithelial morphology and normal Bruch’s membrane. Figure 1B shows the cryosection after microdissection, and figure 1C shows the microdissected cells adherent to the transfer cap. Figure 2 demonstrates spindly appearing “SS” cells (fig 2A) and regular “CS” cells (Fig 2B). “DS” cells had cobblestone morphology with melanin pigment (fig 2C).
Figure 1.
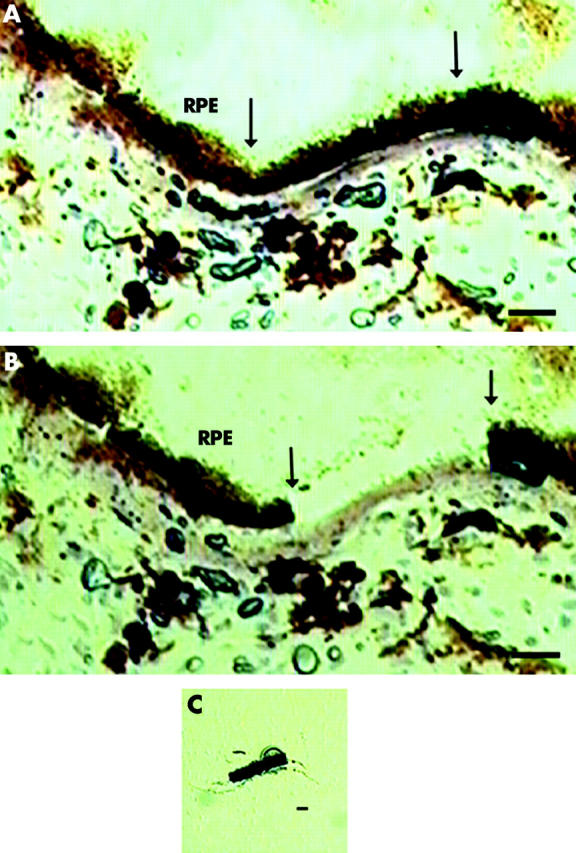
Photomicrograph of macular RPE/Bruch’s membrane with normal morphology before (A) and after (B) laser capture microdissection, as described in the methods. Arrow indicates area of laser dissection. Five laser spots dissected five RPE cells, as seen on the transfer cap (C). Bar = 10 μm.
Figure 2.
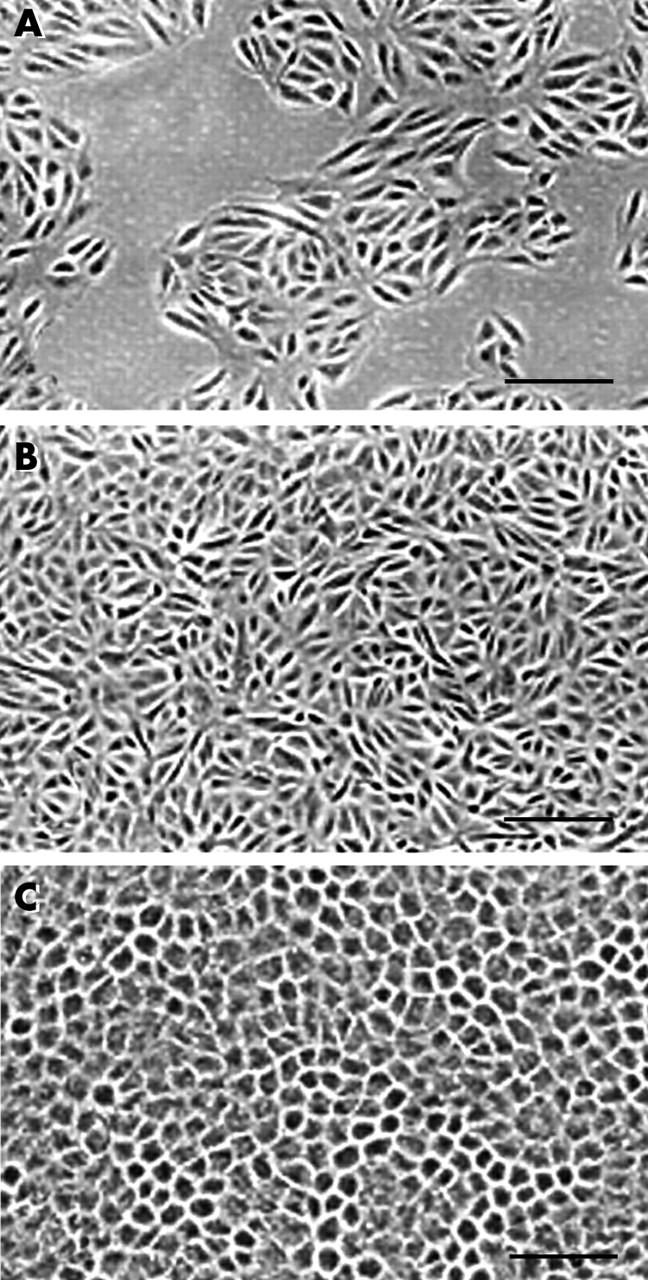
Phase contrast micrograph of ARPE-19 cells grown under various conditions, as outlined in the methods. (A) Subconfluent cells grown in DMEM/F12 + 10% FBS; (B) confluent cells grown in DMEM/F12 + 10% FBS; and (C) differentiated cells grown for 2.5 months in DMEM/F12+10% FBS. Bar = 50 μm.
Scatter plots of microarray analysis for pairwise comparison of native RPE from the five donors, and cultured cells showed reasonable reproducibility (data not shown; R2 = 0.829–0.995 for all comparisons). Using our expression criterion, the number of genes expressed by native RPE was 78% (4177 genes) of the array. The number of genes expressed by ARPE-19 cells ranged from 45.3% (CS), 45.4% (DS), 46.5% (SS), 47.2% (CSW), to 47.7% (DSW).
Table 3 shows the 50 most abundant genes from native RPE cells, of which 48 were also the 50 most abundantly expressed genes by ARPE-19 cells, regardless of culture condition (each separately analysed). While some genes illustrate the multiple functions of the RPE such as melanin biosynthesis (d-dopachrome tautomerase) or antioxidant function (Selenoprotein T), most genes have general cellular function such as protein processing (14%), cytoskeleton (8%), cell cycle (8%), and differentiation (6%). Two class unpaired SAM using a twofold expression differential showed no differentially expressed genes with any false discovery rate (FDR).
Table 3.
50 Most abundant genes expressed by native macular RPE cells
| Gene name | GenBank Acc No | Signal (AU)* | Biological function |
| Cut-like 1, CCAAT displacement protein (Drosophila) | AA284408 | 49.2 | Development |
| cDNA FLJ34046 fis, clone FCBBF2007610 | W15465 | 44.7 | Unknown |
| Solute carrier family 6 (neurotransmitter transporter, betaine/GABA), member 12 | N49856 | 37.7 | Neurotransmitter |
| Nephronophthisis 3 (adolescent) | H93118 | 36.3 | Unknown |
| Junctional adhesion molecule 2 | R68464 | 36.2 | Cell adhesion |
| Prefoldin 4 | AA253430 | 35.9 | Protein folding |
| EST | R32754 | 34.1 | Unknown |
| Selenoprotein T | R78516 | 33.5 | Antioxidant |
| Ubiquitin conjugating enzyme E2I (UBC9 homologue, yeast) | AA487197 | 33.1 | Protein degradation |
| Biogenesis of lysosome related organelles complex-1, subunit 1 | H94857 | 32.2 | Unknown |
| CDC28 protein kinase regulatory subunit 1B | AA459292 | 31.4 | Cell cycle |
| d-dopachrome tautomerase mRNA, complete cds | AA292995 | 31.0 | Unknown |
| Zinc finger protein 258 | AA280676 | 29.4 | Development |
| Endothelin converting enzyme 1 | AA279429 | 28.6 | Cell-cell signalling |
| Multiple endocrine neoplasia I | AA261796 | 27.6 | Transcription regulation |
| CDC28 protein kinase regulatory subunit 2 | AA397813 | 27.0 | Cell cycle |
| Interleukin 24 | AA281635 | 26.5 | Immune response, apoptosis |
| EST | N92646 | 26.0 | Unknown |
| Ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 | AA448184 | 24.9 | electron transport |
| Profilin 1 | AA521431 | 23.5 | Cell structure |
| BCL2 related protein A1 | AA459263 | 23.1 | Apoptosis inhibition |
| EST | R76499 | 23.1 | Unknown |
| Kinesin family member 5B | AA046690 | 21.9 | Organelle transport |
| Rho GTPase activating protein 1† | AA443506 | 21.5 | Cell structure |
| Adaptor related protein complex 1, γ1 subunit | W07300 | 21.5 | Protein sorting |
| NudE nuclear distribution gene E homologue-like 1 (A. nidulans) | R94775 | 21.1 | Unknown |
| EST | H93842 | 20.8 | Unknown |
| Sarcolipin | AA196465 | 20.7 | Intracellular transport |
| Myeloid cell nuclear differentiation antigen | N29376 | 20.7 | Transcription regulation |
| PDGFA associated protein 1† | AA490300 | 20.6 | Cell cycle |
| Guanine nucleotide binding protein (G protein), α inhibiting activity polypeptide 3 | AA490256 | 20.3 | Unknown |
| Chitinase 3-like 1 (cartilage glycoprotein-39) | AA434115 | 20.2 | Metabolism |
| EST | H93906 | 20.1 | Unknown |
| Chromosome 14 open reading frame 2 | T90621 | 20.1 | Unknown |
| Ataxin 2 related protein | AA029963 | 19.9 | Unknown |
| Zinc finger protein 258 | AA280677 | 19.9 | Development |
| DnaJ (Hsp40) homolog, subfamily B, member 1 | AA481758 | 19.8 | Protein folding |
| Vesicle docking protein p115 | AA504342 | 19.5 | Vesicle docking during exocytosis |
| Secretory carrier membrane protein 2 | R32802 | 19.4 | Protein transport |
| Cas-Br-M (murine) ecotropic retroviral transforming sequence | N94234 | 19.4 | Cell growth |
| EST | T60223 | 19.3 | Unknown |
| Chromosome X open reading frame 12 | AA455272 | 18.8 | Unknown |
| EST | R69566 | 18.8 | Unknown |
| Decay accelerating factor for complement (CD55, Cromer blood group system) | R09561 | 18.6 | Complement pathway |
| CTF8, chromosome transmission fidelity factor 8 homologue (S cerevisiae) | N57731 | 18.5 | Unknown |
| EST | R25153 | 18.5 | Unknown |
| Villin 2 (ezrin) | AA411440 | 18.5 | Cell structure |
| Ankyrin repeat domain 1 (cardiac muscle) | AA488072 | 18.5 | Defence response |
| Cytoskeleton associated protein 1 | AA504554 | 18.5 | Cell structure |
| EST | W44701 | 18.3 | Unknown |
*AU, arbitrary units.
†Indicate genes not in the top 50 of cultured RPE cells (any condition).
The majority of the 50 lowest abundance genes expressed by native RPE cells have function related to transcription factors (16%), metabolism (6%), or are unknown (48%). However, a great number of genes had low expression. To further assess the proximity of low abundance genes between native and cultured RPE, 807 genes expressed by native RPE were identified by doubling the arbitrary expression units of the lowest 50 genes. The number of genes expressed by different culture conditions was low: SS (239 genes; 29.6%), CSW (241 genes; 29.8%), CS (223 genes; 27.6%), DSW (263 genes, 32.4%), DS (226 genes; 28.0%).
Cluster analysis of native and cultured RPE
Unsupervised hierarchical cluster analysis showed that CSW or DSW cells clustered closest to native cells and were segregated from SS, CS, and DS cells (fig 3A). Since RPE cells undergo apoptosis and morphological deterioration with ageing, we identified 713 genes involved with differentiation or cell cycle/apoptosis. Supervised cluster analysis was similar to unsupervised cluster analysis (fig 3B).
Figure 3.
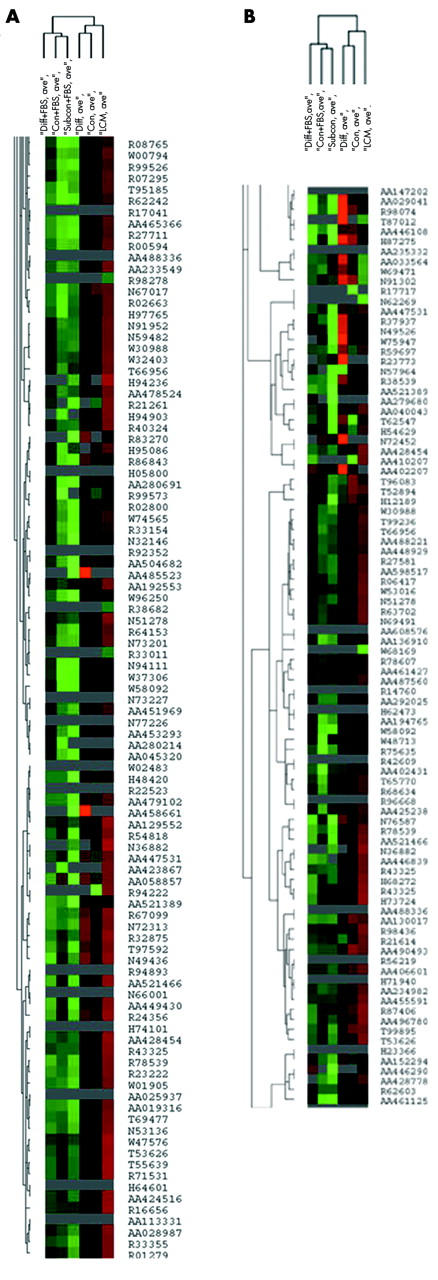
A cluster node of laser capture microdissected RPE cells and ARPE-19 cells grown under five culture conditions. The similar dendrite length indicates relative similarity between conditions. (A) Unsupervised cluster analysis; (B) supervised cluster analysis. Red is log2 intensity value<1, green is log2 intensity value>1 after transformation, black is log2 intensity value = 1, and grey indicates not expressed. “Diff+FBS” is differentiated ARPE-19 cells grown in serum; “Native” is laser captured RPE cells; “Subcon + FBS” is subconfluent ARPE-19 grown in serum; “Con+FBS” is confluent ARPE-19 cells grown in serum; “Con” is confluent serum withdrawn ARPE-19 cells; and “Diff” is differentiated serum withdrawn ARPE-19 cells. Individual genes are designated by ACC number.
Differential expression of native and cultured RPE cells
The expression profiles of CSW and SS cells were used to assess their proximity to native RPE since they were similar and dissimilar, respectively, to native RPE by cluster analysis. Firstly, CSW and SS cultures expressed only 60.5% and 59.6% of the genes, respectively, that were expressed by native RPE. Of the differentially expressed genes identified by SAM (FDR 7%) between native RPE and SS (n = 592 genes), and native RPE and CSW (n = 318 genes), 250 differentially expressed genes were in common, or 42% of SS and 78% of CSW genes, respectively. Of these differentially expressed genes, 36% had no reported function while 7.2% were related to cell cycle/apoptosis, 4.4% to cell structure, 5.6% to metabolism, 7.6% to protein processing, 8.4% to signal transduction, and 6.8% to transcriptional regulation.
Secondly, of 1175 genes unexpressed by native RPE, 324 (27.6%) of these genes were expressed by CSW cells. The function of genes expressed by CSW cells include signal transduction (7.7%), cell cycle/apoptosis (7.7%), transcriptional regulation (7.1%), protein processing (6.5%), intracellular transport (4%), cell adhesion (3.3%), cytoskeleton (1.5%), and unknown function (27.8%). Of genes not expressed by native RPE, 349 genes (29.7%) were expressed by SS cells. The most common function of genes expressed by SS cells included cell cycle/apoptosis (9.7%), protein processing (7.7%), signal transduction (6.9%), transcription regulation (6.3%), small molecule transport (4.9%), cell adhesion (4.3%), immune response (3.4%), RNA processing (2.3%), and unknown function (28.9%).
The number of differentially expressed genes between native and ARPE-19 cells was used as a third assessment of transcriptional proximity. An FDR<10% is a reliable indicator of statistical precision for studies using human tissue.13 Using two class unpaired SAM analysis between native RPE and CSW (FDR 7.2%) or SS cells (FDR 7.4%), 317 and 591 differentially expressed genes respectively, were identified.
The function of differentially expressed genes between native RPE and individual culture conditions was a fourth factor used to evaluate the similarity of expression profiles. The functional annotation of the 317 differentially expressed genes between native RPE and CSW cells included cell cycle/apoptosis (10.7%), protein processing (6.9%), signal transduction (5.7%) and transcription factors (5.7%), metabolism (6.3%), and unknown (42.7%). Analysis using highly stringent conditions (FDR 1.5%) showed that 48 genes were underexpressed and five genes were overexpressed by native RPE compared to CSW cells (table 4). Of this gene set, 21% were related to protein processing.
Table 4.
Differential gene expression between confluent, serum withdrawn cultured and native RPE cells sorted by SAM (FDR 1.5%). Genes are sorted by biological function
| Gene name | GenBank Acc No | Score (d) | Fold change* | Biological function |
| 48 Genes overexpressed by confluent serum withdrawn RPE | ||||
| Lectin, galactoside binding, soluble, 9 (galectin 9) | AA434102 | 4.6899019 | NA† | Cell adhesion |
| Cyclin dependent kinase 4 | AA486312 | 4.0367285 | NA | Cell cycle |
| Pospholipase A2 receptor 1, 180 kDa | R91516 | 3.9299213 | NA | Cell cycle |
| Integrin β1 binding protein 1 | AA456882 | 3.8089025 | NA | Cell-matrix adhesion |
| Integrin, α2 (CD49B, α2 subunit of VLA-2 receptor) | AA463610 | 3.7711619 | NA | Cell-matrix adhesion |
| Actin, β | R44290 | 4.0403841 | NA | Cytoskeleton |
| Caldesmon 1 | AA076063 | 3.7303184 | 1.2 | Cytoskeleton; muscle contraction |
| Oxidase (cytochrome c) assembly 1-like | AA598582 | 3.4692439 | 10.5 | Electron transport |
| Tumour suppressor candidate 3 | N66008 | 5.2150083 | NA | Electron transport |
| Cytochrome c oxidase subunit VIc | AA456931 | 4.3479318 | NA | Electron transport |
| Reproduction 8 | AA465570 | 5.736559 | NA | Fertilisation |
| Sialyltransferase 1 (β galactoside α-2,6-sialyltransferase) | AA598652 | 4.0207485 | NA | Immune response |
| Epstein-Barr virus induced gene 3 | AA425028 | 3.4711393 | NA | Immune response |
| Tumour necrosis factor receptor superfamily, member 5 | H98636 | 3.3610776 | NA | Immune response; apoptosis |
| Fucosyltransferase 4 (α(1,3) fucosyltransferase, myeloid specific) | R28447 | 3.8948373 | NA | Metabolism; carbohydrate metabolism |
| Histidine ammonia-lyase | W86776 | 4.2938508 | NA | Metabolism; histidine catabolism |
| Epoxide hydrolase 2, cytoplasmic | R73525 | 5.1584747 | NA | Metabolism; xenobiotic metabolism |
| Protein phosphatase 1, catalytic subunit, α isoform | AA443982 | 3.7866788 | NA | Metabolism; glycogen metabolism |
| CAZ associated structural protein | R52873 | 3.3870227 | NA | Protein degradation |
| Ubiquitin protein ligase E3C | AA284599 | 4.808889 | NA | Protein degradation |
| Ubiquitin conjugating enzyme E2D 3 (UBC4/5 homologue, yeast) | AA017199 | 4.1638422 | NA | Protein degradation |
| Proteasome regulatory particle subunit p44S10 | AA424807 | 3.8497784 | NA | Protein degradation |
| HECT type E3 ubiquitin ligase | R87212 | 3.2685684 | NA | Protein degradation |
| ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | AA427472 | 16.602038 | 2.8 | Protein degradation; acidification of organelles |
| Protein-O-mannosyltransferase 1 | R13777 | 3.9364101 | NA | Protein modification |
| Aspartylglucosaminidase | R42153 | 3.4922915 | NA | Protein modification |
| Adaptor related protein complex 3, β 2 subunit | H11692 | 4.1620878 | NA | Protein transport |
| SEC13-like 1 (S. cerevisiae) | AA496784 | 4.1282042 | NA | Protein transport |
| α-2-macroglobulin | H06516 | 3.6385137 | NA | Protein transport |
| RAE1 RNA export 1 homolog (S pombe) | AA504128 | 3.4039961 | NA | RNA transport |
| Protein tyrosine phosphatase, non-receptor type 1 | R06605 | 3.5456862 | NA | Signal transduction |
| Zinc finger protein 593 | AA033532 | 3.3121002 | NA | Transcription regulation |
| Signal transducer and activator of transcription 3 interacting protein 1 | N77731 | 3.2713342 | NA | Transcription regulation |
| Zinc finger protein 161 | AA232647 | 6.8052672 | 1.5 | Transcription regulation; cellular defense |
| Topoisomerase (DNA) II alpha 170 kDa | AA504348 | 45.484875 | 17.4 | Transcription regulation; DNA topology |
| Synaptoporin | H98620 | 4.1875559 | NA | Transport |
| ATP binding cassette, subfamily B (MDR/TAP), member 10 | R83875 | 3.3779001 | NA | Transport |
| EST | H66616 | 5.2260079 | NA | Unknown |
| HIV-1 rev binding protein 2 | W52273 | 4.6580643 | 2.6 | Unknown |
| EST | R73909 | 4.2685967 | NA | Unknown |
| EST | AA411554 | 4.1332 | 1.5 | Unknown |
| EST | R02609 | 3.9193518 | 1.5 | Unknown |
| Cisplatin resistance associated | W77812 | 3.8728312 | NA | Unknown |
| Reticulocalbin 1, EF-hand calcium binding domain | AA457719 | 3.3784786 | NA | Unknown |
| Family with sequence similarity 13, member A1 | N51424 | 3.3669014 | NA | Unknown |
| Transcribed sequence with weak similarity to protein ref:NP_060219.1 (H sapiens) hypothetical protein FLJ20294 (H sapiens) | R51835 | 3.3217265 | 10.3 | Unknown |
| Similar to non-histone chromosomal protein HMG-14 (high mobility group nucleosome binding domain 1) (LOC400452), mRNA | R53889 | 3.315474 | NA | Unknown |
| EST | W19429 | 3.2800179 | NA | Unknown |
| 5 genes underexpressed by confluent serum withdrawn RPE | ||||
| Phosphofructokinase, platelet | R38433 | −5.491407 | −2.0 | Metabolism; glycolysis |
| Mannosyl (α-1,6-)-glycoprotein β-1,2-N-acetylglucosaminyltransferase | AA485653 | −3.719321 | −2.0 | Metabolism; oligosaccaride synthesis |
| START domain containing 4, sterol regulated | H11369 | −3.790357 | −2.6 | Metabolism; cholesterol transporter |
| Snail homologue 2 (Drosophila) | H57309 | −3.733474 | −6.3 | Transcription regulation |
| CDNA FLJ42496 fis, clone BRACE2035003 | AA410207 | −4.944185 | −12.2 | Unknown |
*Confluent, serum withdrawn/native RPE; †NA, undetectable in native RPE.
The biological function of the 591 differentially expressed genes between native and SS cells included cell cycle/apoptosis (9.6%), protein processing (8.8%), metabolism (8.3%), signal transduction (7.4%), transcriptional regulation (5.9%), cytoskeleton (4.7%), cell adhesion (3.4%), and unknown (35.4%). Analysis using highly stringent conditions (FDR 1.4%) identified 41 genes that were underexpressed and 5 genes that were overexpressed by native RPE compared to SS cells (table 5). Again, protein processing genes comprised 17% of differentially expressed genes.
Table 5.
Differential gene expression between subconfluent cultured and native RPE cells sorted by SAM (FDR 1.4%). Genes are sorted by biological function
| Gene name | GenBank Acc No | Score (d) | Fold change* | Biological function |
| 41 Genes overexpressed by subconfluent RPE | ||||
| Collagen, type XVI, α1 | R54778 | 6.2086958 | NA† | Cell adhesion |
| Integrin, α2 (CD49B, α2 subunit of VLA-2 receptor | AA463610 | 5.9930352 | NA | Cell adhesion |
| Thrombospondin 2 | H38240 | 5.5952514 | NA | Cell adhesion |
| Insulin-like growth factor binding protein 3 | AA598601 | 6.4324886 | NA | Cell cycle |
| Cyclin D1 (PRAD1: parathyroid adenomatosis 1) | AA487700 | 5.9964532 | NA | Cell cycle |
| v-yes-1 Yamaguchi sarcoma viral related oncogene homologue | R83836 | 9.0139898 | NA | Cell cycle |
| Gap junction protein, β2, 26 kDa (connexin 26) | AA490688 | 5.8023088 | NA | Cell-cell signaling |
| Actin, β | R44290 | 9.7128396 | NA | Cytoskeleton |
| Caldesmon 1 | AA076063 | 7.83121 | 1.4 | Cytoskeleton; Muscle contraction |
| Reproduction 8 | AA465570 | 6.971362 | NA | Fertilisation |
| Tumour necrosis factor receptor superfamily, member 25 | W76376 | 6.1518795 | NA | Immune response, apoptosis |
| Transcription factor 12 (HTF4, helix-loop-helix transcription factors 4) | AA488497 | 7.1312589 | NA | Immune response; development |
| Glutamate receptor, ionotropic, N-methyl d-aspartate 2A | H08933 | 5.6530117 | NA | Ion transport |
| Fucosyltransferase 4 (α(1,3) fucosyltransferase, myeloid specific) | R28447 | 5.7747679 | NA | Metabolism, carbohydrate |
| Microsomal glutathione S-transferase 1 | AA495936 | 6.6792779 | NA | Oxidative stress response |
| HECT type E3 ubiquitin ligase | R87212 | 7.9988818 | NA | Protein degradation |
| CAZ associated structural protein | R52873 | 5.6053736 | NA | Protein degradation |
| 26 serine protease | H04028 | 7.4968725 | NA | Protein degradation; protease |
| Elastase 3B, pancreatic | W40123 | 6.9857814 | NA | Protein degradation; protease |
| Aspartylglucosaminidase | R42153 | 8.4918723 | NA | Protein modification |
| SEC13-like 1 (S cerevisiae) | AA496784 | 10.424023 | NA | Protein transport |
| Adaptor related protein complex 3, β2 subunit | H11692 | 5.6233762 | NA | Protein transport |
| Peroxisome biogenesis factor 1 | AA427472 | 16.184002 | 2.4 | Proton transport |
| Splicing factor proline/glutamine rich (polypyrimidine tract binding protein associated) | AA425853 | 7.7310373 | NA | RNA processing |
| Tyrosine kinase 2 | AA482128 | 7.5206556 | NA | Signal transduction |
| Inositol 1,4,5-triphosphate receptor, type 2 | AA479093 | 7.3995941 | NA | Signal transduction |
| Protein phosphatase 1, catalytic subunit, αisoform | AA443982 | 6.5780078 | NA | Signal transduction |
| Zinc finger protein 161 | AA232647 | 9.8923477 | 1.6 | Transcription regulation; defense response |
| Topoisomerase (DNA) II β170 kDa | AA504348 | 39.16232 | 16.9 | Transcription regulation; DNA topology |
| Synaptoporin | H98620 | 10.04159 | NA | Transport |
| Guanine nucleotide binding protein (G protein), α inhibiting activity polypeptide 3 | AA490256 | 5.6303528 | 1.5 | Transport |
| ATP binding cassette, subfamily B (MDR/TAP), member 10 | R83875 | 5.5977134 | NA | Transport |
| Human neuropeptide Y receptor Y1 (NPYY1) mRNA, exon 2-3 and complete cds. | R19478 | 13.345719 | NA | Unknown |
| Syndecan 2 (heparan sulfate proteoglycan 1, cell surface associated, fibroglycan) | H64346 | 9.7652404 | NA | Unknown |
| Integral membrane protein 2C | AA034213 | 7.76812 | NA | Unknown |
| Cisplatin resistance associated | W77812 | 7.6011771 | NA | Unknown |
| IMAGE:363103 | AA019511 | 7.0814745 | NA | Unknown |
| IMAGE:327676 | W23757 | 6.326244 | NA | Unknown |
| IMAGE:28309 | R40460 | 6.3181421 | 1.5 | Unknown |
| IMAGE:197888 | R96220 | 6.1341687 | NA | Unknown |
| S-phase 2 protein | T69532 | 5.8673496 | NA | Unknown |
| 5 Genes Underexpressed by Subconfluent RPE | ||||
| ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | AA427472 | −6.104330 | −3.6 | Protein degradation; Acidification of organelles |
| Phosphofructokinase, platelet | R38433 | −6.797924 | −2.4 | Metabolism, glycolysis |
| HIV-1 rev binding protein 2 | W52273 | −7.691879 | −12.2 | Unknown |
| CDNA FLJ42496 fis, clone BRACE2035003 | AA410207 | −7.320713 | NA | Unknown |
| Solute carrier family 43, member 3 | N76193 | −6.725273 | −2.2 | Unknown |
*Subconfluent/native RPE; †NA, undetectable in native RPE.
Serum influences the expression of cultured RPE cells
Since serum divided the culture conditions, and native RPE clustered with serum withdrawn conditions, two class unpaired SAM analysis (FDR 9.9%) between serum and serum withdrawn conditions identified 317 differentially expressed genes. Cells that were grown with serum removed had 207 genes overexpressed and 110 genes underexpressed compared to cells grown in serum. The biological function of differentially expressed genes included protein processing (11.7%), signal transduction (11.7%), cell cycle (5.4%), metabolism (5.4%), transcription factors (4.7%), cell adhesion (2.8%), cell structure (1.8%), and unknown (38.8%). The actual differential expression was small, ranging from 15–30%. To evaluate the influence of genes with larger differential expression, SAM analysis with more stringent FDR (4.6%) and a twofold differential expression threshold was performed (table 6). Three apoptosis, and three differentiation genes were identified.
Table 6.
Differential gene expression between cultured RPE cells grown in serum and serum withdrawn conditions sorted by SAM with an FDR of 4.6% and at least a twofold differential expression threshold
| Gene name | GenBank Acc No | Score (d) | Fold change* | Biological function |
| 13 Genes overexpressed by cells grown in serum withdrawn medium | ||||
| Myeloid cell leukaemia sequence 1 (BCL2 related) | AA488674 | 2.87841767 | 11.5 | Apoptosis |
| Phytoceramidase, alkaline | W58013 | 2.60586649 | 2.8 | Apoptosis/ceramide metabolism |
| Reticulocalbin 1, EF-hand calcium binding domain | AA457719 | 2.67862204 | 3.2 | Calcium binding/signal transduction |
| Visinin-like 1 | H65066 | 2.60870158 | 8.6 | Calcium binding/signal transduction |
| Phytanoyl-CoA hydroxylase interacting protein | AA405628 | 3.75618502 | 8.3 | Development/differentiation |
| Leucine rich repeat containing 28 | R95132 | 2.99928312 | 6.9 | Development/differentiation |
| Ephrin-B1 | AA428778 | 2.7292518 | 3.2 | Development/differentiation |
| ATP binding cassette, subfamily C (CFTR/MRP), member 13 | H47929 | 3.63023830 | 6.7 | Ion transport |
| Ubiquitin conjugating enzyme E2D 3 (UBC4/5 homologue, yeast) | AA017199 | 2.72914196 | 3.8 | Protein degradation |
| RNA binding motif protein 4 | AA456271 | 2.79296662 | 2.9 | RNA processing |
| Chromosome 22 open reading frame 8 | H94903 | 3.71707843 | 6.3 | Unknown |
| IMAGE:3895112 | T95342 | 2.66350001 | 4.7 | Unknown |
| Mesoderm development candidate 2 | AA284495 | 2.79383791 | 2.3 | Unknown |
| 2 Genes underexpressed by cells grown in serum withdrawn medium | ||||
| v-myb myeloblastosis viral oncogene homolog (avian)-like 2 | AA456878 | −3.6013598 | −4.5 | Apoptosis/transcription factor |
| IMAGE:295501 | W23543 | −3.4456702 | −2.4 | Unknown |
*Serum withdrawn/serum conditions.
Real time RT-qPCR validation
Real time RT-qPCR was performed on cells grown under identical culture conditions, but separate experiments from the microarray analysis, and native RPE from five different globes to expand the differential expression pattern beyond the eyes used for microarray experiments. Since the purpose of this investigation was to evaluate global gene expression changes, genes were randomly selected. Table 7 shows that all five genes had similar expression patterns as observed on the arrays.
Table 7.
Differential gene expression between cultured and native RPE by real time RT-PCR
| Gene name | GenBank Acc No | Fold change, array* | Fold change, RT-PCR* | p Value |
| Subconfluent v native RPE | ||||
| SEC13-like 1 (S cerevisiae) | AA496784 | 0† | −12.5 | 0.040 |
| ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | AA427472 | −3.6 | −1135 | 0.039 |
| Topoisomerase (DNA) II α170 kDa | AA504348 | 16.9 | ∞‡ | – |
| Confluent, serum withdrawn v native RPE | ||||
| START domain containing 4, sterol regulated | H11369 | −2.5 | −5.7 | 0.0012 |
| Oxidase (cytochrome c) assembly 1-like | AA598582 | 10.5 | 2.71 | 0.0032 |
| Topoisomerase (DNA) II α170 kDa | AA504348 | 17.4 | ∞‡ | – |
*Cultured RPE/native RPE; †0, undetectable in subconfluent ARPE-19 cells; ‡∞, undetectable in native RPE,
DISCUSSION
We showed previously that confluent ARPE-19 cells grown on different matrices had different transcriptional profiles from native RPE, and that cells grown on plastic had the closest transcriptome to native RPE.2 To improve the transcriptional proximity of ARPE-19 cells grown on plastic to native RPE, we varied the culture conditions and evaluated global expression trends, a more informative benchmark than individual gene expression. With this end point, CSW and DSW cells were most similar to native RPE. While each culture condition preserved the expression of the most abundant genes, significant transcriptional differences exist between native and cultured cells including the number of genes on the array that were expressed and not expressed, the number and function of differentially expressed genes, and the number of expressed low abundance genes. Our results suggest that the global expression profiles can be improved by varying the culture conditions, but significant widespread differences remain between cultured and native RPE.
With ageing, the RPE undergoes apoptosis and morphological deterioration. Supervised cluster analysis of 713 genes related to differentiation or cell cycle/apoptosis was similar to unsupervised analysis, further suggesting that CSW or DSW cells are most similar to native RPE. Further analysis of the transcriptome of CSW cells however, showed significant differences from native RPE including the number of genes expressed and not expressed, and differentially expressed genes with a diversity of function. These data collectively suggest that while clustering closest to native RPE, the global transcriptional profile of CSW cells displayed multiple mRNA phenotypic differences which may influence ageing studies.
Culture conditions were segregated by serum. The function of differentially expressed genes, regardless of culture density, was quite wide ranging, which suggests that serum influences multiple cellular functions including protein processing, signal transduction, cell cycle, metabolism, and transcription. Native RPE clustered with cells grown in serum withdrawn medium. Since the outer blood-retinal barrier is composed of the RPE and Bruch’s membrane, the RPE is probably partially shielded from serum by Bruch’s membrane. Our results suggest that serum was a negative factor that separated RPE cells from native cells, and that RPE cells in vivo are not significantly exposed to serum.
Our interpretations are based on a small collection of eyes. The statistical precision of the arrays, however, enabled us to make statistically valid comparisons. While a wide age range was used to reduce age bias, our results may not be generalisable to all native RPE. We were comforted by the validation of our array results by RT-qPCR using a different set of eyes which likely reduces expression differences related to donor to donor variation. We evaluated a single RPE cell line so extrapolation to other cultured RPE cells is unknown. The use of laser microdissection and unamplified RNA for microarray analysis has limitations. While amplification bias is eliminated, RNA quantity prevents a comprehensive validation even with RT-qPCR. The array utilised was not RPE specific, and contains both a relatively limited gene set and uncharacterised genes. With further study, the uncharacterised genes may provide new insights into general or RPE specific functioning. Many investigators use a wide range of culture densities, medium, and duration to study RPE function. Our results provide evidence in support of the long held opinion that culture conditions alter the cellular phenotype and validate the long held assumption that great care must be utilised when extrapolating results of gene expression experiments from cultured to native RPE cells.
Acknowledgments
We thank NDRI and the Sierra Eye and Tissue Bank for donor eyes. Supported by NIH/EY 14005 (JTH), NIH/EY 06473 (LMH), and an unrestricted Research to Prevent Blindness grant to the Wilmer Eye Institute, the Michael Panitch Macular Degeneration Research Fund, and gifts from Aleda Wright, and Rick and Sandy Forsythe. JTH is a recipient of a Clinician Scientist Award from the RPB.
Abbreviations
BSA, bovine serum albumin
CS, confluent serum
CSW, confluent, serum withdrawn
DMEM, Dulbecco’s Modified Eagle medium
DS, differentiated serum
DSW, differentiated serum withdrawn
FBS, fetal bovine serum
FDR, false discovery rate
RPE, retinal pigment epithelium
Commercial relationships: none
REFERENCES
- 1.Dunn KC, Aotaki-Keen AE, Putkey FR, et al. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996;62:155–69. [DOI] [PubMed] [Google Scholar]
- 2.Tian J, Ishibashi K, Handa JT. The expression of native and cultured RPE grown on different matrices. Physiol Genomics 2004;17:170–82. [DOI] [PubMed] [Google Scholar]
- 3.Johnston NL, Cervenak J, Shore AD, et al. Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. Stanley Neuropathology Consortium. J Neurosci Methods 1997;77:83–92. [DOI] [PubMed] [Google Scholar]
- 4.Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem 1990;38:1383–8. [DOI] [PubMed] [Google Scholar]
- 5.Boylan S, Honda S, Hjelmeland LM, et al. An optimized protocol for first strand cDNA synthesis from laser capture microdissected tissue. Lab Invest 2001;81:1167–9. [DOI] [PubMed] [Google Scholar]
- 6.Sgroi DC, Teng S, Robinson G, et al. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res 1999;59:5656–61. [PubMed] [Google Scholar]
- 7.Lee ML, Whitmore GA. Power and sample size for DNA microarray studies. Stat Med 2002;21:3543–70. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–11. [DOI] [PubMed] [Google Scholar]
- 9.Weigel AL, Handa JT, Hjelmeland LM. Microarray analysis of H2O2-, HNE-, or tBH-treated ARPE-19 cells. Free Radic Biol Med 2002;33:1419–32. [DOI] [PubMed] [Google Scholar]
- 10.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998;95:14863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001;98:5116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson DA, Feeney S, Boyle C, et al. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol Vis 2000;6:178–83. [PubMed] [Google Scholar]
- 13.Hwang D, Schmitt WA, Stephanopoulos G. Determination of minimum sample size and discriminatory expression patterns in microarray data. Bioinformatics 2002;18:1184–93. [DOI] [PubMed] [Google Scholar]


