Abstract
Background: Endoscopic recurrence after surgery in Crohn's disease is frequent and unpredictable. Abnormal intestinal production of pro- (interleukin (IL)-1β, tumour necrosis factor α (TNF-α)) and anti- (IL-10) inflammatory cytokines has been associated with severe outcome in experimental models of colitis.
Patients and methods: We evaluated if ileal TNF-α, IL-1β, or IL-10 mRNA levels measured at the time of surgery predict endoscopic recurrence, and if ileal IL-10 levels are associated with particular IL-10 promoter alleles. Ileal biopsies were obtained peroperatively from the healthy neoileum of patients undergoing a right ileocolectomy for Crohn's disease. Mucosal TNF-α, IL-1β, and IL-10 mRNA levels were quantified by competitive polymerase chain reaction. A cut off value was determined using a receiver operating curve. IL-10.G promoter haplotypes were analysed using a polymorphic dinucleotide repeat in the IL-10 promoter region.
Results: Three months after surgery, 53% of patients had endoscopic recurrence while 47% remained free of disease. The risk of endoscopic recurrence correlated with ileal IL-10 mRNA concentrations (r2=0.81). Endoscopic recurrence occurred more frequently in patients classified as low IL-10 producers than in those that were high producers (80% v 40%) (p=0.02). Patients with at least one of the two alleles G7–8 or G10–13 produced, respectively, higher (p=0.006) and lower (p=0.029) ileal IL-10 mRNA. The distribution of IL-10.G microsatellite genotypes was similar in patients with or without endoscopic recurrence.
Conclusion: Low ileal IL-10 mRNA concentration is a good marker of endoscopic recurrence in Crohn's disease but the distribution of IL-10.G haplotypes cannot predict the postoperative evolution of the disease.
Keywords: interleukin 10, Crohn's disease, endoscopic recurrence
Crohn's disease (CD) is a chronic inflammatory disorder of unknown origin. It is characterised by multiple recurrences that may be endoscopic or clinical. Approximately 70% of CD patients will eventually require surgery, and 33–82% may subsequently suffer recurrence, requiring further surgical procedures.1 Following ileocolectomy, the disease usually recurs on the ileal side of the ileocolonic anastomosis (neoileum) and extends proximally. In a series of 89 patients studied one year after ileal resection and ileocolic anastomosis, Rutgeerts et al found that the rate of endoscopic ileal recurrences (ER) was 73%.2 To date, no single test, predictive model, or disease characteristics can predict relapse.
Disturbances in mucosal immunoregulation have previously been demonstrated in CD and may represent an important component of the induction and perpetuation of intestinal inflammation and tissue injury.3 In a recent study, Schreiber et al showed that increased production of tumour necrosis factor α (TNF-α) and interleukin (IL)-1β by intestinal lamina propria cells may predict relapse in patients who are in steroid induced remission.4 Thus far, it has not been demonstrated whether these cytokines are indicators of early postoperative recurrence. In contrast with these proinflammatory cytokines, many observations suggest that IL-10 is a major anti-inflammatory mediator with antifibrotic activity that may modulate disease expression. In vivo, disruption of the IL-10 gene in mice leads to development of colitis5; conversely, IL-10 gene transfer prevents the development of trinitrobenzene sulphonic acid (TNBS) induced colitis6 and injection of IL-10 restores tolerance towards bacterial products abrogated in TNBS induced colitis.7
The purpose of this study was to determine if TNF-α, IL-1β, or IL-10 mRNA concentrations in the ileum at the time of surgery predict ER in CD. As IL-10 production in humans is under genetic control, we also investigated whether intestinal production of IL-10 was associated with particular IL-10 promoter alleles in CD patients.
PATIENTS AND METHODS
Patients
The studies were approved by a local ethics committee and all subjects gave informed consent. The diagnosis of CD was established using standard criteria.8
In the first study, ileal TNF-α, IL-1β, and IL-10 mRNA production was investigated in 36 patients with CD (16 males, 20 females; mean age 29 years) who had a first ileocolectomy and ileocolonic anastomosis. All patients underwent surgery because of disease complications—that is, symptomatic stenosis, fistula, obstruction, or disease activity. No patient received immunosuppressive treatment. If patients were being treated with steroids, treatment was tapered and discontinued four weeks before surgery. During the surgical procedure, ileoscopy and biopsy were systematically performed to assess macroscopic and histological integrity, and to quantify cytokine mRNA production in the ileal mucosa 30 cm above the anastomosis. Eight biopsies were taken from macroscopically non-inflamed ileal mucosa. Some specimens were fixed in formalin and examined and the rest were immediately frozen in liquid nitrogen. No medical treatment (that is, steroids, salicylates, antibiotics, immunosuppressive drugs) except for antidiarrhoeal and antispasmodic drugs was allowed for a period of three months after surgery. Recurrence was evaluated by endoscopy at three months and scored i1–i4 according to the criteria of Rutgeerts et al (table 1 ▶).9
Table 1.
Description of the criteria of Rutgeerts et al for endoscopic recurrence9
| Score | Lesions |
| i0 | No lesions |
| i1 | <5 aphthous lesions |
| i2 | >5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions, or lesions confined to the ileocolonic anastomosis (that is, <1 cm in length) |
| i3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| i4 | Diffuse inflammation with already larger ulcers, nodules, and/or narrowing |
In the second study, ileal IL-10 mRNA production and IL-10 promoter haplotypes were determined in 43 additional patients with CD (18 males, 25 females; mean age 31 years). Biopsies from non-inflamed ileal mucosa were obtained at endoscopy. DNA was extracted from blood samples of all patients in the two studies (n=79, 34 males, 45 females; mean age 30 years) to study microsatellite G allele frequencies of the IL-10 promoter.
Ileal cytokine mRNA quantification by reverse transcription-competitive polymerase chain reaction
Biopsies were homogenised by mechanical dispersion in Trizol (Bioprobe, Montreuil, France) and total RNA was extracted as previously described.10 After treatment at 37°C for 30 minutes with 20–50 units of DNase I RNase-free (Boehringer, Mannheim, Germany), total RNA was reverse transcribed into complementary DNA (cDNA) using Moloney murine leukaemia virus reverse transcriptase (Gibco-BRL, Cergy Pontoise, France), 5 μM oligo-dT16, and 2.5 mM of each of the four dNTP (Pharmacia, Orsay, France) in a final reaction volume of 20 μl in the presence of 1 U/μl of human placenta ribonuclease inhibitor (Promega, Lyon, France). Samples were incubated at 42°C for 60 minutes followed by heating for five minutes at 95°C and stored at −20°C until use. Competitive polymerase chain reaction (PCR) analysis was performed as described by Zou and colleagues11 with some modifications. cDNA (1 μl) was subjected to PCR using primers specific for β-actin (5`-GGGTCAGAAGGATTCCTATG-3`; 5`-GGTCTCAAACATGA TCTGGG-3`), IL-10 (5`-AAATTTGGTTCTAGGCCGGG-3`; 5`-GAGTACAGGGGCATGATATC-3`), TNF-α (5`-ACAAGCCTGT AGCCCATGTT-3`; 5`-AAAGTAGACCTGCCCAGACT-3`), and IL-1β (5`-GGATATGGAGCAACAAGTGG-3`; 5`-ATGTACCA GTTGGGGAACTG-3`). The 20 μl reaction mixture consisted of sense and antisense primers (0.1 μg/μl each), 1 U of Taq polymerase (Perkin Elmer, Courtaboeuf, France), 50 μM of each of the four dNTP, 1×PCR buffer (Perkin Elmer) supplemented with 2.5 mM MgCl2, and grade concentrations of one of the two competitors pQA1 and pQB2.3 Amplification was performed by 40 cycles, consisting of denaturation at 94°C for one minute, primer annealing for one minute at 55°C for IL-10 and β-actin or 58°C for IL-1β and TNF-α, and primer extension at 72°C for 1.5 minutes using a GeneAmp PCR system 9700 (Perkin Elmer). After amplification, separation of the DNA competitor and target cDNA was achieved by electrophoresis in 3% agarose containing ethidium bromide for one hour at 100 V. The amount of PCR products generated by competitor and target cDNA was compared using an image analyser (Bioprofil, Marne-la-Vallée, France). Results of cytokine measurements were expressed in proportion to the number of β-actin cDNA in the same sample.
IL-10.G microsatellite genotyping
DNA extraction was performed using a Wizard kit (Promega). Genotyping at the IL-10.G microsatellite was carried out using the following primer sequences labelled with 6-carboxyfluorescein (6-FAM 5`-GTCCTTCCCCAGGTAGAGC AACACTCC-3`; 5`-CTCCCAAAGAAGCCTTAGTAGTGTTG-3`) with the previously described final reaction mix. After an initial melting time of seven minutes at 95°C, samples were subjected to 30 rounds of 94°C for one minute, 65°C for one minute, 72°C for one minute, with a final extension time of five minutes at 72°C in a GeneAmp PCR sytem 9700. A sample (1 μl) of the PCR products was mixed with formamide, loading buffer E, and 0.5 μl of ROX-500 standard size (PE Applied Biosystems), heated to 95°C for five minutes and then cooled immediately on ice. Fragment sizes were analysed on a sequencing gel containing 6% acrylamide and 6 M urea with a 377XL DNA sequencer (Perkin Elmer).
Statistical methods
Correlation between ileal IL-10 mRNA concentrations and the risk of ER was tested using the Pearson's test. To classify patients as low or high IL-10 producers, an IL-10 mRNA cut off value was determined using a receiver operating characteristic (ROC) curve. The risk of recurrence for low and high producers was compared using the χ2 test.
To evaluate IL-10 mRNA production for each allele, we compared IL-10 mRNA concentrations in patients having the allele with all others, using a Kruskall-Wallis one way ANOVA test.
RESULTS
Prognostic value of ileal TNF-α, IL-1β, and IL-10 mRNA levels in CD recurrence
Nineteen of the 36 patients had ER greater than i1 (53%) and 17 (47%) had no signs of ER. Age, sex, smoking habit, disease duration, disease location, and indications for surgery did not differ between these two groups (table 2 ▶). Despite high tissue concentrations of β-actin mRNA (14 591 (69 587) molecules) in all biopsy specimens, TNF-α and IL-1β mRNA were only detectable in 2/36 and 5/36 samples, respectively. No relation was found between ileal TNF-α and IL-1β mRNA expression at surgery and the risk of recurrence three months later.
Table 2.
Demography and disease history in the 36 patients with (ER+) or without (ER−) postoperative endoscopic recurrence (ER) of Crohn's disease (CD)
| ER− | ER+ | |
| Age (yr) | 30 | 28 |
| Sex ratio (F/M) | 10/7 | 10/9 |
| Smoking history (%) | 50 | 67 |
| CD duration before resection (y) | 5.6 | 4 |
| Disease location | ||
| Ileum | 64% | 44% |
| Colon | 0% | 0% |
| Ileum and colon | 36% | 56% |
| Reason for resection | ||
| Fibrostenosis | 64% | 50% |
| Obstruction | 14% | 0% |
| Fistula/abscess | 14% | 30% |
| Disease activity | 7% | 20% |
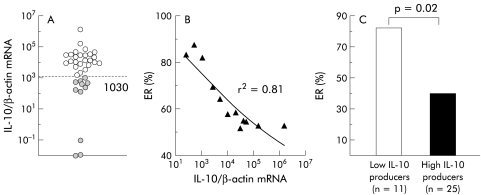
In contrast, IL-10 mRNA expression was found in all biopsies from the 36 patients (mean 5.4×104 molecules/molecule of β-actin), with a large variation in concentrations, ranging from 10−2 to 1.3×106 molecules/molecule of β-actin (fig 1A ▶). IL-10 mRNA concentrations correlated with the risk of ER (r2=0.81) (fig 1B ▶). Using an IL-10 mRNA cut off value of 1030 (as determined using an ROC curve), 11 (31%) patients were classified as low IL-10 producers and 25 (69%) as high producers (fig 1A ▶). ER occurred in 82% (9/11) of the low producers compared with 40% (10/25) in the high producers (p=0.02) (fig 1C ▶). Low ileal IL-10 mRNA concentrations had 88% specificity and 82% positive predictive value for ER. TNF-α and IL-1β mRNA were detected with a similar frequency in low (respectively one and two patients) and high (respectively one and three patients) IL-10 producers.
Figure 1.
(A) Ileal interleukin (IL)-10 mRNA molecules per β-actin molecule in the macroscopically non-inflamed ileal mucosa from 36 patients operated on for Crohn's disease. (B) Correlation between ileal IL-10 mRNA and the risk of endoscopic recurrence (ER). (C) Higher frequency of ER in the group of low IL-10 producers than in the group of high IL-10 producers, as defined by a cut off value of 1030 IL-10 mRNA molecules/molecule of β-actin (broken line in (A)).
Correlation of IL-10.G microsatellite genotypes with spontaneous IL-10 mRNA production and postoperative endoscopic recurrence
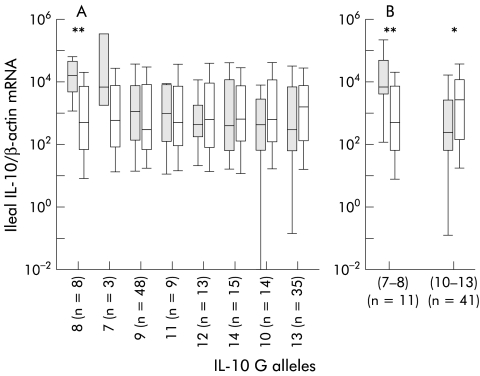
To investigate the possible association between IL-10.G alleles and the ileal concentration of IL-10 mRNA, we quantified IL-10 mRNA in the uninflamed ileum of 43 additional patients with CD. Considering these patients together with the 36 patients included in the first study, levels of ileal IL-10 mRNA in the 79 patients varied with their IL-10.G alleles. As shown in figure 2 ▶, 14% of patients with the G7 or G8 alleles had the highest ileal IL-10 mRNA production whereas the 57% of patients with the G10 or G13 alleles had the lowest IL-10 mRNA production. Differences in IL-10 mRNA synthesis in patients having one allele compared with those with all other alleles was significant only for the G8 allele (16 888 v 504; p=0.0098) (fig 2A ▶). However, compared with an average of all other alleles combined, concentrations of ileal IL-10 mRNA were significantly different in patients with at least one of the two G7–8 (median 6893 v 504; p=0.006) or G10–13 (median 249 v 2711; p=0.029) alleles (fig 2B ▶). The frequencies of the G7–8 and G10–13 alleles were similar in the 36 patients with or without recurrence (table 3 ▶).
Figure 2.
(A) Median values (horizontal bars in box) of ileal interleukin (IL)-10 mRNA in the 79 patients according to their different IL-10.G alleles, plotted in decreasing values of ileal IL-10 mRNA. Differences in IL-10 synthesis in patients with a least one copy of the allele (shaded box) compared with all other patients (open box) was significant only for the G8 allele (**p<0.001). (B) Compared with an average of all other alleles combined (open box), concentrations of ileal IL-10 mRNA were significantly different in patients with at least one of G7–8 (**p<0.001) or G10–13 alleles (shaded box) (*p<0.05).
Table 3.
Interleukin (IL)-10.G microsatellite allele frequencies according to endoscopic recurrence (ER) after surgery in the 36 patients with Crohn's disease
| IL-10.G alleles | ER− (% (n)) | ER+ (% (n)) | Total allele frequencies (n=72) |
| IL-10.G7–8 | 2.9% (1) | 7.9% (3) | 5.5% |
| IL-10.G10–13 | 26.5% (9) | 10.5% (4) | 18% |
ER−, no endoscopic recurrence; ER+, endoscopic recurrence defined as a Rutgeerts score ≥1.
DISCUSSION
Many attempts have been made to identify predictors of recurrence after resection for CD. Patient and disease related factors such as smoking habit,12 site of intestinal involvement, and form of disease13 have been indicated. In our study, age, sex, smoking habit, disease duration and location, and indications for surgery were not predictive of recurrence. However, ileal IL-10 mRNA concentrations quantified by competitive PCR reflected an increased risk of relapse. These results suggest for the first time that determination of ileal IL-10 mRNA concentrations at the time of surgery may define patients at higher risk of relapse requiring more intensive aftercare.
In contrast with the study of Schreiber et al showing that increased secretion of TNF-α and IL-1β may predict clinical relapse in patients in steroid induced remission,4 TNF-α and IL-1β mRNA levels were not predictive of postoperative recurrence in our study. This discrepancy may be explained at least in part by the definition of recurrence (endoscopic v clinical) and the different disease phases of the patients studied. We have previously shown that different cellular and molecular mediators are involved in the early lesions occurring in the neoileum3 and in the established lesions seen in patients after steroid induced remission.4 The fact that different mediators are predictive of early intestinal recurrence after surgery or of clinical relapses in patients with established lesions suggests that different mechanisms are involved in these two different phases of the disease. Another explanation may be that methods used to quantify cytokines were different. Schreiber et al investigated the capacity of isolated lamina propria mononuclear cells to produce TNF-α and IL-1β by ELISA after in vitro stimulation whereas we quantified mRNA of these cytokines in vivo, directly on unstimulated biopsies taken from the healthy ileal mucosa. Under such circumstances TNF-α and IL-1β may be undetectable. As IL-10 downregulates TNF-α and IL-1β secretion,14 it would be interesting to determine whether stimulated lamina propria mononuclear cells produce more proinflammatory cytokines in the low IL-10 producer group than in the high IL-10 producer group.
Many factors are involved in the control of IL-10 production. Analyses of differences in spontaneous IL-10 production in monozygotic and dizygotic twins and non-related individuals suggest that the capacity for IL-10 production has a major genetic component.15 Striking differences among individuals in their ability to produce IL-10 (by lipopolysaccharide activated whole blood culture) are the result of differing rates of IL-10 mRNA synthesis which has been ascribed to polymorphisms in the IL-10 gene promoter. Sixteen alleles in a highly polymorphic microsatellite marker, namely IL-10.G within the IL-10 promoter, have been described.16 Studies related to IL-10 promoter structures in humans have shown that after in vitro stimulation of peripheral blood leucocytes, haplotypes containing the G7 and G14 alleles were respectively associated with lower or higher IL-10 production.17 The question of whether IL-10 promoter structures are associated with spontaneous in vivo production of intestinal IL-10 mRNA remains to be addressed. In the present study, the G14 allele was not significantly associated with higher intestinal IL-10 production. However, we found that G7–8 alleles were associated with the greatest increase in ileal IL-10 mRNA production whereas the G10–13 alleles had the lowest levels. These results suggest that the capacity for intestinal IL-10 production is controlled at least in part by genetic components, which are partially different from those associated with IL-10 production by activated peripheral blood cells. Because IL-10 gene promoter polymorphisms are not correlated with recurrence, we believe that they are not practical genetic markers to predict postoperative ER. The discrepancy between the rate of IL-10 mRNA production and the IL-10 gene promoter polymorphisms in relation to ER may be explained at least in part by the involvement of other genetic influences or environmental factors such as alimentation,18,19 infection,20 or bacterial flora21 which may also influence intestinal IL-10 production.
Acknowledgments
This study was supported by a grant from the Conseil Regional du Nord Pas de Calais and CHU de Lille to B Meresse. The authors would like to thank the IFR 22, C Bisiaux (EPI 0114, CHU Lille, France), C Mouton, and M Crépin (plateau technique de séquencage, CHU Lille, France) for technical assistance.
Abbreviations
CD, Crohn's disease
ER, endoscopic recurrence
TNF-α, tumour necrosis factor α
IL, interleukin
TNBS, trinitrobenzene sulphonic acid
PCR, polymerase chain reaction
ROC curve, receiver operating characteristic curve
REFERENCES
- 1.Rutgeerts PJ. Postoperative recurrence prophylaxis in Crohn's disease: an update. Res Clin Forums 1998;20:49–55. [Google Scholar]
- 2.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 3.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 1997;113:118–26. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber S, Nikolaus S, Hampe J, et al. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet 1999;353:459–61. [DOI] [PubMed] [Google Scholar]
- 5.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74. [DOI] [PubMed] [Google Scholar]
- 6.Barbara G, Xing Z, Hogaboam CM, et al. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut 2000;46:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duchmann R, Schmitt E, Knolle P, et al. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol 1996;26:934–8. [DOI] [PubMed] [Google Scholar]
- 8.Gower-Rousseau C, Salomez JL, Dupas JL, et al. Incidence of inflammatory bowel disease in northern France (1988–1990). Gut 1994;35:1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 10.Estaquier J, Idziorek T, Zou W, et al. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med 1995;182:1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W, Durand-Gasselin I, Dulioust A, et al. Quantification of cytokine gene expression by competitive PCR using a colorimetric assay. Eur Cytokine Netw 1995;6:257–64. [PubMed] [Google Scholar]
- 12.Yamamoto T, Keighley MR. Smoking and disease recurrence after operation for Crohn's disease. Br J Surg 2000;87:398–404. [DOI] [PubMed] [Google Scholar]
- 13.Williams JG, Wong WD, Rothenberger DA, et al. Recurrence of Crohn's disease after resection. Br J Surg 1991;78:10–19. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, O'Garra A, de Waal Malefyt R, et al. Interleukin-10. Annu Rev Immunol 1993;11:165–90. [DOI] [PubMed] [Google Scholar]
- 15.Westendorp RG, Langermans JA, Huizinga TW, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 1997;349:170–3. [DOI] [PubMed] [Google Scholar]
- 16.Eskdale J, Kube D, Gallagher G. A second polymorphic dinucleotide repeat in the 5' flanking region of the human IL10 gene. Immunogenetics 1996;45:82–3. [DOI] [PubMed] [Google Scholar]
- 17.Eskdale J, Gallagher G, Verweij CL, et al. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA 1998;95:9465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudo N, Aiba Y, Takaki A, et al. Dietary nucleic acids promote a shift in Th1/Th2 balance toward Th1-dominant immunity. Clin Exp Allergy 2000;30:979–87. [DOI] [PubMed] [Google Scholar]
- 19.Kleemann R, Scott FW, Worz-Pagenstert U, et al. Impact of dietary fat on Th1/Th2 cytokine gene expression in the pancreas and gut of diabetes-prone BB rats. J Autoimmun 1998;11:97–103. [DOI] [PubMed] [Google Scholar]
- 20.Huang DS, Lopez MC, Wang JY, et al. Alterations of the mucosal immune system due to Cryptosporidium parvum infection in normal mice. Cell Immunol 1996;173:176–82. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Isomaki P, Rimpilainen M, et al. Human cytokine responses induced by gram-positive cell walls of normal intestinal microbiota. Clin Exp Immunol 1999;118:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]