Abstract
Background and aims: The prevalence of Helicobacter pylori colonisation in populations in developed country has been declining, as shown by community based serological surveys of adults in Vammala, Finland in 1973 and 1994. In this study, we determined whether the proportion of subjects colonised by cagA+ or cagA− H pylori strains has changed as the overall prevalence of H pylori+ has declined.
Methods: We examined 911 sera from Vammala's study for antibodies to the CagA antigen of H pylori using a truncated CagA protein as the antigen in an ELISA and we examined the trend in acquisition and carriage of cagA+ strains.
Results: As expected, the prevalence of carriage of both cagA+ and cagA− strains fell between 1973 and 1994 (p<0.001). However, the prevalence of cagA+ strains among those <45 years declined (34% to 8%) significantly (p<0.001) more than for cagA− strains (12% to 6%). Of 221 subjects with paired serum samples, 12 (5.4%) changed H pylori status; the estimated seroconversion and reversion rates were 0.4% and 0.13% per year, respectively. Except for the few individuals who changed serostatus, absolute antibody levels to H pylori antigens, including CagA, changed little over the 21 year period.
Conclusions: The decline in CagA seroprevalence predominantly reflects declining acquisition of cag+ strains in younger subjects. In addition, these data confirm that H pylori acquisition chiefly occurs during childhood but continues to occur during adulthood, albeit at low rates, in developed countries.
Keywords: Helicobacter pylori, cag A strains, seroconversion
There is increasing evidence that gastric colonisation of humans with Helicobacter pylori has been present at least for centuries, and is probably ancient (summarised by Blaser1). In developing countries, nearly all adults are H pylori+2, 3 but in developed countries the prevalence of H pylori is much lower.4 In all populations the prevalence of H pylori increases with age5–9 which is best explained by a birth cohort phenomenon with diminished acquisition during childhood as socioeconomic development has occurred.10, 11 In a Finnish population, the prevalence of H pylori colonisation substantially decreased between 1973 and 1994.12 Colonisation decreased markedly between 1973 and 1994 from 56% to 31% (p=0.001).
H pylori strains are either cagA+ or cagA− based on whether or not they possess a 128 kDa protein that is a marker for the presence of the 35–40 kb cag island.13, 14 This is a fundamental dichotomy among H pylori strains, associated with important differences in clinical outcome in Western populations.15–18 A serological response to the CagA protein is strongly predictive that a patient is carrying a cagA+ strain.16, 17, 19 In general, although individuals are usually colonised with either cagA+ or cagA− strains, mixed populations may be present, particularly in developing countries.20, 21 Elements in the cag island are responsible for the type IV secretion system mediated injection of the CagA protein into epithelial cells (see for example Odenbreit and colleagues22), explaining at least in part why CagA is an immunodominant antigen. Using the stored serum specimens from the Finnish population studied earlier,12 we determined whether the proportion of subjects colonised by cagA+ or cagA− H pylori strains has changed as the overall prevalence of H pylori+ has declined.
MATERIALS AND METHODS
Serum samples
Sera had been collected from 911 healthy subjects randomly selected from the population over 14 years of age in Vammala, Finland in 1973 (n=408) and 1994 (n=503). The two populations tested in 1973 and 1994 came from the same sample of 16 000 inhabitants of Vammala which had not had any marked shifts in socioeconomic status. The population in Vammala was approximately 16 000 in both years, and descriptions of the study design have been reported.12 We also examined paired serum samples from 221 healthy subjects obtained in both 1973 and 1994 that had been collected as part of this study.12
Serological assays
IgG and IgA antibodies to H pylori had been measured previously by an ELISA using an acid-glycine extract as the antigen, as previously reported.12 The sensitivity and specificity of these assays were 94% and 93% for IgG and 73% and 95% for IgA, respectively.12 Antibodies to the H pylori CagA antigen were measured using a recombinant CagA fragment by ELISA, and an optical density ratio (ODR) of ≥0.35 was considered positive, as described previously.16 The sensitivity and specificity of the assay were 94.4% and 92.5%, respectively, in a US population.16 All assays were performed at least in duplicate.
Statistical analysis
H pylori positive status was defined as a positive result in any of the three serological assays whereas H pylori negative status was defined when all three assays were negative. In subjects from whom paired specimens were available from 1973 and 1994, seroconversion or reversion was defined on the basis of IgG response to the glycine extracted H pylori antigens. Differences in frequencies of response to CagA antigen among groups were examined by χ2 analysis. The magnitude of the responses in different groups was analysed using the Student's t test for independent samples.
RESULTS
Validation of CagA threshold in the tested population
As gastric biopsies were not obtained from these healthy subjects, we sought an alternative way to determine whether the threshold for positivity in the CagA assay was valid in this population. To address this question, we used specimens from 65 subjects who were positive in both years for both the IgG and CagA assays as a gold standard to define subjects persistently colonised by cagA+ H pylori strains. For these 130 specimens, the mean (SD) ODR in the CagA assay was 0.78 (0.22). By subtracting two intervals of SD, we defined a level that was 97.5% likely to represent truly positive CagA status. This value (0.34) was nearly identical to the a priori value (0.35) determined based on a group of H pylori negative healthy children in the USA; this threshold represented the mean value plus 3 SD for the negative children.16 Thus the results of the two methods were nearly identical, validating the threshold for CagA positivity used for this study population.
Prevalence of CagA antibodies in the 1973 and 1994 populations sampled
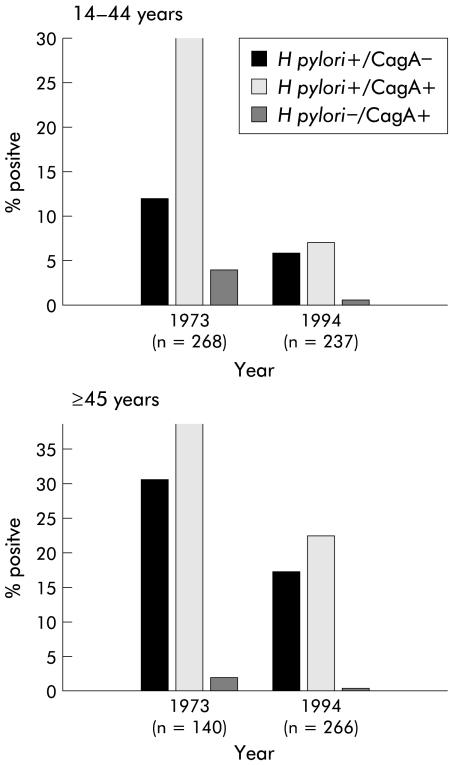
The prevalence of CagA antibodies in the sampled population significantly (p<0.001) declined from 36.5% in 1973 to 20.4% in 1994, which paralleled the overall decline in H pylori seroprevalence in the community during that time.12 The decline was substantially the same for males and females (data not shown). Among subjects 14–44 years old, the CagA seroprevalence rate declined precipitously (34.3% to 8.0%; p<0.0001) (fig 1 ▶). Among subjects over 45 years, the decline (40.7% to 31.6%) was less substantial. The proportion of subjects seropositive for H pylori but negative for CagA fell less markedly between 1973 and 1994 in those aged 14–44 years (11.6% to 5.9%; p=0.03) and over 45 years (30.7% to 24.1%; NS). Thus although the proportion of subjects who were H pylori seropositive fell in both age groups, and declined for both strain types (CagA+ or CagA− ), the largest effect was the decrease in CagA positivity among subjects aged 14–44 years. As the number of older individuals differed significantly in the two years studied, with a higher proportion (52.5%) of the population of Vammala in 1994 older than 45 years than in 1973 (34.3%), we calculated the proportion of cagA seropositivity in the 60–80 year age group at both points in the study, and the results were almost identical (35% in 1973 v 32.5% in 1994). In contrast, the prevalence of cagA seropositivity in the 45–59 year age group was greater in 1973 (41.3%) than in 1994 (29.9%). This is also consistent with the decline in cagA seropositivity observed in younger individuals, and suggests that the decline has been ongoing for decades.
Figure 1.
Change in seroprevalence of Helicobacter pylori carriage between 1973 and 1994 by age and cagA status (defined by CagA seropositivity). Both H pylori and cagA status were determined, and the 911 study subjects were stratified by age (14–44 and ≥45 years). Data are shown for specimens obtained in 1973 (n=408) and 1994 (n=503). The decline in seroprevalence of H pylori cagA+ strains in subjects 14–44 years old was significantly (p<0.001) greater than that for H pylori cagA− strains.
We found 14 (3.4%) subjects who were cagA+ but H pylori negative in 1973. Similarly, we found 20 (3.9%) subjects who were H pylori negative but cagA+ in 1994. The prevalence of cagA+ in the absence of H pylori antibody response in both years was slightly lower than the usual prevalence reported in other studies.23, 24 Subjects carrying H pylori may have substantial immune responses to the group antigen or to individual antigens (for example, CagA), reflecting the heterogeneity in the host responses in our outbred human population.
Seroprevalence in the cohort sampled 21 years apart
Sera from each of 221 subjects obtained both in 1973 and 1994 were examined to determine whether H pylori or CagA serostatus had changed. Of the 663 assays performed on sera obtained in 1994, 53 (8.0%) showed a change from 1973. In 23 (10.4%) subjects whose initial H pylori status was positive, one or more assays showed a change in status (in nine cases from positive to negative and in 14 cases from negative to positive) but the other assays did not change. Thus overall H pylori status for that subject remained positive. In total, only 12 (5.4%) subjects had a change in overall status over the 21 years (table 1 ▶). Of 115 subjects who were seronegative in 1973, nine (7.8%) seroconverted in one or more (mean 2.7 (0.7)) assays over the next 21 years, giving a crude seroconversion rate of 0.4% per year. Of the nine seroconverters, seven also became positive in the IgA assay, and eight also became positive in the CagA assay. The mean (SD) ages in 1973 of persistently seronegative subjects (31.7 (10.9) years) and seroconvertors (28.6 (7.9) years) were very similar, as were sex distributions. Of the 106 subjects who were defined as seropositive in 1973, only three (2.8%) seroreverted; each of these subjects had only been positive for H pylori IgG in 1973. The three serorevertors were not significantly different in age or sex distribution from the 103 serofast subjects. The crude seroreversion rate was 0.13% per year.
Table 1.
Changes in status in Helicobacter pylori assays among 221 subjects in Vammala, Finland, examined in both 1973 and 1994
| Assay that changed in status | ||||||
| Serological status | Total No of subjects affected | IgG | IgA | CagA | Any | Mean (SD) No of assays with changes |
| Negative→positive* | 9 | 9 | 7 | 8 | 24 | 2.7 (0.7) |
| Positive→negative† | 3 | 3 | 0 | 0 | 3 | 1.0 (0.0) |
| Total (%)‡ | 12 (5.4) | 12 (5.4) | 7 (3.2) | 8 (3.6) | 27 (4.1) | 2.3 (1.0) |
*This change in status was defined as seroconversion.
†This change in status was defined as seroreversion.
‡Numbers in parentheses indicate the proportion of the total population of 221 represented by the subjects who showed a change in status in the specified assay.
Stability of antibody responses
We next sought to determine the degree of fluctuation in the level of antibodies present among H pylori+ subjects over the 21 year period (table 2 ▶). For the 95 subjects who were IgG+ for H pylori antibodies in 1973, 87 (91.6%) remained positive in 1994 and the overall reciprocal geometric mean titre (RGMT) decreased from 3179 to 3009 (5.3%) by 1994. Excluding the three subjects who seroreverted in 1994 and the five other subjects who only reverted in IgG but H pylori status did not change, for the remaining 87 subjects the RGMT changed from 3203 to 3411 (6.1% increase; NS), representing a mean increase of 0.29% in antibody level per year. Among the 59 subjects who also were IgA+ in 1973, 54 (91.5%) remained positive in 1994, and the overall RGMT also changed little (table 2 ▶), representing a mean annual increase of 0.6%. All 69 subjects who were CagA+ in 1973 remained positive in 1994, with a mean annual increase of 0.3% in measurable antibody levels (table 2 ▶).
Table 2.
Stability of antibody responses to Helicobacter pylori antigens over 21 years in 95 subjects* who were IgG seropositive in 1973
| No of subjects positive in that assay | Antibody level | |||
| Assay | 1973 | 1994 | 1973 | 1994 |
| H pylori IgG† | 95 | 87 | 3179 | 3009 |
| H pylori IgA† | 59 | 54 | 180 | 201 |
| CagA IgG‡ | 69 | 69 | 0.75 (0.24) | 0.80 (0.21) |
*In 95 subjects (mean age 35.0 (12.0) years) who were IgG seropositive in 1973.
†Shown as reciprocal geometric mean titres (RGMT).
‡Shown as mean optical density ratio units (SD).
DISCUSSION
The major observation of this study is that over the 21 year period, the seroprevalence of cagA+ H pylori strains declined faster than that for cagA− H pylori strains, especially among subjects less than 45 years of age. This difference may be related to differential acquisition and loss rates in later birth cohorts. That among the 106 seropositive subjects in 1973 there were very few seroreversions (2.8%) by 1994 suggests that the overall decline in H pylori seroprevalence in Vammala between 1973 and 1994 predominantly reflected decreased acquisition of H pylori colonisation. However, as the subjects we tested were all over 14 years, our findings did not directly examine H pylori loss during early childhood. In many developed countries, the intensity of antibiotic exposure during childhood has been increasing markedly.25, 26 Even with the minimal success of antimicrobial monotherapy against H pylori,27, 28 the effects of repeated antibiotic regimens over the course of childhood may be substantial.29 The accumulating evidence that cagA+ strains are more susceptible than cagA− strains to eradication treatment30, 31 is consistent with the trends (fig 1 ▶) we observed.
Addition of the CagA serological assay to those for antibodies to H pylori acid-glycine extracted antigens increases the sensitivity of detecting H pylori carriage as there are subjects with strong responses to the immunodominant CagA antigen who do not meet the criteria for positivity in the assays using the acid extracted antigens.32 Despite differences in the primary structure of cagA related to the geographic origin of strains,33, 34 host response to CagA is well preserved, as described previously,19 and as further validated in this study. The nearly identical serum antibody levels to CagA (and to the acid-glycine extracted antigens) in the same individuals 21 years apart (table 2 ▶) confirms both the stability of these responses and the longstanding equilibrium between hosts and their specific H pylori populations.35 These results also indicate that there are no substantial age related changes among adults in serological responses to CagA antigens that could explain the declining prevalences observed between 1973 and 1994. These data confirm that H pylori acquisition chiefly occurs during childhood2, 3, 5 but continues to occur during adulthood, albeit at low rates, in developed countries.10, 12, 25, 36
An alternate but not mutually exclusive hypothesis is that with socioeconomic development, transmission of cagA+ strains has become proportionally less efficient than for cagA− strains in this community. However, the observation that among the nine subjects who seroconverted nearly all were CagA positive contradicts this hypothesis. However, we have not examined a prior time period, and it is possible that transmission of cagA+ strains during adulthood was even more efficient previously than during the period 1973 to 1994. Another possible explanation is that as socioeconomic status increases, children become less important as a source of infection of adults (and adult to adult transmission thus becomes relatively more important). As such the few adult seroconversions that occur mirror existing adult infections (from early cohorts) their present childhood infections.
There has been a differential decline in transmission of the various Shigella species with improving hygienic conditions during the 20th century in which the most virulent strains (S dysenteriae) have disappeared most quickly.37 Among populations in developing countries, in which nearly all adults are H pylori +, most are also CagA seropositive.38–40 In contrast, in developed countries in which H pylori seroprevalence is lower, higher proportions of strains are cagA−.38, 41, 42 Our present data suggest that this change in proportion has been a dynamic process that occurred during socioeconomic development, probably as a birth cohort phenomenon, resulting from differential acquisition and/or loss rates between cagA+ and cagA− strains. Since this study is the first to report the decline in cagA+ strains in developed countries, it will be important to seek independent confirmation. As cagA+ strains have more intense interactions with human epithelial cells than cagA− strains,15, 43, 44 and are associated with increased risk of both duodenal ulceration17, 42 and gastric cancer,15, 45 and with a decreased risk of oesophageal diseases,23, 46 the consequences of enhanced loss of cagA+ strains for human health may be profound.
Acknowledgments
Supported in part by RO1 DK 53704 from the National Institute of Health, and by the Medical Research Service of the Department of Veterans Affairs. We thank Dr OP Heinonen for help in acquiring the serum specimens.12
Abbreviations
ODR, optical density ratio
RGMT, reciprocal geometric mean titre
Footnotes
Conflict of interest: Dr Blaser has a potential royalty interest in CagA diagnostics (licensed by Vanderbilt University) based on his discovery of CagA.
REFERENCES
- 1.Blaser MJ. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology in the modern era. Gut 1998;43:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Megraud F, Brassens Rabbe MP, Denis F, et al. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol 1989;27:1870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-Pérez GI, Bodhidatta L, Wongsrichanalai J, et al. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis 1990;161:1237–41. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DN, Parsonnet J. Epidemiology and natural history of Helicobacter pylori infection. In: Blaser MJ, Ravdin JJ, Breenber HB, et al, eds. Infections of the gastrointestinal tract. New York: Raven Press, 1995:551–63.
- 5.Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infections. Epidemiologic Rev 1991;13:42–59. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer B, Kaldor J, Tee W, et al. Antibody response to Campylobacter pylori in diverse ethnic groups. Scand J Infect Dis 1988;20:349–50. [DOI] [PubMed] [Google Scholar]
- 7.Morris A, Nicholson G, Lloyd G, et al. Seroepidemiology of Campylobacter pyloridis. N Z Med J 1986;99:657–9. [PubMed] [Google Scholar]
- 8.Sitas F, Forman D, Yarnell JW, et al. Helicobacter pylori infection rates in relation to age and social class in a population of Welsh men. Gut 1991;32:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendall MA, Goggin PM, Molineaux N, et al. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet 1992;339:896–7. [DOI] [PubMed] [Google Scholar]
- 10.Parsonnet J, Blaser MJ, Pérez-Pérez GI, et al. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterology 1992;102:41–6. [DOI] [PubMed] [Google Scholar]
- 11.Banatvala N, Mayo K, Megraud F, et al. The cohort effect and Helicobacter pylori. J Infect Dis 1993;168:219–21. [DOI] [PubMed] [Google Scholar]
- 12.Kosunen TU, Aromaa A, Knekt P, et al. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect 1997;119:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akopyants NS, Clifton S W, Kersulyte D, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol 1998;28:37–53. [DOI] [PubMed] [Google Scholar]
- 14.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA 1996;93:14648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peek RM Jr, Miller GG, Tham KT, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest 1995;71:760–70. [PubMed] [Google Scholar]
- 16.Blaser MJ, Pérez-Pérez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 1995;55:2111–15. [PubMed] [Google Scholar]
- 17.Crabtree JE, Taylor JD, Wyatt JI, et al. Mucosal IgA recognition of Helicobacter pylori 120kDa protein, peptic ulceration, and gastric pathology. Lancet 1991;338:332–5. [DOI] [PubMed] [Google Scholar]
- 18.Kolho K-L, Karttunen R, Heikkila P, et al. Gastric inflammation is enhanced in children with CagA positive Helicobacter pylori infection. Pediatr Infect Dis J 1999;18:337–41. [DOI] [PubMed] [Google Scholar]
- 19.Hook-Nikanne J, Perez-Perez GI, Blaser MJ. Antigen characterization of Helicobacter pylori strains from different parts of the world. Clin Diagn Lab Immunol 1997;4:592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold BD, vanDoorn LJ, Guarner J, et al. Genotypic, clinical and demographic characteristics of children infected with Helicobacter pylori. J Clin Microbiol 2001;39:1348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales-Espinosa R, Castillo-Rojas G, Gonzalez-Valencia G, et al. Colonization with Mexican patients by multiple Helicobacter pylori strains with different vacA and cagA genotypes. J Clin Microbiol 1999;37:3001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000;287:1497–500. [DOI] [PubMed] [Google Scholar]
- 23.Chow WH, Blaser MJ, Blot WJ, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric adenocarcinoma. Cancer Res 1998;58:588–90. [PubMed] [Google Scholar]
- 24.Torres J, Perez-Perez GI, Leal-Herrera Y, et al. Infection with cagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int J Cancer 1998;78:298–300. [DOI] [PubMed] [Google Scholar]
- 25.Fawcett JP, Shaw JP, Brooke M, et al. Seroprevalence of Helicobacter pylori in a longitudinal study of New Zealanders at age 11 and 21. Aust NZ J Med 1998;28:585–9. [DOI] [PubMed] [Google Scholar]
- 26.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995;273:214–19. [PubMed] [Google Scholar]
- 27.Rauws EA, Langenberg W, Houthoff HJ, et al. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology 1988;94:33–40. [PubMed] [Google Scholar]
- 28.Peterson WL, Graham DY, Marshall B, et al. Clarithromycin as monotherapy for eradication of Helicobacter pylori: A randomized, double-blind trial. Am J Gastroenterol 1993;88:1860–4. [PubMed] [Google Scholar]
- 29.Blaser MJ. The changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis 1999;179:1523–30. [DOI] [PubMed] [Google Scholar]
- 30.Van Doorn LJ, Quint W, Schneeberger P, et al. The only good Helicobacter pylori is a dead Helicobacter pylori. Lancet 1997;350:71–2. [DOI] [PubMed] [Google Scholar]
- 31.Marais A, Monteiro L, Lamouliatte H, et al. Cag negative status of Helicobacter pylori is a risk factor for failure of PPI-based triple therapies in non-ulcer dyspepsia. Gastroenterology 1998;114:A214. [Google Scholar]
- 32.Perez-Perez GI, Brown WR, Cover TL, et al. Corelation between serological and mucosal inflammatory response to Helicobacter pylori. Clin Diag Lab Immunol 1994;1:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Doorn L-J, Figueiredo C, Ssanna R, et al. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J Clin Microbiol 1999;37:2306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaoka Y, El-Zimaity HMT, Gutierrez O, et al. Relationship between the cagA 3` repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology 1999;117:342–9. [DOI] [PubMed] [Google Scholar]
- 35.Blaser MJ, Kirschner D. Dynamics of Helicobacter pylori colonization in relation to the host response. Proc Natl Acad Sci USA 1999;96:8359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor DN, Sanchez JL, Smoak BL, et al. Helicobacter pylori infection in Desert storm troops. Clin Infect Dis 1997;25:979–82. [DOI] [PubMed] [Google Scholar]
- 37.Kostrzewski J, Stypulkowska-Misiurewicz H. Changes in the epidemiology of dysentery in Poland and the situation in Europe. Arch Immunol Ther Exp 1968;16:429–51. [PubMed] [Google Scholar]
- 38.Perez-Perez GI, Bhat N, Gaesbauer J, et al. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer 1997;72:453–6. [DOI] [PubMed] [Google Scholar]
- 39.Yamaoka Y, Kodama T, Kashima K, et al. Variants of the 3` region of the cagA gene in Helicobacter pylori isolates from different H. pylori associated diseases. J Clin Microbiol 1998;36:2258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Z-L, van der Hulst RWM, Feller M, et al. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis associated dyspepsia. J Clin Microbiol 1997;35:1344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerhard M, Lehn N, Neumayer N, et al. Clinical relevance of the Helicobacter pylori gene for blood group antigen-binding adhesin. Proc Nat Acad Sci USA 1999;96:12778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cover TL, Dooley CP, Blaser MJ. Characterization and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun 1990;58:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crabtree JE, Covacci A, Farmery SM, et al. Helicobacter pylori induced interleukin-8 expression in gastric epthelial cells is associated with Cag A-positive phenotype. J Clin Pathol 1995;48:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma SA, Tummuru MKR, Miller GG, et al. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun 1995;63:1681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsonnet J, Friedman GD, Orentreich N, et al. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997;40:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology 1998;115:50–7. [DOI] [PubMed] [Google Scholar]