Abstract
Aim: To evaluate the efficacy of power Doppler sonography (US) in depicting internal fistulae and their vascularity, and to study the characteristics of blood flow within the fistula wall.
Patients and methods: The study involved 45 consecutive patients with Crohn's disease and suspected internal fistulae detected by grey scale US. The fistulae were subsequently evaluated using power Doppler US to reveal any areas of increased vascularity, and the results were compared with radiographic, endoscopic, or intraoperative findings. Whenever feasible, we also performed spectral analysis of blood flow revealed by power Doppler US, calculated its resistance index (RI), and analysed its characteristics, reproducibility, and relationship with biochemical and clinical variables (Crohn's disease activity index, disease duration, location, and abdominal complications).
Results: Power Doppler US revealed vascularity in all of the internal fistulae that where subsequently confirmed by diagnostic procedures. In the case of intra-abdominal abscesses in the vicinity of the fistula, vascular signals were detected mostly around and not within the lesions. The intensity and distribution of the signals differed within the fistulae tracks and had only slight day to day reproducibility; furthermore, there was no significant correlation with clinical or biochemical variables. Spectral analyses of blood flow within the fistulae revealed arterial flow in 96.7% of patients (median RI 0.715). RI was a more reproducible parameter and significantly correlated with clinical (r= 0.54) and biochemical activity (r= 0.56) of CD. It was also higher in fistulae complicated by abscesses.
Conclusion: Power Doppler US can reveal the presence of vasculature within the wall of internal fistulae and therefore enhance grey scale US performance. The RI characteristics of blood flow within the fistulae are reproducible and correlate with biochemical and clinical disease activity.
Keywords: Crohn's disease, ultrasonography, fistulae
Internal fistulae and fissures are well known features of Crohn's disease (CD) and form part of the spectrum of intestinal complications leading to peri-intestinal abscesses, loop adhesion, malabsorption, and ultimately stricture formation. Radiographic detection of internal fistulae is still unsatisfactory: they are frequently discovered only at laparotomy in CD patients and a number of retrospective studies showed that barium radiology identified only 30–50% of fistulae.1–3
It has recently been shown that ultrasonography (US) is accurate in detecting internal fistulae in CD patients4 and may be superior to barium radiology. However, US peri-intestinal signs compatible with fissures and fistulae may be difficult to interpret and differentiate from small peri-intestinal abscesses of small peri-intestinal lymph nodes.
Histologically, fistulae and fissures are inflammatory lesions made by granulation tissue and are characterised by a rich microvasculature.
Power Doppler US is a new and highly sensitive technique for detecting the presence of flow in vessels that are poorly imaged by conventional colour Doppler, and revealing inflammatory hyperaemia or increased and irregular flow in tumours.5 In particular, it has significant advantages over colour Doppler in detecting tissue vasculature because of its reduced noise, angle independence, and lack of aliasing, as has already been demonstrated in focal lesions of the gastrointestinal tract.6
It may therefore enhance the performance of grey scale US in detecting internal fistulae complicating CD by showing the presence of flow in peri-intestinal lesions that are compatible with fissures and fistulae, and may also provide a new means of studying vascularisation within internal fistula walls in vivo. Despite the clinical relevance of fistulae in CD, only a few studies have considered the pathobiology of fistula formation and no data are available concerning the in vivo characteristics of internal fistula vascularity.
The aim of this study was therefore to evaluate the efficacy of power Doppler US in confirming the ultrasonographic suspicion of internal fistulae and revealing their vasculature in CD patients. We also evaluated the characteristics of vascularity within the wall of the fistulae and their reproducibility and possible relationships with clinical and biochemical findings, as a preliminary step towards investigating whether power Doppler US can be used: (1) to assess changes in internal fistula flow, such as those due to therapy; and (2) to evaluate its usefulness in studying the pathobiology and mechanisms of angiogenesis of fistulae in CD patients in vivo.
PATIENTS AND METHODS
Between August 1999 and August 2000, 45 consecutive CD patients with suspected internal fistulae at grey scale US (14 women, 29 men; mean age 44.8 years (range 21–71)) were enrolled in the study, including three with a previous contrast radiography diagnosis of internal fistula.
All patients were evaluated in terms of disease site, duration, and clinical and biochemical activity according to the Crohn's disease activity index (CDAI), and C reactive protein, as previously described.7
Patients first underwent a grey scale US scan, and were then evaluated using power Doppler US to demonstrate any areas of vascularity within the suspected fistulous tracks. Depending on the clinical setting, the US results were compared with the results of radiographic, tomographic, endoscopic, and/or intraoperative diagnostic procedures performed to assess the presence and type of internal fistulae. Only intraoperative findings were considered as the reference standard for defining the presence or absence of internal fistulae; radiographic, tomographic, and endoscopic findings were used to confirm, but not exclude, the presence of fistulae because of the possibility of false negative results.1 Radiographic diagnosis of internal fistulae was defined according to conventional radiographic criteria.8 Intra-abdominal abscesses were diagnosed on the basis of intraoperative findings (purulent fluid) or tomographic findings according to usual criteria.8
US examinations were carried out by the same sonographer using a real time scanner (Hitachi EUB 525, Tokyo, Japan) with a convex 3.5 MHz and (for detailed evaluation) a linear 7.5 MHz transducer. As previously reported, the internal fissures and fistulae were sonographically defined as peri-intestinal hypoechoic areas or a hypoechoic tract, with or without hyperechoic content, seen between loops or between a loop and the urinary bladder.4,9,10
All Doppler examinations were performed using a convex 3.5 MHz transducer. The specific power Doppler parameters included a PRF of 400–800 Hz, the lowest possible wall filter, and a CD power gain of 6–18 (mean 13.2). To detect vascularity within the lesions, the manufacturer's pre-set power gain optimisation was verified as previously described11 and, when suspected, fistulous tracts were seen within the colour box, the power gain was slightly increased or adjusted until artefacts were seen.
Intralesional blood flow was diagnosed only if vascularity was demonstrated within the fistula wall and continuous observations showed that it was consistently reproducible in the same location. The intensity of the vascularity was subjectively categorised as mild (small focal area of colour signal), moderate (multiple areas of weak colour signal), or marked (multiple areas of colour signal).12,13 Vascularisation of the fistulae was recorded on colour prints and subjectively re-evaluated and categorised by the same sonographer at the end of the study.
Spectral analyses of intralesional blood flow were obtained whenever possible to document arterial or venous flow within the fistula wall. The spectral results were improved by first identifying the flow within the fistula using power Doppler US and then positioning the 5 mm sampling volume at the centre of the colour signal. Whenever an arterial waveform was obtained, the resistance index (RI) was calculated as RI=(S–D)/S, where S=peak systolic velocity and D=end peak diastolic velocity. We used this semiquantitative index because it is related to impedance of the arterial bed (which includes resistance, compliance, and congestion of the vascular bed) and its value does not depend on the insonation angle. RI was evaluated from at least three different points of the same fistula, and the mean value was used in the analysis. When more than one fistula was suspected at US, more evaluations were performed to have at least three different measurements for each suspected fistula.
In 15 patients, the power Doppler evaluation and spectral analysis of intralesional blood flow were repeated at intervals of 2–5 days to assess the day to day reproducibility of the findings.
The distribution of individual characteristics was evaluated by simple descriptive statistics. Differences among distributions of selected variables were evaluated using Fisher's exact tests for categorical data. The Wilcoxon rank sum test was performed for statistical evaluation of the significant difference of the distributions of continuous variables between two groups. The Spearman rank correlation coefficient was used to determine the correlation.
Multiple linear regression was performed to explore the relationship between RI and other continuous variables. Regression analyses were also performed excluding outlier values. Deviation from linearity was tested adding quadratic terms in the model.
All analyses were performed using SAS statistical software. Statistical tests were adjusted for the effect of multiple tests by multiplying the observed p values by the number of tests done. All statistical tests and p values were two tailed.
RESULTS
We could not find intralesional vascular signals in three of the original 45 patients with suspected internal fistulae at grey scale US: the presence of internal fistulae was intraoperatively excluded in one case and not radiographically detected in the others.
Power Doppler US revealed vascularity in all of the remaining fistulous tracks (fig 1 ▶): the presence of internal fistulae was not confirmed by diagnostic procedures in five cases but was documented in the other 37 patients by contrast radiography (21 patients), computed tomography (11), colonoscopy (7), and/or intraoperative findings (17). In five patients who underwent surgery, the fistulae were only detected by US and power Doppler US (contrast radiography was not performed due to intestinal occlusion) in two, and detected by US and power Doppler US but missed by standard radiography, computed tomography, and colonoscopy in the other three.
Figure 1.
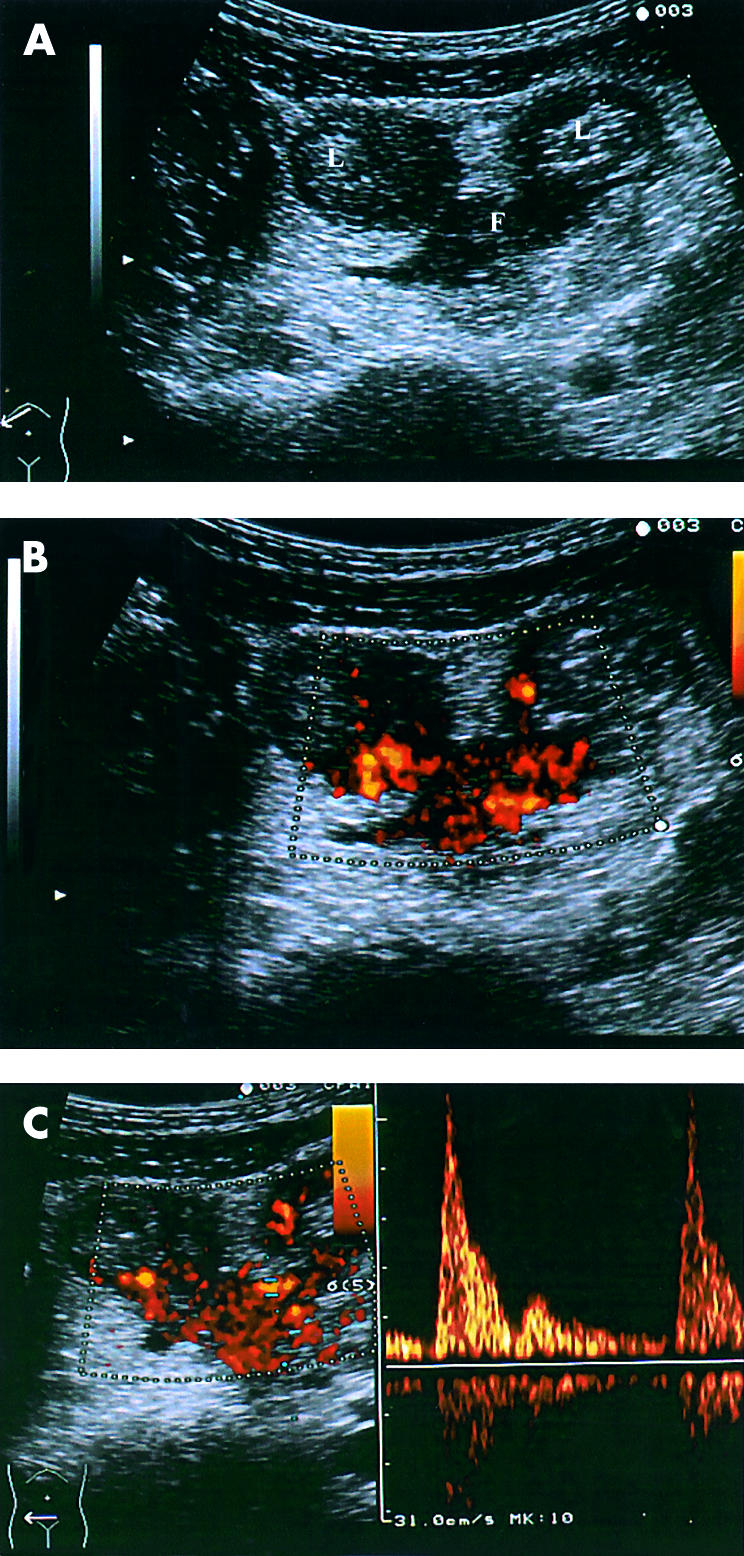
Grey scale ultrasound (A), power Doppler imaging (B), and spectral analysis (C) of the flow of an internal fistula (F) extending between two ileal loops (L).
The demographic and clinical characteristics of the patients in whom an internal fistula was confirmed are shown in table 1 ▶.
Table 1.
Demographic, clinical, and biochemical characteristics of patients
| Parameter | Median (min−max) | n (%) |
| Female | 11 (29.7) | |
| Age (y) | 34 (20–71) | |
| Duration of CD (months) | 72 (1–256) | |
| Previous surgery | 16 (43.2) | |
| CD intestinal location | ||
| Small bowel only | 21 (56.8) | |
| Small bowel and colon | 16 (43.2) | |
| Radiographic evidence of stenosis | 34 (91.9) | |
| Radiographic evidence of abscesses | 9 (24.3) | |
| Active disease (CDAI >150) | 21 (56.8) | |
| CDAI (best) | 207 (50–432) | 34 |
| C reactive protein (ng/ml) | 3.5 (0.2–18.5) | 36 |
CDAI, Crohn's disease activity index.
In nine patients, grey scale US revealed typical anechoic lesions compatible with intra-abdominal abscesses in the vicinity of the fistula. All abscesses were subsequently confirmed by computed tomography and at laparotomy in eight patients who underwent surgery. Power Doppler US showed the absence of signals in the centre of these lesions and increased vascularity around their periphery in continuance with the fistulous track (fig 2 ▶).
Figure 2.
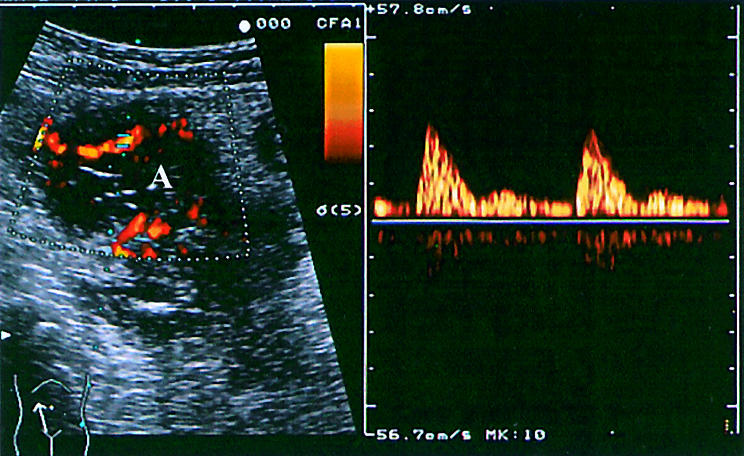
Power Doppler imaging of a deep mesenteric abscess (A) showing increased vascularity around the periphery of the lesion and spectral analysis of flow.
Characteristics of lesion vascularity
The distribution and intensity of vascularity within the fistulae were not homogeneous (particularly at a low level of colour gain), and the colour signals did not completely fill the fistulous tracks imaged at grey scale US in any patient (fig 1 ▶). The intensity of vascularity was mild in 10 patients, moderate in 13, and marked in 14; it was reproducible from day to day in 11/15 patients (73.3%).
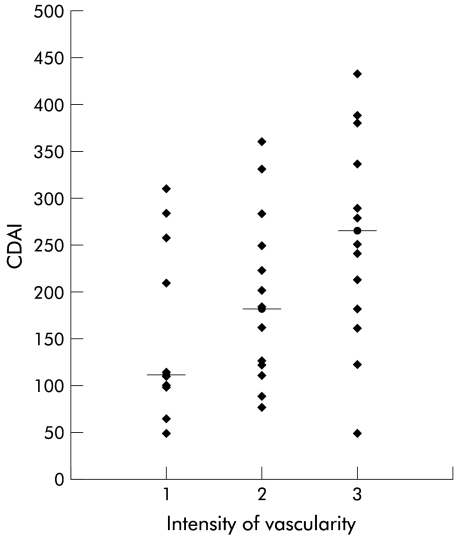
There was no significant relationship between the intensity of vascularisation and demographic, clinical, or biochemical parameters, although there was a trend towards an increase in the median CDAI from mild to marked vascularity (112 v 183 v 266; p=0.07) (fig 3 ▶).
Figure 3.
Relationship between the intensity of vascularity within the wall of the fistula (median) and Crohn's disease activity index (CDAI). 1=Mild, 2=moderate, 3=marked.
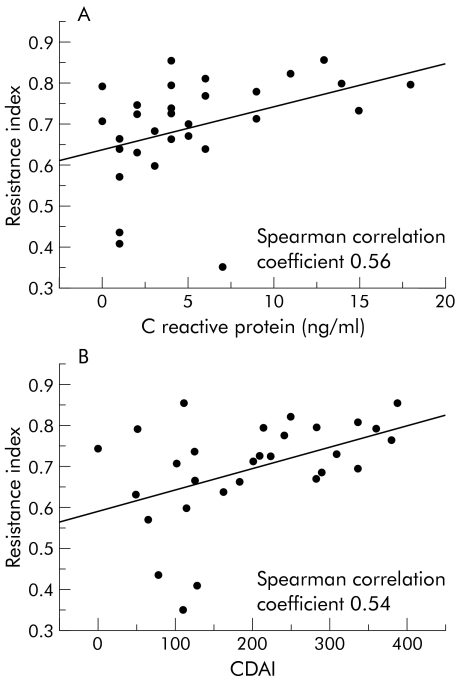
Spectral analyses of blood flow within the wall of the fistulous tract were obtained in 31 patients: one showed a venous waveform, 16 an arterial waveform, and 14 both. The median RI of arterial flow was 0.715 (minimum−maximum 0.350–0.853); the median intraindividual variation in the RI measurements made from different points in the fistulous tracks and expressed by means of the coefficient of variation was 5.3%. The day to day reproducibility of the mean RI values was good, with a Pearson correlation coefficient (r) of 0.88 between the two measurements. RI values significantly correlated with severity and biochemical activity of the disease, being higher in patients with more active disease (fig 4 ▶).
Figure 4.
Correlation between resistance index and C reactive protein levels (A) and Crohn's disease activity index (CDAI) (B). C reactive protein in one patient and CDAI in one patient were not available.
RI was also higher in patients whose fistulae were complicated by abscesses than in those without abscesses. There was no significant relationship between RI values and sex, disease duration, age, previous surgery, or intestinal CD site (table 2 ▶).
Table 2.
Comparison of resistance index (RI) by demographic and clinical variables
| Variable | RI | p Value |
| Sex | ||
| Male (n=20) | 0.716 (0.139) | |
| Female (n=10) | 0.678 (0.139) | 0.7 |
| Age (y) | ||
| <35 (n=15) | 0.727 (0.084) | |
| ≥35 (n=15) | 0.658 (0.145) | 0.2 |
| CD intestinal location | ||
| Small bowel only (n=16) | 0.720 (0.079) | |
| Small bowel and colon (n=14) | 0.657 (0.157) | 0.5 |
| Evidence of abscesses | ||
| Yes (n=9) | 0.781 (0.058) | |
| No (n=21) | 0.652 (0.125) | 0.002 |
| Stenosis | ||
| No (n=3) | 0.544 (0.215) | |
| Yes (n=27) | 0.707 (0.104) | 0.3 |
| Disease duration (months) | ||
| <72 | 0.699 (0.127) | |
| ≥72 | 0.680 (0.128) | 0.7 |
| Previous surgery | ||
| Yes (n=13) | 0.690 (0.112) | |
| No (n=17) | 0.691 (0.135) | 0.8 |
Values are mean (SD).
According to linear regression analysis, there were significant relationships between RI and C reactive protein and CDAI, even after excluding three outlier values (table 3 ▶). Because of the high correlation between C reactive protein and CDAI (Spearman correlation coefficient 0.69) and limited sample size, more complex models were not proved. No deviation from linearity was found after inclusion of quadratic terms in the model.
Table 3.
Linear regression estimates and Spearman correlation coefficient for resistance index, including and excluding outlier values
| 29 subjects | 26 subjects (excluding 3 outliers) | |||||
| Regression coefficient–SE | p Value | Sperman correlation coefficient | Regression coefficient−SE | p Value | Sperman correlation coefficient | |
| C reactive protein | 0.01178–0.004528 | 0.01 | 0.56 | 0.008921–0.002700 | 0.003 | 0.59 |
| CDAI | 0.000626–0.000199 | 0.004 | 0.54 | 0.000353–0.000135 | 0.01 | 0.47 |
DISCUSSION
Over the past few years it has been increasingly found that power Doppler US is an interesting means of revealing neovascularisation and inflammatory hyperaemia, and assessing therapy related vascular changes in various clinical, neoplastic, and non-neoplastic conditions.5
Its role in CD has so far been evaluated in only a few preliminary studies which have found that increased vascularity of the diseased bowel wall correlates with disease activity and is probably due to inflammatory responses and neovascularisation5,14; however, no other data have been published concerning the usefulness of power Doppler US in detecting and evaluating fistulae in CD patients.
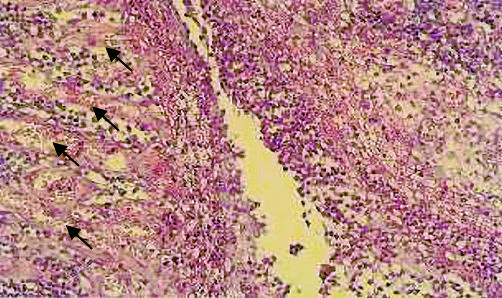
Fistulae and fissures, including those complicating CD, are inflammatory lesions made by granulation tissue and characterised by a rich microvasculature (fig 5 ▶), which may explain the fistula vascularity revealed by power Doppler US in this study. Furthermore, our results fit well with recent findings by Oberhuber et al who showed that internal fistulae appear to pass through the muscularis propria, along piercing mesenteric vessels up to 1 mm in diameter,15 which are detectable by power Doppler sonography.
Figure 5.
Histological findings of a fistula showing tissue granulation with diffuse inflammatory infiltrate and a high density of microvessels (arrows). (Photograph by courtesy of Dr L Carsana, Pathology Unit, L Sacco Hospital, Milan, Italy)
In clinical practice, a non-invasive estimate of the vascularity of suspected fistulous tracks could be used to strengthen the diagnostic accuracy of grey scale US in the detection of internal fistulae. Although our study was not designed to determine the diagnostic accuracy of power Doppler US (the fistulae were already suspected at grey scale US), we found that it was diagnostically sensitive and, in the few patients who underwent surgery, did not lead to false positive results.
We also found that it revealed increased vascularity around abscesses and the absence of internal vascularity, results that are in line with those of previous studies of superficial septic abscesses using colour and power Doppler US.13,16,17 In the case of CD patients, this characteristic is conceivable if we consider that abscesses arise in or around a fistulous track: although it is difficult to separate fistulae tracts from the abscess walls, we believe that power Doppler can be used to differentiate abscesses from inflammatory masses or fistulae, although larger scale studies are necessary to confirm these preliminary findings.
The possibility of detecting fistula vascularisation using power Doppler US prompted our interest in defining the characteristics of blood flow. To this end, we considered two previously used parameters: the intensity of vascularity (a qualitative and subjective index) and RI obtained by performing spectral analysis of blood flow (a semiquantitative index).6,12,18,19 Before attempting to establish any correlation between these indices and clinical or biochemical parameters, we tested their reproducibility: the intensity and distribution of the vascular (colour) signals within the fistulous tracks were patchy, dishomogeneous, and had a moderate level of day to day reproducibility but the RI value within the fistulae was more reproducible and stable over a short period of time (2–5 days).
We did not find any relevant correlation between the intensity of the vascular signals within the fistulae and disease activity but there was a trend towards a relationship between increased disease severity and greater vascularisation. However, although it is simple and practical to use, the qualitative nature of this index does not allow us to draw any definite conclusions. On the other hand, the fistula RI was significantly higher in patients with more severe and biochemically active disease.
It is difficult to interpret these findings because the significance of intra-tissue RI values and the intensity of vascular colour signals is still uncertain and, in particular, it is not clear whether they are useful parameters for in vivo assessment of inflammatory hyperaemia or neovascularisation.18,20,21 Furthermore, there are very few published data concerning pathological or biochemical assessment of angiogenesis and vascularity of the bowel wall or internal fistulae in CD patients.
The vascularisation pattern in internal fistulae may be related to the intensity of angiogenesis, inflammatory activity, or (probably) both. It is difficult to prove whether the intensity of vascularity and the RI value are related to angiogenesis because this has never been evaluated in CD. Furthermore, studies of tumour angiogenesis and intratumoral RI have led to conflicting results insofar as both low and high RI values have been found. To date, only one study has assessed the vascularity of gastrointestinal tract diseases by means of intrarectal colour Doppler and Doppler flow analysis: the results showed a high RI and hypervascularity in malignancies, and a lower RI and hypovascularity in a few benign rectal diseases.18
It may be hypothesised that intrafistula vascularity and blood flow patterns are due to the intense neovascularisation related to the marked inflammatory infiltration and biochemical and clinical activity of the disease. It has been shown that active CD is associated with high serum levels of basic fibroblast growth factor and endothelial growth factor, and probably with more intense intestinal angiogenesis.22,23 If this is the case, the high degree of vascularisation and high RI values in the fistulae may be the effect of an increase in the number of vessels compressed by the inflammatory infiltrate, which could be more pronounced in active disease (fig 5 ▶).
Another possible explanation for our findings is that the vascular pattern may be due to the pre-existing vessels at the site of fistula formation. Partly destroyed or severely damaged piercing mesenteric arteries, with a high degree of vasculitis and vessel-like structures, were found in almost 50% of patients by Oberhuber and colleagues.15 Intestinal microvascular endothelium has been shown to contribute to the cytokine network of the intestinal mucosa with the ability to respond to locally generated cytokines and to produce potent inflammatory mediators.24 It can therefore be hypothesised that this could lead to the growth of the piercing vessel along the fistula tract or to production of new vessels as a consequence of inflammatory infiltration and disruption of the piercing vessel itself. In this regard it would be essential to study histological findings in resected specimens. Unfortunately, specific histological evaluation of diseased bowel and fistulous tract was not an aim of this study, and most operated patients underwent miniresection and strictureplasties.
However, as we do not yet have histological data concerning fistulae, our findings must be considered preliminary and exploratory. Further studies are required to clarify the role of power Doppler US in detecting piercing vessels, in quantifying tissue perfusion, and eventually neoangiogenesis within diseased bowel and fistula walls in CD. Also, studies are needed based on second harmonic imaging in conjunction with power Doppler US and contrast agents, and the histological evaluation of angiogenesis, the results of which could answer the question of whether power Doppler US is really a useful means of assessing CD patients prone to the development of fistulae and monitoring the effect of therapeutic agents such as anti-tumour necrosis factor α and thalidomide which have been found to have potential activity in angiogenesis.25–27
In conclusion, our findings showed that power Doppler US may be a new method of assessing internal fistulae in CD patients. The possibility of detecting and imaging the vascularity of fistulous tracks could extend our knowledge of the pathobiology, evolution, and therapeutic response of internal fistulae complicating CD.
Abbreviations
CD, Crohn's disease
US, ultrasonography
CDAI, Crohn's disease activity index
RI, resistance index
REFERENCES
- 1.Michelassi F, Stella M, Balestracci T, et al. Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn's disease. Ann Surg 1993;218:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finke M. Enteroclisis: double contrast examination of the small bowel. Radiol Technol 1987;59:143–9. [PubMed] [Google Scholar]
- 3.Glass RE, Ritchie JK, Lennard-Jones JE, et al. Internal fistulae in Crohn's disease. Dis Col Rectum 1985;28:557–61. [DOI] [PubMed] [Google Scholar]
- 4.Gasche C, Moser G, Turetschek K, et al. Transabdominal bowel sonography for detection of intestinal complication in Crohn's disease. Gut 1999;44:112–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinoli C, Pretolesi F, Crespi G, et al. Power Doppler sonography: clinical applications. Eur J Radiol 1998;28:S133–40. [DOI] [PubMed] [Google Scholar]
- 6.Engle TC, Jeffrey RB, Li KCP, et al. Power Doppler imaging of focal lesion of the gastrointestinal tract: Comparison with conventional color Doppler imaging. J Ultrasound Med 1996;15:63–6. [PubMed] [Google Scholar]
- 7.Maconi G, Parente F, Bollani S, et al. Factors affecting splanchnic haemodynamics in Crohn's disease: a prospective study using Doppler ultrasound. Gut 1998;43:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelvin MF, Herlinger H. Crohn's Disease. In: Herlinger H, Maglinte DDT, Birnbaum BA, eds. Clinical imaging of the small intestine, 2nd Edn. New York: Springer-Velag, 1999:259–89.
- 9.Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn's disease. Dig Dis Sci 1996;41:1643–8. [DOI] [PubMed] [Google Scholar]
- 10.Sarrazin J, Wilson SR. Manifestation of Crohn's disease at US. Radiographics 1996;16:499–520. [DOI] [PubMed] [Google Scholar]
- 11.Bude RO, Rubin JM. Power Doppler sonography. Radiology 1996;200:21–3. [DOI] [PubMed] [Google Scholar]
- 12.Quillin SP, Siegel MJ. Gastrointestinal inflammation in children: color Doppler ultrasonography. J Ultrasound Med 1994;13:751–6. [DOI] [PubMed] [Google Scholar]
- 13.Arslan H, Sakarya ME, Bozkurt M, et al. The role of power Doppler sonography in the evaluation of superficial soft tissue abscesses. Eur J Ultrasound 1998;8:101–6. [DOI] [PubMed] [Google Scholar]
- 14.Stavros AT, Rapp CL, Thichman D. Sonography of inflammatory conditions. Ultrasound Q 1995;13:1–26. [Google Scholar]
- 15.Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn's disease. Virchows Arch 2000;437:293–7. [DOI] [PubMed] [Google Scholar]
- 16.Latifi HR, Siegel MJ. Color Doppler flow imaging of paediatric soft tissue masses. J Ultrasound Med 1994;13:165–9. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DG, Merton DA, Liu JB, et al. Superficial masses with color Doppler imaging. J Clin Ultrasound 1991;19:555–60. [DOI] [PubMed] [Google Scholar]
- 18.Joosten FBM, Jansen JBMJ, Joosten HJM, et al. Use of intra-rectal ultrasound with color Doppler and Doppler flow analysis in differentiation of benign and malignant rectal disease. Eur J Ultrasound 1996;3:25–32. [Google Scholar]
- 19.Lassau N, Paturel-Asselin C, Guinebretiere JM, et al. New hemodynamic approach to angiogenesis: color and pulsed Doppler ultrasonography. Invest Radiol 1999;34:194–8. [DOI] [PubMed] [Google Scholar]
- 20.Cheng WF, Lee CN, Chu JS, et al. Vascularity index as a novel parameter for the in vivo assessment of angiogenesis in patients with cervical carcinoma. Cancer 1999;85:651–7. [DOI] [PubMed] [Google Scholar]
- 21.Emoto M, Iwasaki H, Mimura K, et al. Differences in the angiogenesis of benign and malignant ovarian tumors, demonstrated by analyses of color Doppler ultrasound, immunohistochemistry, and microvessel density. Cancer 1997;80:899–907. [PubMed] [Google Scholar]
- 22.Bousvaros A, Leichtner A, Zurakowski D, et al. Elevated serum vascular endothelial growth factor in children and young adults with Crohn's disease. Dig Dis Sci 1999;44:424–30. [DOI] [PubMed] [Google Scholar]
- 23.Bousvaros A, Zurakowski D, Fishman SJ, et al. Serum basic fibroblast growth factor in pediatric Crohn's disease. Implications for wound healing. Dig Dis Sci 1997;42:378–86. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen EM, Johansen FE, Jahnsen FL, et al. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut 1998;42:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands BE, Podolsky DK. New life in a sleeper: thalidomide and Crohn's disease. Gastroenterology 1999;117:1485–8. [DOI] [PubMed] [Google Scholar]
- 26.D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 1994;91:4082–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Haens GR. Infliximab (RamicadeTM), a new biological treatment for Crohn's disease. Ital J Gastroenterol Hepatol 1999;31:519–20. [PubMed] [Google Scholar]