Abstract
Background: Colorectal adenomatous and, probably, hyperplastic polyp development requires epithelial remodelling and stratification, with loss of E-cadherin expression implicated in adenoma formation. We have shown that P-cadherin, normally expressed in stratified epithelia and placenta, is aberrantly expressed in disturbed epithelial architecture associated with colitis.
Aims: (i) To investigate the role of P-cadherin in colonic polyp formation. (ii) To ascertain whether expression of P-cadherin is independent of or correlated with expression of its associated proteins— E-cadherin, β-catenin, and γ-catenin. (iii) To determine if P-cadherin is functional regarding catenin binding in polyps.
Methods: Expression and localisation of cadherins (E- and P-) and their associated catenins (β- and γ-) were determined in aberrant crypt foci (ACF), in polyps with hyperplastic morphology (hyperplastic polyps and serrated adenomas), and in adenomatous polyps by immunohistochemistry, western blotting, and mRNA in situ hybridisation. Assessment of cadherin-catenin binding was evaluated by co-immunoprecipitation. Adenomatous polyposis coli (APC) mutation was assessed in adenomatous polyps.
Results: P-cadherin was expressed from ACF through to hyperplastic and adenomatous polyps. Alterations in E-cadherin and catenin expression occurred later, with variant patterns in (i) ACF, (ii) hyperplastic polyps and serrated adenomas, and (iii) adenomatous polyps. P-cadherin present in adenomas was functional with regard to catenin binding, and its expression was independent of APC mutational status.
Conclusions: P-cadherin is aberrantly expressed from the earliest morphologically identifiable stage of colonocyte transformation, prior to changes in E-cadherin, catenin, and APC expression/mutation. P-cadherin expression alone does not predict tissue morphology, and such expression is independent of that of associated cadherins and catenins.
Keywords: cadherin, catenin, polyp, remodelling, colorectal cancer
Aberrant crypt foci (ACF) are the earliest identified neoplastic lesions in the colon.1 Apart from a high incidence of K-ras mutations,2,3 mutations of genes associated with colorectal tumorigenesis, particularly adenomatous polyposis coli (APC), rarely occur in ACF.4–6 The finding of unstable X chromosome methylation in ACF implies that epigenetic events may also play a role in their formation.7 Aberrant crypt formation and the epithelial remodelling necessary for polyp development most likely entail modified cell-cell and cell-matrix adhesion. Indeed, transgenic mouse models have established the significance of cadherin cell adhesion molecules in maintenance of normal intestinal crypt biology and architecture.8–10
Cell-cell adhesion in colonic crypts is performed by E-cadherin transmembrane proteins.11 Classical cadherins bind through their cytoplasmic domain to β- and γ-catenin12,13 which ultimately link cadherins to the actin cytoskeleton,14 helping to regulate cell morphology and polarity. Catenins are present in membranous (bound to cadherins) and cytoplasmic/nuclear (“free”) pools in cells. β-catenin present in the second of these pools can transcribe target genes such as c-myc15 and cyclin D116 on binding to T cell factor (TCF) and lymphocyte enhancing factor (LEF) transcription factors. The level of gene activation via β-catenin/TCF is regulated by the formation of a complex of APC, glycogen synthase kinase-3β, and axin, leading to β-catenin ubiquitination and degradation.17–21 Most APC mutations in colorectal adenomas lead to the formation of a protein truncated proximal to the β-catenin binding domains,22,23 thus eliminating APC mediated β-catenin degradation.
P-cadherin is expressed in placenta and stratified squamous epithelia24 but not in normal colon. P-cadherin null mice develop mammary gland hyperplasia, dysplasia, and abnormal lymphoid infiltration,25 demonstrating that loss of normal P-cadherin expression leads to cellular and glandular abnormalities. Our group has shown that P-cadherin is aberrantly expressed in inflamed and dysplastic colitic mucosa, with concomitant E-cadherin downregulation.26 Reduced E-cadherin expression in breast carcinoma and cervical squamous intraepithelial neoplasia is similarly accompanied by aberrant P-cadherin expression.27,28 These studies suggest interdependence of expression of E- and P-cadherin in some circumstances. E-cadherin is downregulated in colonic adenomas29 but expression of P-cadherin has not been studied in these lesions.
We sought to investigate whether P-cadherin was aberrantly expressed during aberrant crypt formation in the colon, and if such expression persisted during polyp development. Subsequently, we analysed if P-cadherin expression was correlated with or independent of alterations in expression and localisation of associated cadherins, catenins, and APC.
MATERIALS AND METHODS
Tissue specimens
Formalin fixed paraffin embedded sections of colonic hyperplastic (n=20) and sporadic adenomatous (n=22) polyps were obtained from the archives of University Hospital Birmingham, UK.
Adenomatous polyps were subclassified as follows: tubular n=11, tubulovillous n=11, mild dysplasia n=12, moderate dysplasia n=5, severe dysplasia n=5, size <1 cm n=17, size >1 cm n=5, right sided n=4, and left sided n=18.
Formalin fixed paraffin embedded sections of ACF (n=23) were provided from the archives at Case Western Reserve University, Cleveland, Ohio, USA. As most human ACF display some morphological alterations in histological sections, the ACF in this study were designated as follows: those with alterations that were mild and failed to meet the accepted criteria for dysplasia were classified as ACF with atypia and those with alterations that were more severe and met the previously defined criteria for dysplasia were classified as ACF with dysplasia.30
Formalin fixed paraffin embedded sections of serrated adenomas (n=20) were provided from the archives of University Hospital Birmingham and St Marks Hospital, London, UK. Sections of formalin fixed normal human skin and colon were used as positive and negative tissue controls for immunohistochemistry, immunofluorescence, and mRNA in situ hybridisation.
Frozen normal colon and adenomatous polyp tissue, stored at −80°C, was gained from the Department of Surgery tissue bank, Birmingham University, UK. These samples were derived from patients undergoing endoscopic bowel examination at University Hospital, Birmingham, UK.
HT 29 colorectal cancer cells were purchased from American Type Culture Collection (Manassas, USA) and maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum (Life Technologies, Paisley, UK) in a 5% CO2 humidified atmosphere at 37°C. These cells were used as positive cell controls for western blotting and co-immunoprecipitation experiments.
Immunohistochemistry
The streptavidin-biotin indirect immunoperoxidase method was used for immunohistochemistry. Sections (5 μm) were dewaxed, dehydrated, and endogenous peroxidase activity blocked by incubation in 3% H2O2 in methanol for 15 minutes. Microwave antigen retrieval was undertaken for 2× five minutes in 0.01 M trisodium citrate buffer, pH 6. Non-specific immunoreactivity was blocked with 20% normal goat serum in phosphate buffered saline (PBS), pH 7.3, for 30 minutes, and then sections were incubated overnight with primary antibody. Primary antibodies P-cadherin (IgG1, clone 56), E-cadherin (IgG2a, clone 36), β-catenin (IgG1, clone 14), and γ-catenin (IgG2a, clone 15) were used at a dilution of 1:300 (Transduction Labs, Lexington, Kentucky, USA). The following isotype matched control antibodies to cytokeratins were used in immunohistochemistry. Monoclonal IgG2a mouse antibody to Cam 5.2 was obtained from Becton Dickinson (Mountain View, California, USA) and used at 8 μg/ml. Monoclonal IgG1 mouse antibody to Ber EP4 was obtained from Dako (Copenhagen, Denmark) and used at 2.5 μg/ml. After washing with PBS, sections were incubated with biotinylated goat antimouse/rabbit IgG (Dako) according to the manufacturer's instructions for 20 minutes. After washing with PBS, sections were incubated with streptavidin-peroxidase conjugate (Dako) for 20 minutes and washed with PBS. Incubation of sections with diaminobenzidine tetrahydrochloride (Sigma, Poole, UK) at 1 mg/ml plus 1 μl/ml H2O2 was used to develop the peroxidase reaction for 10 minutes. Sections were counterstained with haematoxylin and mounted in depex (Sigma) after being taken through ethanol and xylene, and analysed on a light microscope.
In addition, absorption experiments to test antibody specificity were performed using cell lysates supplied by the manufacturer. Briefly, antibodies were reacted with a 10-fold excess of cell lysate (∼100 μg/ml) on a wheel at 4°C for 16 hours. The resulting solution was then used as per protocol for immunohistochemistry. The subsequent specificity of the antibody was determined by the absence of immunoreactivity on appropriate paraffin sections.
Isotype matched antibody controls employed for P-cadherin, E-cadherin, β-catenin, and γ-catenin demonstrated no difference between normal colon, ACF, hyperplastic, adenomatous, or serrated adenomatous polyps (data not shown).
Absorbance controls demonstrated specificity of antibodies by lack of immunoreactivity in control sections: skin for P-cadherin, and normal colon for E-cadherin, β-catenin, and γ-catenin.
Evaluation of immunohistochemistry
Immunostained material was assessed by light microscopy and scored by three independent observers (RGH, JAZJ, DSAS). The number of positive staining samples was noted, as was the percentage of positive crypts per lesion, subcellular localisation of immunoreactivity, and correlation between positive immunoreactivity and tissue phenotype/morphology. Immunoreactivity was compared with that in control sections of colon and skin which had been screened independently and deemed normal by a pathologist (DSAS). The presence of morphologically normal crypts within each section served as additional internal negative/positive controls depending on the antibody used.
Immunofluorescence
Tissue sections were blocked in 5% normal goat serum in 1% bovine serum albumin in PBS for 20 minutes and reacted with primary antibodies. Antibodies used were mouse anti-P-cadherin and E-cadherin (Transduction) used at 1:300, and rabbit polyclonal anti-β-catenin (Sigma) used at 1:200. Sections were washed with PBS and incubated for one hour with secondary antibody. Texas Red linked antimouse IgG1 and FITC linked antimouse IgG2a (Sigma) were used at a 1:800 dilution, and FITC linked antirabbit IgG (Stratech Scientific, Luton, UK) was used at a dilution of 1:200. Nuclei were stained with 4,6-diaminido-2-phenylindole (DAPI; Sigma) at a concentration of 0.1 μg/ml for one minute. Sections were washed in PBS, allowed to air dry, and mounted in immunofluorescence mounting medium (Sigma). Sections were analysed on an Olympus BX40 fluorescence microscope.
Western blotting
Adenomatous polyp biopsies taken from a −80°C tissue bank were defrosted on ice. The mucosa was dissected from the adherent connective tissue and cut into two approximately equal parts. One half was homogenised in protein lysis/sample buffer (0.0675 M Tris, pH 6.7, 2% sodium dodecyl sulphate, 5% β-mercaptoethanol, 10% glycerol, 0.001% bromophenol blue) and heated to 100°C for five minutes; the other was homogenised in immunoprecipitation buffer (20 mM Hepes, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 100 mM NaF). Residual insoluble debris from both aliquots was removed by centrifugation at 13 000 rpm for five minutes. Briefly, protein lysis samples were separated on an 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel and proteins were transferred onto Hybond PVDF nitrocellulose membranes (Amersham, High Wycombe, UK). Membranes were blocked overnight at 4°C in 10% Marvel in Tris buffered saline Tween (TBST), and incubated with the following primary antibodies at concentrations of 1:100 (P-cadherin rabbit polyclonal; Santa Cruz, Santa Cruz, USA), 1:200 (E-cadherin rabbit polyclonal; Santa Cruz), 1:4000 (β-catenin; Sigma), and 1:200 (cyclin D1, mouse IgG2a, clone DCS-6; Sigma) in TBST for one hour. Blots were washed extensively with TBST and probed with antimouse or rabbit horseradish peroxidase conjugated secondary antibody (Amersham) at a concentration of 1:10 000 for 30 minutes. After extensive washing in TBST, ECL reagent (Amersham) was used according to the manufacturer's instructions.
Co-immunoprecipitation
Adenoma samples lysed in immunoprecipitation buffer were precleared with 1% w/v protein-A sepharose (Sigma) for 30 minutes at 4°C. Supernatants were then incubated with polyclonal rabbit anti-β-catenin antibody (Sigma) for one hour at 4°C, followed by addition of 1% w/v protein-A sepharose for one hour. Samples were centrifuged at 7000 rpm for two minutes, beads were washed in TNT buffer (0.5% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.5) five times and resuspended in 250 μl protein lysis/sample buffer. Beads were then incubated at 100°C for five minutes and centrifuged at 13 000 rpm for five minutes. The resultant supernatant was analysed by western blotting.
mRNA in situ hybridisation
Sections (7 μm) were cut from normal colon and skin, and hyperplastic and adenomatous colorectal polyps, and mounted onto silanated slides.31 A 784 base pair cDNA fragment of P-cadherin was generated by digesting human P-cadherin cloned into the EcoRI site of pBR322 (a gift from S Hirohashi, National Cancer Centre Research Institute, Tokyo, Japan) with BlnI. This cDNA was random prime labelled to a specific activity of approximately 1×108cpm/μg 35S dCTP using the Amersham Megaprime labelling system. The prehybridisation treatments were as detailed previously.32,33 Briefly, these included sequential immersion in 0.2 M HCl (20 minutes); 0.2× standard saline citrate, 10 minutes; 5 μg/ml of proteinase K, pH 7.5, one hour at 37°C; 0.2% glycine in PBS; 0.4% paraformaldehyde in PBS, pH 7.0, 20 minutes; and freshly prepared 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8. Following proteinase treatment adjacent serial sections were also reacted with 1 mg/ml RNAse A in 0.5× standard saline citrate for one hour at 37°C. All sections were prehybridised for one hour at 37°C in 50% formamide, 1 mg/ml bovine serum albumin, 0.02% Ficoll, 0.02% polyvinyl pyrrolidone, 0.6 M NaCl, 0.2 mg/ml sheared salmon sperm DNA, 10 mM Tris (pH7.4), 0.5 mM EDTA, 10 mM dithiothreitol, and 10% dextran sulphate. Hybridisation with heat denatured 35S labelled probe (100 ng/ml prehybridisation solution) was carried out at 37°C overnight. After hybridisation the tissue sections were washed with a series of high stringency washes and dehydrated in 70% and 90% ethanol with 0.3 M ammonium acetate and air dried. Autoradiography was performed with Ilford K5 emulsion melted at 40°C and diluted 1:1 with distilled water. Slides were exposed at 4°C for 14 days and developed in Kodak D19 developer for five minutes, rinsed, fixed for five minutes and counterstained with haematoxylin and eosin.
Protein truncation test
The protein truncation test (PTT) was used for analysis of two overlapping exon 15 segments of the APC gene of adenoma samples from which DNA was extracted by standard methods. Two primer pairs amplified the overlapping segments 2 and 3, containing codons 658–1283 and 1099–1701, respectively. The nucleotide sequences for each primer pair were: segment 2: forward, 5`-GAGAACAACTGTCTACAAACT-3` and reverse, 5`-AGCTGATGACAAAGATGATAATG-3`; segment 3: forward, 5`-GTTTCTCCATACAGGTCACG-3` and reverse, 5`-GAGCC TCATCTGTACTTCTGC-3`. Forward primers were T7 modified by addition of a GGATCCTAATACGACTCACTATAGGGAGACCACCATG sequence to the 5` end containing a T7 promoter/translation initiation signal. PTT was performed using a kit (Promega, Southampton, UK), as previously described.34 Protein products were resolved by 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and bands visualised by autoradiography.
RESULTS
P-cadherin is expressed prior to disturbances in E-cadherin, catenins, or APC—expression in ACF
P-cadherin was expressed in 15/23 (65%) ACF. P-cadherin expression status was similar in ACF with only atypia (9/13 or 69%) versus ACF with dysplasia (6/10 or 60%). Such expression was both membranous and cytoplasmic in subcellular localisation, and was confined to histologically abnormal crypts. Staining was typically of greater intensity in the superficial crypt regions, particularly in those with obvious hyperplastic morphology (fig 1A ▶). This was seen against a background of normal E-cadherin expression and localisation in all examples (fig 1B ▶).
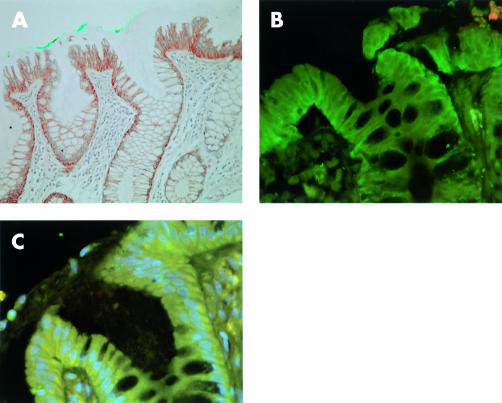
Figure 1.
Cadherin and catenin expression in aberrant crypt foci. (A) P-cadherin was expressed in cytoplasm and along cell boundaries, as demonstrated by immunohistochemistry (aberrant crypt marked with green dye) (magnification ×100). (B) Immunofluorescence image using FITC labelled E-cadherin and Texas Red labelled P-cadherin demonstrating general preserved membranous E-cadherin reactivity with cytoplasmic P-cadherin reactivity (magnification ×200). (C) Immunofluorescence image using FITC labelled β-catenin, Texas Red labelled P-cadherin, and 4,6-diaminido-2-phenylindole (DAPI) stained nuclei. Image shows loss of β-catenin from cell membranes with corresponding cytoplasmic/perinuclear staining (pale blue nuclei) and extensive cytoplasmic P-cadherin staining (yellow) (magnification ×200).
Although there were no ACF with marked nuclear β-catenin staining, β-catenin occasionally showed cytoplasmic localisation in abnormal crypts (fig 1C ▶) of P-cadherin positive and negative samples. γ-Catenin cytoplasmic translocation occurred in one sample, a dysplastic P-cadherin negative ACF. No nuclear γ-catenin was seen in any sample.
ACF with atypia and dysplasia thus showed similar patterns of cadherin and catenin expression (table 1 ▶), with an initial disturbance in P-cadherin expression in many cases.
Table 1.
Pattern of cadherin and catenin expression in aberrant crypt foci with atypia and dysplasia, categorised by P-cadherin expression status
| n | E-cadherin membranous (reduced) | β-Catenin cytoplasmic | γ-Catenin cytoplasmic | |
| ACF: atypia | ||||
| P-cadherin+ | 9 | 0 | 1 | 0 |
| P-cadherin− | 4 | 0 | 2 | 0 |
| ACF: dysplasia | ||||
| P-cadherin+ | 6 | 0 | 0 | 0 |
| P-cadherin− | 4 | 0 | 2 | 1 |
Universal P-cadherin expression in lesions with hyperplastic morphology
All hyperplastic polyps (n=20) and serrated adenomas (n=20) showed extensive P-cadherin immunoreactivity in hyperplastic regions of both lesions (fig 2A ▶–C) and dysplastic regions of serrated adenomas, by both immunohistochemistry and immunofluorescence (fig 3A, B ▶). This immunoreactivity was seen to be both membranous and cytoplasmic in nature. E-cadherin (fig 3C ▶), β-catenin, and γ-catenin maintained their normal basolateral membranous localisation in all samples except for one serrated adenoma which demonstrated nuclear β-catenin (fig 3D ▶).
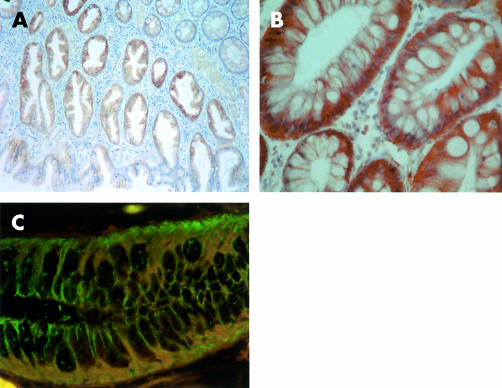
Figure 2.
Cadherin and catenin expression in hyperplastic colonic polyp. (A, B) P-cadherin was extensively expressed along membranes of cells in hyperplastic crypts, as demonstrated by immunohistochemistry (magnification ×60, ×200). (C) Immunofluorescence image using FITC labelled E-cadherin and Texas Red labelled P-cadherin hyperplastic crypt demonstrating (i) membranes staining purely for E-cadherin (green), (ii) membranes where P-cadherin and E-cadherin were colocalised (orange), and (iii) cytoplasmic P-cadherin immunoreactivity (red) (magnification ×200).
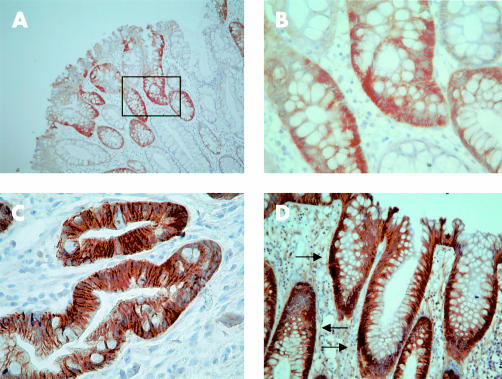
Figure 3.
Cadherin and catenin expression in serrated adenomatous colonic polyp. (A) P-cadherin was expressed along membranes and in the cytoplasm of areas displaying serrated architecture (magnification ×100). (B) Magnified (×200) photograph from boxed section of (A) showing membranous and cytoplasmic P-cadherin expression. (C) E-cadherin shows maintained membranous expression in all areas (magnification ×200). (D) β-catenin nuclear immunoreactivity (arrowed) in crypts with serrated architecture (magnification ×100).
Hyperplastic polyps and serrated adenomas defined by hyperplastic morphology therefore demonstrated analogous patterns of cadherin and catenin expression.
Expression of P-cadherin is independent of E-cadherin and catenin expression and localisation in adenomatous polyps
Our analysis of adenomatous polyps (n=22) demonstrated decreased membranous E-cadherin (fig 4A ▶) and β-catenin expression, with an increase in cytoplasmic/nuclear β-catenin expression, localised to dysplastic areas.
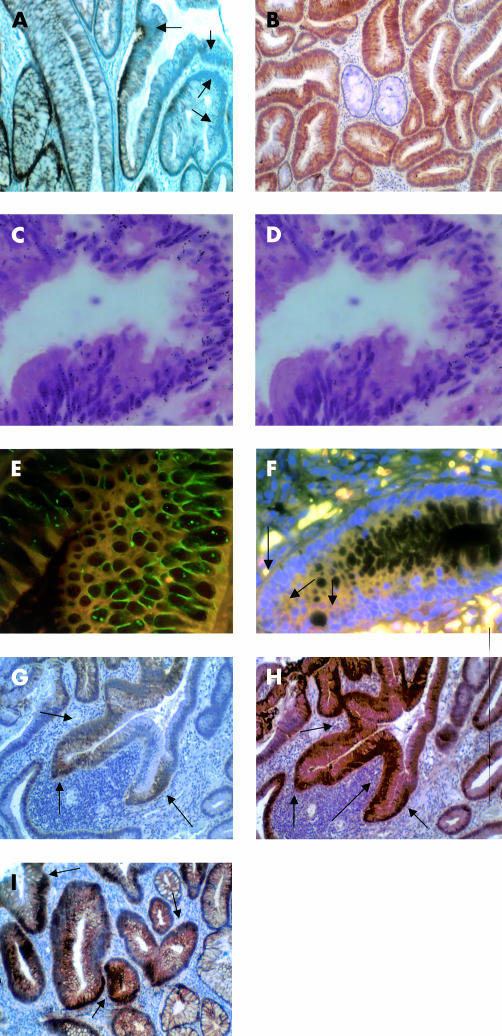
Figure 4.
Cadherin and catenin expression in adenomatous colonic polyps and bifurcating gland. (A) E-cadherin showed downregulation at membranes in dysplastic crypts (arrows) (magnification ×100). (B) P-cadherin was expressed along membranes and in the cytoplasm of dysplastic crypts but not in more morphologically normal crypts (centre) (magnification ×100). (C) P-cadherin mRNA (black grains) was seen throughout this dysplastic crypt with some serrated features (magnification ×100). (D) P-cadherin mRNA grains largely absent from RNAse treated serial section of a crypt seen in fig 3C ▶ (magnification ×100). (E) Immunofluorescence image using FITC labelled E-cadherin and Texas Red labelled P-cadherin dysplastic crypt demonstrating (i) membranes staining purely for E-cadherin (green), (ii) membranes where P-cadherin and E-cadherin are colocalised (orange), and (iii) cytoplasmic P-cadherin immunoreactivity (red) (magnification ×200). (F) Immunofluorescence image using FITC labelled β-catenin, Texas Red labelled P-cadherin, and 4,6-diaminido-2-phenylindole (DAPI) stained nuclei showing colocalised cytoplasmic β-catenin and P-cadherin in a dysplastic area of a crypt (yellow), with nuclear β-catenin reactivity in the same dysplastic areas (arrows) (magnification ×200). (G, H) Bifurcating dysplastic gland showing extensive cytoplasmic and membranous P-cadherin staining (G) (arrows) with coexisting cytoplasmic and nuclear β-catenin localisation (H) (arrows) (both magnification ×100). (I) γ-Catenin was translocated from the membrane to the cytoplasm and nucleus in dysplastic crypts (arrows) (magnification ×100).
Immunohistochemical and immunofluorescence data demonstrated P-cadherin expression in crypts with both mildly, moderately, and severely dysplastic phenotypes in 18/22 (82%) adenomas. The percentage of crypts demonstrating P-cadherin immunoreactivity varied from 25% to 100% among adenomas, with no trends on comparing tubular and tubulovillous examples, or polyp site or size. As with hyperplastic polyps, this expression was both cytoplasmic and membranous in nature (fig 4B ▶). In situ hybridisation demonstrated P-cadherin mRNA in dysplastic crypts (fig 4C ▶) but not in RNAse treated negative control serial sections (fig 4D ▶). P-cadherin expression did not correlate with position along the crypt axis. No obvious phenotype characterised adenomas that did not express P-cadherin.
In many dysplastic crypts, P-cadherin was coexpressed with E-cadherin but in some dysplastic crypts only P-cadherin or E-cadherin was expressed (fig 4E ▶). In a few severely dysplastic crypts, there was generally a lack of either P-cadherin or E-cadherin expression.
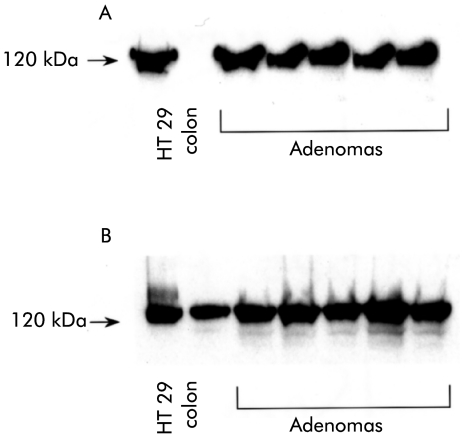
To support the immunohistochemistry and immunofluorescence data, western blots of adenoma and normal colon samples were run. Bands corresponding to P-cadherin (120 kDa) and E-cadherin (120 kDa) were demonstrated in adenomas but only E-cadherin was present in normal colon (fig 5 ▶).
Figure 5.
Western blot of HT 29 colorectal cell line (positive control), human normal colon, and colorectal adenomas for (A) P-cadherin (120 kDa) and (B) E-cadherin (120 kDa). E-cadherin was expressed in normal colon whereas P-cadherin was not. Both P-cadherin and E-cadherin were expressed in all adenomas analysed.
In all adenomas analysed, β-catenin was translocated from the membrane to the cytoplasm/nucleus in most dysplastic crypts (fig 4F ▶). This translocation occurred in both P-cadherin positive and negative crypts. P-cadherin expression and β-catenin cytoplasmic/nuclear localisation were also seen in all glands undergoing bifurcation (n=5) (fig 4G, H ▶).
Cytoplasmic and nuclear translocation of γ-catenin was identified in both P-cadherin positive and negative tubular adenomas (fig 4I ▶) but γ-catenin translocation from the membrane was present only in P-cadherin positive tubulovillous adenomas (n=8). As with β-catenin, γ-catenin translocation was seen in both P-cadherin positive and negative crypts. No nuclear γ-catenin localisation was seen in bifurcating glands.
Table 2 ▶ summarises expression patterns of cadherins and catenins in adenomas.
Table 2.
Pattern of cadherin and catenin expression in tubular and tubulovillous adenomas, categorised by P-cadherin expression status
| n | E-cadherin membranous (reduced) | β-Catenin cytoplasmic/ nuclear | γ-Catenin cytoplasmic/ nuclear | |
| Tubular adenomas | ||||
| P-cadherin+ | 9 | 9 | 9 | 6 |
| P-cadherin− | 2 | 2 | 2 | 1 |
| Tubulovillous adenomas | ||||
| P-cadherin+ | 9 | 9 | 9 | 8 |
| P-cadherin− | 2 | 2 | 2 | 0 |
P-cadherin is functional as regards catenin binding, and its expression is independent of APC mutation in adenomas
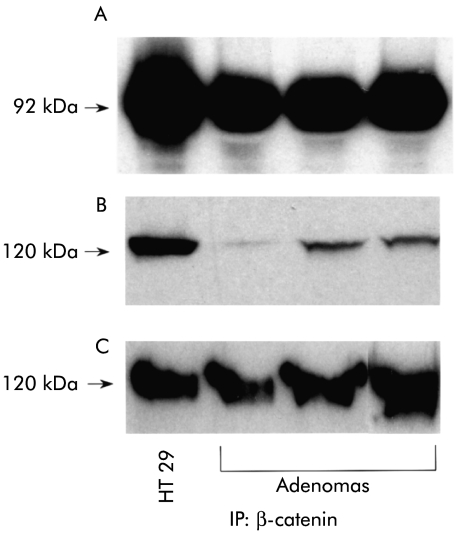
To assess whether P-cadherin was functional with regard to catenin binding, we examined whether aberrantly expressed P-cadherin formed complexes with β-catenin in adenomas by co-immunoprecipitation. In all instances where β-catenin was efficiently immunoprecipitated from adenomas, both P-cadherin and E-cadherin co-immunoprecipitated (fig 6 ▶), demonstrating that β-catenin binds to both P-cadherin and E-cadherin in these adenomas. Absence of bands on probing with anti-cyclin D1 (which does not bind cadherins or catenins) confirmed the absence of non-specific binding (data not shown).
Figure 6.
Western blot of HT 29 colorectal cell line (positive control) and colonic adenomas for (A) β-catenin (92 kDa), (B) P-cadherin (120 kDa), and (C) E-cadherin (120 kDa). β-catenin primary immunoprecipitation with subsequent immunoblotting for β-catenin, P-cadherin, and E-cadherin. Both P-cadherin and E-cadherin co-immunoprecipitated with β-catenin in the adenomas analysed.
Data obtained in hyperplastic polyps suggest that P-cadherin expression is independent of APC status. To test this hypothesis, PTT analysis of APC was carried out in four adenomas shown to express P-cadherin immunohistochemically. Of the four adenomas analysed, three showed a homozygous truncation in the mutation cluster region of exon 15 but one showed only wild-type expression from the mutation cluster region (fig 7 ▶). These data indicate that P-cadherin expression is an APC independent phenomenon.
Figure 7.
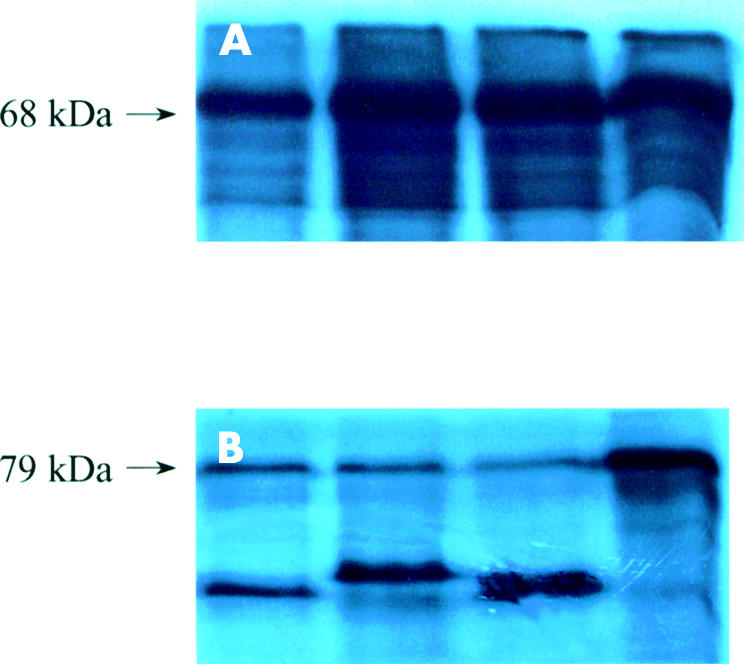
Autoradiograph showing protein truncation test products of APC exon 15 (A) segment 2 and (B) segment 3 of colonic adenomas. All adenomas showed homozygous wild-type expression from segment 2. Adenomas 1–3 demonstrated heterozygous truncation from segment 3 whereas the fourth adenoma showed homozygous wild-type expression from this segment.
DISCUSSION
We have shown that P-cadherin is aberrantly expressed at the earliest stages of aberrant colonic crypt formation, before disturbances in E-cadherin, β-catenin, and γ-catenin expression and localisation occur. Current knowledge would also indicate that such expression occurs prior to APC mutation, although we did not directly assess APC mutation in our samples. Such aberrant P-cadherin expression persists throughout polyp development in the colon. Common patterns of P-cadherin expression in the morphologically diverse lesions studied implies that P-cadherin expression alone cannot determine tissue architecture.
The data gained from the lesions analysed suggest that P-cadherin expression is regulated by a different mechanism to that of E-cadherin during colonocyte transformation. The usual regulation of differential spatial expression of the two proteins thus appears to be preserved in the lesions studied, and P-cadherin expression is not purely a response to downregulation of E-cadherin. Furthermore, our data imply that P-cadherin expression and APC mutation/catenin nuclear translocation are independent events, corroborated by the PTT data. We further demonstrated that P-cadherin expressed in adenomas binds β-catenin. The presence of catenin translocation from the membrane in adenomas implies however that P-cadherin expression is unable to prevent such catenin translocation in this situation.
The novel observation that P-cadherin was expressed in hyperplastic polyps, lesions thought to have low malignant potential, indicates that P-cadherin expression in polyps is unlikely to be directly implicated in the neoplastic process. Patterns of cadherin and catenin expression in these lesions were almost identical to those seen in serrated adenomas. Hyperplastic polyps and serrated adenomas display similarities in morphology,35 pattern of mucin secretion,36,37 and mutator phenotype38 and have been suggested to represent a histogenetic continuum.37 Our data on cadherin and catenin expression in these lesions are consistent with this hypothesis.
Near absence of nuclear β-catenin in serrated adenomas and ACF, together with a low reported incidence of APC mutation in the latter lesions, suggests that dysplastic clones present in these lesions may initially evolve by an alternative pathway from that requiring APC mutation.
Most adenomatous colonic polyps studied displayed a very high incidence of aberrant P-cadherin expression. A small number of adenomas did not express P-cadherin however. Possible explanations for this include: (i) P-cadherin expression occurred during evolution of the adenoma but this expression was transient and had been lost when the polyp was biopsied. (ii) Other proteins involved in stratified epithelia formation can substitute for P-cadherin in polyp formation. (iii) P-cadherin is a more labile antigen than the other cadherins and catenins that were expressed in these same lesions.
Our novel demonstration of γ-catenin nuclear translocation in adenomas raises the question of whether γ-catenin is leading to target gene transcription in these lesions. Previous reports support39 and refute40 a role for γ-catenin in oncogenesis. The transcription targets of γ-catenin are uncertain, and it is unclear therefore whether γ-catenin is involved in adenoma growth in the manner that β-catenin appears to be.
In conclusion, we have demonstrated aberrant P-cadherin expression from the earliest stage of abnormal crypt morphology, before alteration in E-cadherin, catenins, and APC occur, with persistence throughout polyp development. P-cadherin expression appears to be independent of expression of associated cadherins, catenins, and APC, and is unable to determine tissue morphology alone. Subsequent challenges will be to dissect the molecular mechanisms leading to aberrant P-cadherin expression and to elicit their downstream effects. In this manner manipulation of such molecules may become possible, leading to the prospect of therapies designed to reverse or prevent colorectal polyp development and progression.
Acknowledgments
This work was funded by a Research Training Fellowship from the Wellcome Trust to Robert Hardy, and in part by a Public Health Service grant CA66725 from the National Cancer Institute to Theresa Pretlow.
Abbreviations
ACF, aberrant crypt foci
APC, adenomatous polyposis coli
LEF, lymphocyte enhancing factor
TCF, T cell factor
PBS, phosphate buffered saline
TBST, Tris buffered saline Tween
DAPI, 4,6-diaminido-2-phenylindole
PTT, protein truncation test
REFERENCES
- 1.Siu I-M, Robinson DR, Schwartz S, et al. The identification of monoclonality in human aberrant crypt foci. Cancer Res 1999;59:63–6. [PubMed] [Google Scholar]
- 2.Pretlow TP, Brasitus TA, Fulton NC, et al. K-ras mutations in putative preneoplastic lesions in human colon. J Natl Cancer Inst 1993;85:2004–7. [DOI] [PubMed] [Google Scholar]
- 3.Nucci R, Robinson CR, Longo P, et al. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol 1997;28:1396–407. [DOI] [PubMed] [Google Scholar]
- 4.Jen S, Powell SM, Papadopoulos N, et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994;54:5523–6. [PubMed] [Google Scholar]
- 5.Smith AJ, Stern HS, Penner M, et al. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res 1994;54:5527–30. [PubMed] [Google Scholar]
- 6.Otori K, Konishi M, Sugiyama K, et al. Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer 1998;83:896–900. [DOI] [PubMed] [Google Scholar]
- 7.Sakurazawa N, Tanaka N, Onda M, et al. Instability of X chromosome methylation in aberrant crypt foci of the human colon. Cancer Res 2000;60:3165–9. [PubMed] [Google Scholar]
- 8.Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: Essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 1995;129:489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 1995;270:1203–7. [DOI] [PubMed] [Google Scholar]
- 10.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev 1996;10:985–96. [DOI] [PubMed] [Google Scholar]
- 11.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Ann Rev Biochem 1990;59:237–52. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA 1990;87:4246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jou TS, Stewart DB, Stappert J, et al. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA 1995;92:5067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimm DL, Koslov ER, Kebriaei P, et al. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA 1995;12:8813–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T-C, Sparks AB, Rago C, et al. Identification of c-myc as a target of the APC pathway. Science 1998;281:1509–12. [DOI] [PubMed] [Google Scholar]
- 16.Tetsu O, McCormick F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999;398:422–6. [DOI] [PubMed] [Google Scholar]
- 17.Hart MJ, de los Santos R, Albert IN, et al. Downregulation of beta-catenin by human axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 1998;8:573–81. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda S, Kishida S, Yamamoto H, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3 beta and beta-catenin and promotes GSK-3 beta-dependent phosphorylation of beta-catenin. EMBO J 1998;17:1371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol 1998;8:591–4. [DOI] [PubMed] [Google Scholar]
- 20.Kishida S, Yamamoto H, Ikeda S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stability of beta-catenin. J Biol Chem 1998;273:10823–6. [DOI] [PubMed] [Google Scholar]
- 21.Sakanaka C, Weiss JB, Williams LT. Bridging of beta-catenin and glycogen-synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci USA 1998;95:3020–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet 1992;1:229–33. [DOI] [PubMed] [Google Scholar]
- 23.Smith KJ, Johnson KA, Bryan TM, et al. The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA 1993;90:2846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoyama Y, Yoshida T, Terada M, et al. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol 1989;109:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radice GL, Ferreira-Cornwell MC, Robinson SD, et al. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol 1997;139:1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski JA, Bedford FK, Boulton RA, et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn's colitis. Lab Invest 1998;78:1155–67. [PubMed] [Google Scholar]
- 27.Palacios J, Benito N, Pizarro A, et al. Anomolous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression and pathological features. Am J Pathol 1994;146:605–12. [PMC free article] [PubMed] [Google Scholar]
- 28.De Boer CJ, van Dorst E, van Krieken H, et al. Changing roles of cadherins and catenins during progression of squamous intraepithelial lesions in the uterine cervix. Am J Pathol 1999;155:505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malizadeh A, Karayiannakis AJ, El-Hariry I, et al. Expression of E-cadherin-associated molecules (α-, β-, and γ-catenins and p120) in colorectal polyps. Am J Pathol 1997;150:1977–84. [PMC free article] [PubMed] [Google Scholar]
- 30.Siu I-M, Pretlow TG, Amini SB, et al. Identification of dysplasia in human colonic aberrant crypt foci. Am J Pathol 1997;150:1805–13. [PMC free article] [PubMed] [Google Scholar]
- 31.Rentrop M, Knapp B, Winter M, et al. Amino-lysilane treated glass slide support for ISH of keratin cDNAs to frozen sections under varying fixation and pretreatment conditions. Histochem J 1986;18:271–6. [DOI] [PubMed] [Google Scholar]
- 32.Hoyland JA, Freemont AJ, Sharpe P. Interleukin 6, IL-6 receptor and IL-6 nuclear factor gene expression in Paget's disease. J Bone Miner Res 1994;9:75–80. [DOI] [PubMed] [Google Scholar]
- 33.Hoyland JA, Sharpe P. Upregulation of c-fos proto oncogene expression in Pagetic osteoclasts. J Bone Miner Res 1994;9:1191–4. [DOI] [PubMed] [Google Scholar]
- 34.Van der Luijt RB, Khan PM. Protein truncation test for presympyomatic diagnosis of familial adenomatous polyposis. In: Adolph KW, ed. Methods in molecular genetics. New York: Academic Press, 1996:97–112.
- 35.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic polyps: serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 1990;14:524–37. [DOI] [PubMed] [Google Scholar]
- 36.Yao T, Kouzuki T, Kajiwara M, et al. `Serrated' adenoma of the colorectum, with reference to its gastric differentiation and its malignant potential. J Pathol 1999;187:511–17. [DOI] [PubMed] [Google Scholar]
- 37.Biemer-Huttmann A-E, Walsh MD, McGuckin MA, et al. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem 1999;47:1039–47. [DOI] [PubMed] [Google Scholar]
- 38.Iino H, Jass JR, Simms LA, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999;52:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolligs FT, Kolligs B, Hajira KM, et al. γ-catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of β-catenin. Genes Dev 2000;14:1319–31. [PMC free article] [PubMed] [Google Scholar]
- 40.Simcha I, Geiger B, Yehuda-Levenberg S, et al. Suppression of tumorigenicity by plakoglobin: an augmenting effect of N-cadherin. J Cell Biol 1996;133:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]