Abstract
Background and aims: Sigma ligands display antisecretory activity against various secretagogues, suggesting antidiarrhoeal properties. In this study, we evaluated: (i) the antidiarrhoeal effect of JO 2871, a high affinity sigma ligand, in three models of toxigenic diarrhoea in mice; and (ii) the site and mechanism of action of this compound.
Methods: Faeces were collected after toxin or vehicle administration in male DBA2 or NMRI mice. Diarrhoea was determined by cumulative stool weight (mg) over a 120 minute period. Diarrhoea was induced by intravenous administration of Salmonella enteriditis lipopolysaccharide (LPS), or oral administration of Escherichia coli heat stable (E coli-sta) or Clostridium difficile toxins. Two sigma ligands, igmesine and JO 2871, were administered either orally or intravenously, 60 and 30 minutes before the toxins, respectively. JO 2871 was also given orally 30 minutes after E coli-sta. In addition, JO 2871 was administered intracerebroventricularly five minutes before LPS and E coli-sta. BMY 14802 (1000 μg/kg orally), a sigma receptor antagonist, or cyclosomatostatin (CSS 1 μg/kg intravenously), a somatostatin antagonist, were given five minutes prior to JO 2871 in LPS, E coli-sta, and C difficile toxin treated mice. Gastric emptying and intestinal transit were evaluated after oral JO 2871 and BMY 14802 and intravenous CSS.
Results: Stool weight measured 120 minutes after administration of the toxins was significantly increased. Oral JO 2871 and igmesine dose dependently inhibited toxigenic diarrhoea in all models. ED50 values obtained using JO 2871 (1–20 μg/kg) were more than 40 times lower than those obtained with igmesine. Oral JO 2871 given after E coli-sta also inhibited diarrhoea in a dose dependent manner (ED50 50 μg/kg). Both sigma ligands were active by the intravenous route on LPS and E coli-sta induced stool weight increases. JO 2871 administered intracerebroventricularly failed to block this effect at any dose tested. Both BMY 14802 and CSS reversed the antidiarrhoeal effect of oral JO 2871. JO 2871, BMY 14802, and CSS did not affect transit parameters.
Conclusions: JO 2871 exerts a potent oral antidiarrhoeal effect, acting peripherally through sigma sites and somatostatin release.
Keywords: sigma ligands, diarrhoea, Escherichia coli heat stable toxin, Clostridium difficile toxins, Salmonella enteriditis lipopolysaccharides, somatostatin
Diarrhoea is one of the most common maladies facing medicine. Intestinal pathogenic bacteria such as Escherichia coli (E coli), Salmonella enteriditis (S enteriditis), and Clostridium difficile (C difficile) cause diarrhoeal diseases by producing highly potent enterotoxins. Binding of these enterotoxins to the intestinal epithelium results in activation of cyclic nucleotide second messengers leading to intestinal water secretion. In contrast, intestinal water secretion depends on neural regulation, including intrinsic and extrinsic components. A large number of studies have indicated a regulatory effect of the enteric nervous system in intestinal secretion induced by intraluminal secretagogues. Thus intramural nervous reflexes have been shown to be responsible for a major part of secretion elicited by luminal cholera toxin,1 heat stable E coli enterotoxin (E coli-sta),2 bile salt,3 and secretion induced by noxious stimulation of the intestinal serosa.4 Extrinsic innervation is also involved in this regulation. Indeed, several peptides injected centrally have been shown to suppress toxins and prostaglandin E2 induced diarrhoea.5,6 Further capsaicin sensitive afferents are involved in E coli-sta induced diarrhoea.7 Secretion of water and electrolytes from the intestine results in hypovolaemia and decreased circulation to vital organs which causes high morbidity and mortality, especially in children and in the elderly. To date, oral rehydratation salts are widely used for the treatment of diarrhoea (cholera). In addition, acute but not profuse diarrhoea of various origins is commonly treated with orally active antidiarrhoeal drugs, such as opiates. However, opiate drugs, such as loperamide, have more pronounced effects on gut motility than on epithelial functions.8 Other drugs such as octreotide, a peptidergic analogue of somatostatin, must be administered subcutaneously or intravenously in severe cases, but this peptide exhibits side effects such as steatorrhoea and gall bladder stasis.9 There is still a need for a specific antisecretory agent which improves the severity of diarrhoeal illness without any related gastrointestinal motor effects or other side effects.
σ1 Receptors are a subtype of σ receptor known to bind diverse classes of pharmacological agents with high affinity. These agents include neurosteroids, antipsychotics, and dextrorotatory benzomorphans.10,11 σ1 Receptors are currently thought to be involved in various biological processes, such as learning, memory, and analgesia.12–15 The σ1 receptor has been cloned16,17 and its amino acid sequence does not resemble that of any mammalian protein. The sequence of σ1 receptors contains an endoplasmic reticulum retention signal close to the N terminus, a binding domain for steroids, and one putative transmembrane region.
Sigma sites are widely distributed in the organism. They have been characterised in the central nervous system, liver, endocrine organs, lymphocytes, and also in the digestive tract. For example, using binding studies, Roman and colleagues18 provided evidence for the presence of σ receptors in the myenteric plexus of guinea pig small intestine. Moreover, autoradiographic studies19 revealed a dense distribution of σ sites in the mucosa and the submucosal plexus of guinea pig gastrointestinal tract.
Igmesine, a potent and selective sigma ligand,20 was used as a probe to demonstrate the effects of sigma receptor activation on modulation of intestinal secretion. Indeed, igmesine can exert a proabsorptive effect on jejunal mucosa in vitro through a neuronal pathway.21 Similarly, igmesine reverses the vasoactive intestinal polypeptide (VIP) induced increase in short circuit current22 and inhibits prostaglandin induced intestinal secretion in humans.23 Recently, we have shown that igmesine displays antidiarrhoeal activity in S enteriditis lipopolysaccharide (LPS), E coli-sta enterotoxin, and C difficile toxin induced diarrhoea in mice.24 In addition, Turvill and colleagues25 showed that igmesine inhibited cholera toxin and E coli-sta induced jejunal secretion in rats. However, as stimulated intestinal water secretion can be centrally mediated5,6 and igmesine can act centrally to modulate visceral functions,26,27 we have evaluated the site of action (central v peripheral) of JO 2871 (a follow up compound of igmesine) in this study.
Hence the aims of the present study were: (i) to evaluate the potent antidiarrhoeal effect of JO 2871 ((E)-3-(1-cyclopropylmethyl-2-azinanyl) - 1 - (3,4-dichlorophenyl) - 1 - propene (-)-isomer, HCl salt) compared with igmesine in three models of toxigenic diarrhoea (S enteriditis LPS, E coli-sta, and C difficile toxins); (ii) to determine its site of action (central v peripheral); and (iii) to attempt to elucidate its mechanism of action.
METHODS
Binding assays
Cloning
Human σ1 receptor was cloned from the neuroblastoma cell line SK-N-MC using reverse transcription-polymerase chain reaction (PCR) methods. Specific primers were designed according to the published sequence (Genebank accession number HSU75283). The entire cDNA sequence obtained was sequenced to check for the absence of PCR induced mutation, and was proved to be identical to the published one.
Transfection
Transfections were performed using Fugene 6 reagent (Roche, Meylan, France) according to the manufacturer's protocol. Briefly, COS7 cells were seeded in flasks (75 cm2) at a density of 700 000, and two days before transfection 10 μg DNA were added to Fugene 6 and incubated with the cells for six hours. Cells were harvested for performing the binding assays 24–48 hours following transfection in Tris HCl 50 mM, EDTA 1 mM, pH 7.4.
Binding experiments
Cell membrane suspensions (5 μg protein/tube) were incubated (120 minutes at 37°C) with [3H]-(+)- pentazocine (2.5 nM final concentration; 28 and 58 Ci/mmol; NEN, Paris, France) in a final volume of 500 μl. The buffer used as incubation medium was 50 mM Tris HCl, 1 mM EDTA, pH 7.4. Incubations were performed in borosicated glass tubes.
Non-specific binding was determined in the presence of haloperidol 1 μM. Increasing concentrations of the compounds (from 0.001 nM to 300 nM) were added to the incubation medium.
Incubation was stopped by washing the membranes on GF/B filters previously soaked in the incubation buffer with 0.5% polyethyleneimine added. Washing was performed with 3×2 ml of the incubation buffer maintained at 4°C.
Filters were transferred to scintillation picovials (Packard Meridien, USA) and added to 5 ml of Emulsifier Scintillator Plus (Packard). Counting of scintillation was perormed in a Tri-carb sprectometer (Packard).
Calculations
Inhibitor concentration 50 (IC50) values were determined by non-linear regression from the inhibition values of specific binding using GraphPad Prism software. Ki values were calculated from IC50 values according to Cheng and Prussoff.28 The Hill slope corresponding to each curve was calculated by the software.
In vivo experimental design
Faecal output measurement
Animals
Male DBA2 mice (20–25 g body weight; Janvier, Le Genest St Isle, France) or NMRI mice (30–35 g body weight; Harlan, Gannat, France) were individually housed in propylene cages and kept in a temperature controlled room (21 (1)°C). They were allowed free access to water and fed ad libitum with laboratory pellets (UAR Epinay-sur-Orge, France).
Faeces were collected and weighted every 30 minutes over a 120 minute period after administration of S enteriditis LPS, E coli-sta, or C difficile toxins A and B. Faecal output was determined by measuring cumulative stool weight (mg) over a 120 minute period.
The activity of the pharmacological agents tested was calculated according to the following formula:
 |
where T=mean stool weight (mg), collected over 120 minutes after toxin administration; P=mean stool weight under treatment (mg), 120 minutes after toxin administration; C=mean stool weight of control groups (mg), 120 minutes after vehicle administration.
S enteriditis LPS
Twenty eight groups of 10–12 male DBA2 were used. Diarrhoea was induced by intravenous administration of S enteriditis LPS (15 mg/kg). For oral treatment, JO 2871 (1–100 μg/kg), igmesine (0.1–1 mg/kg), or vehicle (saline 0.2 ml) were administered 60 minutes before induction of diarrhoea. For intravenous pretreatment, JO 2871 (0.3×10−3 to 3×10−3 μg/kg), igmesine (0.1–1 mg/kg), or saline (0.1 ml) were administered 30 minutes prior to S enteriditis LPS administration. In another series of experiments, JO 2871 (10−4 to 10 μg/kg) or saline was also given by the intracerebroventricular route five minutes before S enteriditis LPS. The somatostatin receptor antagonist cyclo (7-aminoheptanoyl-PHE-D-TRP-LYS-THR(BZL)) (cyclosomatostatin (CSS) 1 μg/kg intravenously)29 or the sigma receptor antagonist BMY 14802 (1 mg/kg orally)30 were given to S enteriditis LPS treated mice five minutes prior to JO 2871 (10 μg/kg orally). The effect of these two antagonists (CSS, BMY 14802) alone was also evaluated in two distinct groups of mice.
E coli-sta
Twenty five groups of 10–12 male NMRI mice were used. Diarrhoea was induced by oral administration of E coli-sta (70 μg/kg). JO 2871 (10–1000 μg/kg), igmesine (0.5–1 mg/kg), or saline (0.2 ml) was administered orally 60 minutes prior to induction of diarrhoea. JO 2871 or saline was also injected intracerebroventricularly (5 μl at doses from 0.1×10-3 to 10 μg/kg) or intravenously (0.1 ml at doses of 0.1–100 μg/kg) five and 30 minutes, respectively, before gavage with the E coli-sta solution. Igmesine was also given intravenously (500–3000 μg/kg) 30 minutes prior to E coli-sta administration. In two other groups, CSS (1 μg/kg intravenously) and BMY 14802 (1000 μg/kg orally) were given five minutes before JO 2871 (50 μg/kg orally) or saline in E coli-sta treated mice.
Preventive versus curative effect
In a separate series of experiments, five groups of 10–12 male NMRI mice were used. JO 2871 (1–1000 μg/kg) or saline (0.2 ml) was administered orally 30 minutes after oral administration of E coli-sta (70 μg/kg) and faecal output was evaluated over 120 minutes after toxin administration, as previously described.
C difficile toxins
Eleven groups of 10–12 male NMRI mice (30–35 g body weight) were used. Diarrhoea was induced by oral administration of a C difficile A and B toxin mixture (5 ng/mice). JO 2871 (0.01–1000 μg/kg), igmesine (500–3000 μg/kg), or saline (0.2 ml) were administered orally 60 minutes before the C difficile toxins. CSS (1μg/kg intravenously) and BMY (1000 μg/kg orally) were also administered five minutes before JO 2871 (50 μg/kg orally) in C difficile toxin treated animals.
Transit evaluation
Four groups of 10–12 male NMRI mice were used. Gastric emptying (GE) and small intestinal transit were assessed according to the technique described by Porreca and Burks.31 Animals received an oral test meal consisting of 0.5 ml of reconstituted milk (1 g of milk powder in 3 ml of water containing 1 μCi/ml of 51Cr sodium chromate). Thirty minutes later, the animals were sacrificed by cervical dislocation and the stomach and small bowel removed. These organs were placed on a ruled template, and the intestine was cut into 10 segments of equal length. Then the stomach, intestinal segments, and proximal colon were placed into individual test tubes and counted in a gamma radiation counter for two minutes. GE was calculated as a per cent of the total counts found in the small intestine and colon. Intestinal transit was evaluated by the described geometric centre (GC) technique32 according to the following formula:
 |
JO 2871 (1000 μg/kg orally) and BMY 14802 (1000 μg/kg orally) were administered 60 minutes prior to the test meal. CCS (1 μg/kg intravenously) was given 30 minutes before the test meal.
Drugs and chemicals
S enteriditis LPS, E coli-sta, and CSS were purchased from Sigma Chemical Co (St Louis, Missouri, USA). C difficile toxin A and B were provided by Dr G Corthier (INRA Jouy en Josas, France). BMY 14802 was synthesised at Pfizer Global R&D, Fresnes Laboratories.
Statistical analysis
Differences in stool weight between toxin treated mice and controls, as well as transit parameters between JO 2871, BMY14802, and CCS treated mice and controls were determined using ANOVA followed by the unpaired t test. The criterion for statistical significance was set at p<0.05. Comparisons between the different treatments were performed using Dunnett's test. The same criteria for statistical significance were applied.
RESULTS
Binding
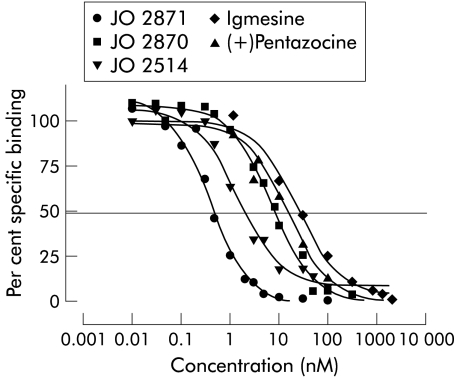
JO 2871 displayed a Ki value (0.26 (0.10) nM) on cloned human σ1 receptor that was approximately 30-fold higher than that of its corresponding stereoisomer JO 2870 (Ki 6.7 (4.31) nM), 70-fold higher than that of igmesine (Ki 18 (3) nM), and 40-fold higher than that of (+) pentazocine (Ki 8.2 (1.2) nM). The racemate (JO 2514) corresponding to JO 2871 displayed a Ki of 1.36 (0.81) nM (table 1 ▶).
Table 1.
Comparative affinities for cloned human σ1 receptor of JO 2871, its stereoisomer JO 2870, and the corresponding racemate JO 2514, with igmesine and pentazocine
| Compound | Ki (nM) | nH |
| JO 2871 | 0.26 (0.10) | 1.34 (0.35) |
| JO 2870 | 6.7 (4.3) | 0.98 (0.24) |
| JO 2514 | 1.4 (0.8) | 1.3 (0.2) |
| Igmesine | 18 (3) | 1.03 (0.05) |
| (+)Pentazocine | 8.2 (1.2) | 0.84 (0.13) |
Results are mean (SEM) of three separate determinations.
All of these compounds dose dependently displaced the specific binding of [3H]-(+)-pentazocine with total inhibition of specific binding with increasing concentrations. The Hill slope (nH) was very close to 1 in all cases (table 1 ▶), suggesting that the competition curves were following the law of mass action (fig 1 ▶).
Figure 1.
Dose dependent displacement curves of specific binding of JO 2871, its stereoisomer (JO 2870), and the racemate (JO 2514) on cloned human σ1 receptor compared with igmesine and (+)pentazocine. The curves represent displacement of [3H]-(+)-pentazocine by increasing concentrations of the compounds. Values are means (SEM) of three separate experiments.
Influence of toxins in mice
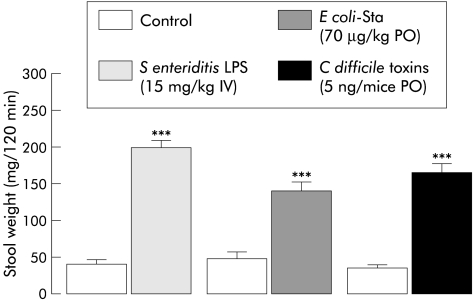
All toxins used in this study resulted in emission of watery faeces and an increase in faecal output over the 120 minute period after their administration (fig 2 ▶). Stool weight increases compared with controls after S enteriditis LPS, E coli-sta, and C difficile toxin A and B were 452%, 360%, and 390%, respectively.
Figure 2.
Effect of Salmonella enteriditis lipopolysaccharide (LPS) (15 mg/kg intravenously (IV)), Escherichia coli heat stable toxin (E coli-sta 70 μg/kg orally (PO)), and Clostridium difficile toxin (5 ng/mice PO) administration on 120 minute total faecal output in mice (mean (SEM), n=10–12). ***Significantly different (p<0.001) from control.
Oral antidiarrhoeal effect of JO 2871 versus igmesine
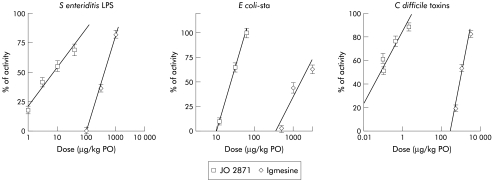
Both sigma ligands (JO 2871 and igmesine) given orally reduced 120 minute faecal output in a dose dependent manner in the three models of toxigenic diarrhoea tested. However, JO 2871 exerted its effect at significantly lower doses than igmesine in the three models. Indeed, for S enteriditis LPS induced diarrhoea, efficacy dose 50 (ED50) values were 10 and 415 μg/kg for JO 2871 and igmesine, respectively (fig 3 ▶). JO 2871 and igmesine also blocked E coli-sta induced diarrhoea with ED50 values of 20 and 1603 μg/kg, respectively (fig 3 ▶). Finally, they inhibited C difficile toxin induced diarrhoea in NMRI mice with an ED50 of 1 μg/kg for JO 2871 and 1090 μg/kg for igmesine (fig 3 ▶).
Figure 3.
Comparative efficacy (% of activity) of JO 2871 and igmesine administered orally (PO) on Salmonella enteriditis lipopolysaccharide (LPS), Escherichia coli heat stable toxin (E coli-sta), and Clostridium difficile toxin induced diarrhoea. Note the efficacy of JO 2871 administered orally on all toxigenic diarrhoeas tested, at doses 40–1000 times lower than those of igmesine.
JO 2871 administered at a maximal dose active on the models of diarrhoea (1000 μg/kg) did not affect basal faecal output (table 2 ▶).
Table 2.
Comparative influence of oral and intravenous administrations of JO 2871 on basal faecal output
| Vehicle | JO 2871 (1000 μg/kg orally) | JO 2871 (10 μg/kg intravenously) | |
| Faecal output (mg) 0–120 min | 34 (12) | 35 (7) | 42 (9) |
Values correspond to the total amount of faeces collected from 0 to 120 minutes after treatment with JO 2871.
Preventive versus curative effect of JO 2871
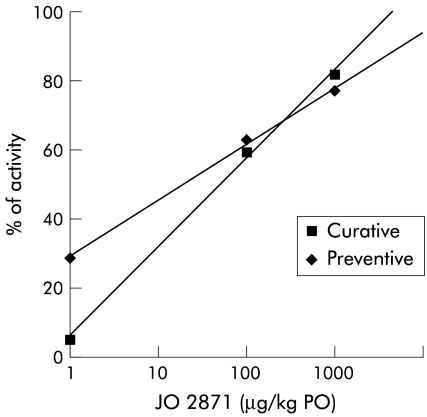
JO 2871 given orally after toxin administration (E coli-sta) also reduced faecal output over 120 minutes in a dose dependent manner in this model of toxigenic diarrhoea. The efficacy of JO 2871 was also observed at low doses (fig 4 ▶). However, the ED50 value in this curative treatment (50 μg/kg) was 2.5-fold higher than the ED50 value corresponding to preventive treatment (fig 4 ▶).
Figure 4.
Comparative efficacy (% of activity) of JO 2871 administered orally (PO) before (preventive) and after (curative) Escherichia coli heat stable toxin on this toxigenic diarrhoea. Note the efficacy of both oral treatments in this model at low doses. The ED50 value of curative treatment was 2.5-fold higher than the ED50 value obtained for the preventive treatment.
Effect of JO 2871, BMY 14802, and CSS on transit
The effect of JO 2871 on transit parameters (GE and GC) was tested after oral administration of this compound at a maximal dose active on the models of diarrhoea (1000 μg/kg). The same parameters were evaluated after oral BMY 14802 and intravenous CSS administration at the doses used, which significantly antagonised the antidiarrhoeal effect of JO 2871. None of these compounds exhibited any effect on transit parameters (table 3 ▶).
Table 3.
Effect of oral (JO 2871 and BMY 14802) and intravenous (cyclosomatostatin (CSS)) administration on gastric emptying (GE) and intestinal transit in mice
| Gastric emptying (GE) (%) | Intestinal transit (GC units) | |
| Vehicle (saline 0.2 ml PO) | 44.8 (5.1) | 6.6 (0.3) |
| JO 2871 (1000 μg/kg PO) | 45.1 (4.9) | 6.4 (0.3) |
| BMY 14802 (1000 μg/kg PO) | 43.7 (4.4) | 5.9 (0.4) |
| CSS (1 μg/kg IV) | 44.2 (4.8) | 6.2 (0.1) |
Values for GE and geometric centre (GC) were determined 60 minutes after oral (PO) JO 2871 and BMY 14802 administration and 30 minutes after CSS intravenous (IV) administration.
Antagonism of JO 2871 antidiarrhoeal effect
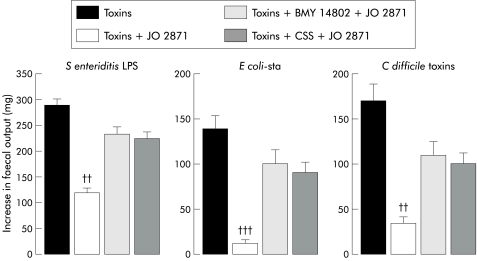
Administered orally five minutes before JO 2871 (10 μg/kg), BMY 14802 (1 mg/kg orally) reduced (by 66%) the antidiarrhoeal effect of JO 2871 on S enteriditis LPS induced increase in faecal output (fig 4 ▶). At the same dose, BMY 14802 orally also reduced (by 70% and 55%) the antidiarrhoeal effect of JO 2871 (50 μg /kg) on E coli-sta and C difficile toxin induced diarrhoea, respectively (fig 5 ▶).
Figure 5.
Antagonism by BMY 14802 and cyclosomatostatin (CSS) of the effect of JO 2871 on faecal output in mice. Salmonella enteriditis lipopolysaccharide (LPS), Escherichia coli heat stable toxin (E coli-sta), and Clostridium difficile toxins increased faecal output in mice. BMY 14802 (1 mg/kg orally) and CSS (1μg/kg intravenously) were administered five minutes prior to JO 2871 (10 μg/kg orally) in S enteriditis LPS (15 mg/kg intravenously) treated mice. BMY 14802 and CSS at the same doses were administered five minutes prior to JO 2871 (50 μg/kg orally) in E coli-sta (70 μg/kg) and C difficile toxin (5 ng/mice) treated mice. Significantly different (††p<0.01 and †††p<0.001) from toxins.
CSS (1 μg/kg) administered intravenously five minutes before JO 2871 (10 μg/kg) reduced (by 62%) the antidiarrhoeal effect of JO 2871 on S enteriditis LPS induced increase in stool weight (fig 4 ▶). At the same dose CSS also reduced (by 62% and 49%) the antidiarrhoeal effect of JO 2871 (50 μg/kg) on E coli-sta and C difficile toxin induced diarrhoea, respectively (fig 5 ▶).
Site of action of JO 2871
Intravenous treatment with JO 2871 dose dependently inhibited S enteriditis LPS induced diarrhoea. The per cent reductions were 27%, 38%, and 55% for 0.3, 1, and 3×10−3 μg/kg (table 4 ▶). In contrast, administrated by the intracerebroventricular route at doses of 0.03–10−3 μg/kg, JO 2871 did not modify S enteriditis LPS induced diarrhoea (table 4 ▶). Similarly, JO 2871 given intravenously dose dependently blocked E coli-sta induced diarrhoea (table 4 ▶) with per cent reductions of 50%, 80%, and 87% for 0.1, 1, and 10 μg/kg, respectively. At the highest dose tested (10 μg/kg), JO 2871 did not affect basal faecal output. When injected intracerebroventricularly at doses of 0.01–10 μg/kg, JO 2871 failed to inhibit the increased faecal output induced by S enteriditis LPS or E coli-sta (table 4 ▶).
Table 4.
Comparative efficacy of JO 2871 on Escherichia coli heat stable toxin (E coli-sta) and Salmonella enteriditis lipopolysaccharide (LPS) induced diarrhoea according to the route of administration (oral (PO) v intravenous (IV) v intracerebroventricular (ICV))
| ED 50 (μg/kg) | |||
| IV | PO | ICV | |
| E coli-sta (70 μg/kg PO) | 0.1 | 20 | >10 |
| S enteriditis LPS (15 mg/kg IV) | 4×10−4 | 10 | >10 |
DISCUSSION
The high affinity sigma ligand JO 2871, a follow up compound of igmesine, dose dependently inhibited toxigenic diarrhoea. This study shows for the first time that this effect was exerted through a sigma receptor and involved a somatostatin pathway. Interestingly, oral pretreatment with JO 2871 exerted an antidiarrhoeal effect at very low doses with an ED50 value of approximately 1 μg/kg. Igmesine, which displays lower affinity than JO 2871 for sigma receptors, also blocked toxigenic diarrhoea when administered orally but with approximately 1000 times lower efficacy (ED50 in the order of mg/kg). Furthermore, the oral antidiarrhoeal effect of JO 2871 was also demonstrated in established diarrhoea (induced by E coli-sta) at low doses since ED50 obtained for the curative treatment was 2.5-fold higher than the ED50 corresponding to preventive treatment.
The antidiarrhoeal properties of both sigma ligands shown in this study are in agreement with previous findings illustrating that selective sigma ligands, such as igmesine, decrease basal Isc in Ussing chamber experiments as an indication of their proabsorptive action in the mouse jejunum.21 Igmesine reduces both VIP induced intestinal secretion in vitro22 and prostaglandin induced secretion in healthy volunteers.23 More recently, it was shown that igmesine also inhibits neurally mediated enterotoxigenic secretion without affecting basal net water movements25 and exhibits potent antidiarrhoeal effects on various models of toxigenic diarrhoea.24
Although the structure of σ1 receptors is known, the biochemical basis subserving the action of σ1 receptors remains elusive. However, several lines of evidences have suggested that σ1 receptors may be related to regulation of intracellular Ca2+ in cardiac myocytes33–35 or in rat brain synaptosomes.36 More recently, the role of σ1 receptors in regulating intracellular Ca2+ was examined in NG108 cells.37 In this study, nanomolar concentrations of sigma ligands potentiated the bradykinin induced increase in cytosolic free Ca2+ concentration and this effect was blocked by a 21-mer antisense oligodeoxynucleotide against the cloned σ1 receptor. This potentiation may be linked to induction of β and β2 cPKC translocation from cytoplasmic to membrane compartments.38 Moreover, (+)pentazocine triggers the translocation of σ1 receptors from the endoplasmic reticulum towards the cytoplasmic membrane where σ1 receptors could exert a subsequent, powerful, and rapid regulation of neuronal excitability through a heterotrimeric G protein/PLC/PKC cascade. This finding could evidence a yet undescribed mode of rapid recruitment of membrane bound second messenger cascade (involving PLC and PKC) via activation of an intracellular single transmembrane domain receptor (σ1 receptor). As a functional complement to these studies, recent results39 have demonstrated that sigma ligands modulate electrical activity of isolated neurones in primary culture. This result is in agreement with a possible neuronal site for the antisecretory effects of igmesine, as evidenced by its blockade by tetrodotoxin (TTX).21 Igmesine binds σ1 sites in guinea pig brain cells20 and in cloned human recombinant brain cells with a Ki value of 10.4 nM. JO 2871 exhibits higher affinity for sigma receptors compared with igmesine with a Ki of 0.26 nM for the cloned receptors. The antidiarrhoeal effect of JO 2871 is antagonised by BMY 14802, a sigma/5-HT1A receptor antagonist, supporting an action through the sigma receptor. Furthermore, since the dose of BMY 14802 used did not affect transit parameters, we can suggest that sigma receptors are involved in the antisecretory effect of JO 2871. Autoradiographic studies demonstrated the presence of a dense population of σ binding sites in the mucosal and submucosal plexus of guinea pig gastrointestinal tract.19 However, no clear relationship between this specific localisation and the functional role of the σ receptors in the digestive tract has yet been evidenced. Previous studies have shown that igmesine peripherally administered stimulates a colonic motor response to feeding but without affecting colonic motility in the fasted state.40 Furthermore, such igmesine induced stimulation of postprandial motility accelerates colonic transit in rats.27 Also, in the present study we showed that JO 2871 administered orally in mice did not modify stool weight or transit parameters (GE and intestinal transit). Taken together, these observations reinforce that hypothesis that sigma ligands exert an antisecretory effect as acceleration of colonic transit seems to be inconsistent with the antidiarrhoeal effect observed. The antisecretory effect of igmesine has already been demonstrated after systemic administration23–25 even when administered after the secretory agent.25 In this study, we first demonstrated that JO 2871 displayed antidiarrhoeal activity when given orally in a preventive and curative way. The preventive effect was reproduced by intravenous but not intracerebroventricular administration, suggesting that JO 2871 exerts its action on intestinal secretion through a peripheral mechanism. This activity may involve the myenteric plexus and/or intrinsic sensory nerves as the antisecretory action of other sigma ligands such as igmesine was demonstrated to be TTX sensitive in vitro21 and in vivo.41
Enterotoxins bind enterocyte receptors, triggering intracellular events (for example, increase in cyclic nucleotide concentration) leading to water secretion. For example, E coli-sta binds cell surface receptors and activates two membrane associated cyclic GMP dependent kinases.42 This activation initiates a secretory signalling cascade characterised by a rise in intracellular concentrations of cyclic GMP.43 The secretory effect of C difficile toxin A has also been described to be mediated through a specific epithelial cell receptor, which has been characterised subsequently as an α-galactose and N-acetylglucosamine containing glycoprotein receptor coupled to a pertussis toxin sensitive G protein.44 Despite local binding to enterocytes, the secretory effect of enterotoxins in vivo depends mainly on local innervation.45 This neurally mediated secretory reflex may involve three enteric neurones: (i) a sensory neurone, which has dendrites extending from the mucosa and relaying information to an interneurone in the submucosal and myenteric plexus, (ii) an interneurone projecting to a secretomotor efferent and receiving additional enteric and extrinsic neuronal inputs to modulate this reflex. Indeed lidocaine, tetrodotoxin, and hexamethonium block the effects of bacterial enterotoxins such as E coli-sta and S typhimurium toxin.46 Moreover, the diarrhoeal effect of E coli-sta also involves sensory innervation and mast cell degranulation,47 underlying the fact that immune cells of the lamina propria participate in neuroimmune regulation of enterotoxin secretory effects in the gut. Similarly, capsaicin sensitive sensory afferent neurones and mast cells are involved in the secretory mechanism of C difficile toxin A.48 More recently a neuronal pathway has also been demonstrated to be involved in rotavirus induced intestinal secretion.49 Taken together, these findings reinforce the concept of activation of neuroimmune pathways in the effects of enterotoxins, and other secretory pathogens and sigma ligands may act at this level to suppress the secretory effect of pathogens.
Somatostatin, a peptide released by neuroendocrine cells, has inhibitory effects on gastric, pancreatic,9 and intestinal secretion of water and electrolytes.50 In addition, somatostatin and its analogue octreotide have been shown to be potent antidiarrhoeal agents in refractory diarrhoea.51 The main source of circulating immunoreactive somatostatin is the gastrointestinal tract52 and projections of somatostatin-immunoreactive neurones are localised predominantly in the submucous plexus with fewer fibres in the myenteric plexus.53 Rat colonic epithelium expresses multiple subtypes of somatostatin receptors, and activation of the SST-R2 subtype mediates inhibition of cAMP dependent ion secretion by both somatostatin and octreotide.54
Somatostatin and igmesine have already being shown to exert similar antisecretory effects on interleukin 1β induced colonic hypersecretion in rats and the effect of igmesine is blocked by CSS.55 Similarly, the antidiarrhoeal activity of JO 2871 is antagonised by CSS. Moreover, CSS had no effect on transit parameters. Taken together, these results suggest the involvement of a somatostatin pathway in the antisecretory mechanism of action of JO 2871. Consequently, we can hypothesise that JO 2871 acts peripherally on sigma receptors, probably located within the gut on somatostatinergic neurones, triggering release of somatostatin which in turn acts directly or indirectly on receptors located on enterocytes or on secretomotor neurones to alleviate toxin induced intestinal secretion. The hypothesis of a direct and indirect mechanism involved in the action of somatostatin is supported by previous findings showing a parallel reduction in cholera toxin induced net fluid secretion and in VIP release from the small intestine of the cat.56 Activation of sigma receptors inhibits acetylcholine release57 and favours noradrenaline release,58 and such effects may contribute to its antisecretory action. However, the lack of an anti-transit effect of JO 2871 is not in agreement with this possible mechanism of action.
In conclusion, the high affinity sigma ligand JO 2871 is an effective antidiarrhoeal agent against various toxigenic diarrhoeas. It prevents toxigenic diarrhoea but is also effective as a curative agent. Its activity at very low oral doses against a large spectrum of pathogenic diarrhoeas suggest that it acts distally via a common peripheral neuronal pathway. Based on all of these data we can speculate that JO 2871 provides a new basis for the development of potent antidiarrhoeal therapies.
Abbreviations
E coli-sta
Escherichia coli heat stable toxin
CSS, cyclosomatostatin
LPS, lipopolysaccharide
ED50, efficacy dose 50
IC50, inhibitor concentration 50
TTX, tetrodotoxin
GE, gastric emptying
GC, geometric centre
VIP, vasoactive intestinal polypeptide
REFERENCES
- 1.Cassuto J, Siewert A, Jodal M, et al. The involvement of intramural nerves in cholera toxin induced intestinal secretion. Acta Physiol Scand 1983;117:195–202. [DOI] [PubMed] [Google Scholar]
- 2.Eklund S, Jodal M, Lundgren O. The enteric nervous system participates in the secretory response to the heat stable enterotoxins of Escherichia coli in rats and cats. Neuroscience 1985;14:673–81. [DOI] [PubMed] [Google Scholar]
- 3.Karlström L. Mechanisms of bile salt-induced secretion in the small intestine. Acta Physiol Scand 1986;126:1–48. [PubMed] [Google Scholar]
- 4.Sjöqvist A, Cassuto J, Jodal M, et al. The effect of intestinal fluid transport of exposing the serosa to hydrochloric acid. Acta Physiol Scand 1982;116:447–54. [DOI] [PubMed] [Google Scholar]
- 5.Brown DR, Miller RJ. CNS involvement in the antisecretory action of (Met5)enkephalinamide on the rat intestine. Eur J Pharmacol 1983;17:441–4. [DOI] [PubMed] [Google Scholar]
- 6.Primi MP, Bueno L. Central nervous system influence of prostaglandin E2 on jejunal water and electrolyte transport in conscious dogs. Gastroenterology 1986;91:1427–32. [DOI] [PubMed] [Google Scholar]
- 7.Theodorou V, Marche P, Fioramonti J, et al. Sensitive innervation and mast cells are involved in E. col ista-induced diarrhea in mice. Neurogastroenterol Mot 1996;8:A193. [Google Scholar]
- 8.Kachel G, Ruppin H, Hagel J, et al. Human intestinal motor activity and transport: effects of a synthetic opiate. Gastroenterology 1986;90:85–93. [DOI] [PubMed] [Google Scholar]
- 9.Battershill PE, Clissold SP. Octreotide: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in conditions associated with excessive peptide secretion. Drugs 1989;38:658–702. [DOI] [PubMed] [Google Scholar]
- 10.Snyder SH, Largent BL. Receptor mechanisms in antipsychotic drug action: focus on sigma receptors. J Neuropsychiatry Clin Neurosci 1989;1:7–15. [DOI] [PubMed] [Google Scholar]
- 11.Su TP. Sigma receptors. putative links between nervous, endocrine and immune systems. Eur J Biochem 1991; 200:633–42. [DOI] [PubMed] [Google Scholar]
- 12.Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J Pharmacol Exp Ther 1994;271:1583–90. [PubMed] [Google Scholar]
- 13.Maurice T, Su TP, Parish DW, et al. Pre-084, a sigma selective pcp derivative, attenuates mk-801-induced impairment of learning in mice. Pharmacol Biochem Behav 1994;49:859–69. [DOI] [PubMed] [Google Scholar]
- 14.Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25-35 amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 1998;83:413–28. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard P, Maurice T, St Pierre S, et al. Neuropeptide Y and the calcitonin gene-related peptide attenuate learning impairments induced by mk-801 via a sigma receptor-related mechanism. Eur J Neuroscience 1997;9:2142–51. [DOI] [PubMed] [Google Scholar]
- 16.Hanner M, Moebius FF, Flandorfer A, et al. Purification, molecular cloning, and expression of the mammalian sigma (1)-binding site. Proc Natl Acad Sci U S A 1996;93:8072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad PD, Li HW, Fei YJ, et al. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J Neurochem 1998;70:443–51. [DOI] [PubMed] [Google Scholar]
- 18.Roman F, Pascaud X, Vauché D, et al. Evidence for a non-opioid sigma binding site in the guinea pig myenteric plexus. Life Sci 1988;42:2217–22. [DOI] [PubMed] [Google Scholar]
- 19.Roman F, Pascaud X, Chomette G, et al. Autoradiographic localization of sigma opioid receptors in the gastrointestinal tract of the guinea pig. Gastroenterology 1989;97:76–82. [DOI] [PubMed] [Google Scholar]
- 20.Roman F, Pascaud X, Martin B, et al. JO 1784, a potent and selective ligand for rat and mouse brain σ-sites. J Pharm Pharmacol 1990;42:439–40. [DOI] [PubMed] [Google Scholar]
- 21.Riviere PJM, Rao RK, Pascaud X, et al. Effects of neuropeptide Y, peptide YY, and sigma ligands on ion transport in mouse jejunum. J Pharmacol Exp Ther. 1993;264:1268–74. [PubMed] [Google Scholar]
- 22.Rao RK, Riviere PJM, Pascaud X, et al. Suppression of VIP-induced increases in ileal transport by sigma ligands. Gastroenterology 1995;108:A170. [Google Scholar]
- 23.Roze C, Bruley des Varannes S, Shi G, et al. Inhibition of prostaglandin-induced intestinal secretion by igmesine in healthy volunteers. Gastroenterology 1998;115:591–6. [DOI] [PubMed] [Google Scholar]
- 24.Theodorou V, Chovet M, Dassaud M, et al. Antidiarrheal effects of igmesine on various models of toxigenic diarrhea. Gastroenterology 1999;116:A868. [Google Scholar]
- 25.Turvill JL, Kasapidis P, Farthing MJG. The sigma ligand, igmesine, inhibits cholera toxin and Escherichia coli enterotoxin induced jejunal secretion in the rat. Gut 1999;46:564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gue M, Bueno L. Brain CCKA receptors mediate the colonic motor response to feeding in dogs. Peptides 1991;12:523–7. [DOI] [PubMed] [Google Scholar]
- 27.Gue M, Del Rio-Lacheze C, Junien JL, et al. Central CCK-8s receptors trigger the sigma ligand and 5HT1A agonists-induced acceleration of post-prandial colonic transit in rats. Neurogastroenterol Mot 1994;6:29–35. [Google Scholar]
- 28.Cheng Y, Prussoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–108. [DOI] [PubMed] [Google Scholar]
- 29.Fries Jl, Murphy WA, Sueiras-Diaz J, et al. Somatostatin antagonist analog increases GH, insulin, and glucagon release in the rat. Peptides 1982;5:811–14. [DOI] [PubMed] [Google Scholar]
- 30.Pascaud XB, Chovet M, Soulard P, et al. Effects of a new σ ligand, JO 1784, on cysteamine ulcers and duodenal alkaline secretion in rats. Gastroenterology 1993;104:427–34. [DOI] [PubMed] [Google Scholar]
- 31.Porreca F, Burks T. The spinal cord as a site of opioid effects on gastrointestinal transit in mouse. J Pharmacol Exp Ther 1983;227:22–7. [PubMed] [Google Scholar]
- 32.Miller M, Galligan JJ, Burks T. Accurate measurements of intestinal transit in the rat. J Pharmacol Meth 1981;6:211–17. [DOI] [PubMed] [Google Scholar]
- 33.Ela C, Barg J, Vogel Z, et al. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J Pharmacol Exp Ther 1994;269:1300–9. [PubMed] [Google Scholar]
- 34.Novakova M, Ela C, Barg J, et al. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur J Pharmacol 1995;286:19–30. [DOI] [PubMed] [Google Scholar]
- 35.Novakova M, Ela C, Bowen WD, et al. Highly selective sigma-receptor ligands elevate inositol 1,4,5-trisphosphate production in rat cardiac myocytes. Eur J Pharmacol 1998;353:315–27. [DOI] [PubMed] [Google Scholar]
- 36.Brent PJ, Herd L, Saunders H, et al. Protein phosphorylation and calcium uptake into rat forebrain synaptosomes—modulation by the sigma ligand, 1,3-ditolylguanidine. J Neurochem 1997;68:2201–11. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Maurice T, Su TP. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J Pharmacol Exp Ther 2000;293:788–98. [PubMed] [Google Scholar]
- 38.Morin-Surun MP, Collin T, Denavit-Saubie M, et al. Intracellular sigma1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc Natl Acad Sci U S A 1999;96:8196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriani O, Le Foll F, Galas L, et al. The sigma ligand (+)-pentazocone depresses M current and enhances calcium conductances in frog melanotrophs. Am J Physiol 1999;277:E73–80. [DOI] [PubMed] [Google Scholar]
- 40.Junien JL, Gué M, Pascaud X, et al. Selective stimulation of colonic motor response to a meal by sigma ligands in dogs. Gastroenterology 1990;99:684–9. [DOI] [PubMed] [Google Scholar]
- 41.Fargeau H, Riviere PJM, Junien JL, et al. Igmesine inhibition of VIP-induced jejunal hypersecretion in rats; involvement of endogenous somatostatin and nervous pathways. J Neurogastro Motil 1998;10:443. [Google Scholar]
- 42.Donowitz M, Welsh MJ. Regulation of mammalian small intestinal electrolyte secretion. In: Physiology of the gastrointestinal tract, 2nd edn. New York: Raven, 1987:1351–90.
- 43.Guarino A, Cohen M, Thompson M, et al. T84 cell receptor binding and guanyl cyclase activation by Escherichia coli heat-stable toxin. Am J Physiol 1987;253:G775–80. [DOI] [PubMed] [Google Scholar]
- 44.Percy WH, Burakoff R, Rose K, et al. In vitro evidence that rabbit colonic muscularis mucosae has a Clostridium difficile toxin receptor. Am J Physiol 1998;275:G402–9. [DOI] [PubMed] [Google Scholar]
- 45.Tantisira MH, Jodal M, Lundgren O. Effects of heat-stable Escherichia coli enterotoxin on intestinal alkaline secretion and transepithelial potential difference in the rat intestines in vivo. Scand J Gastroenterol 1990;25:19–28. [DOI] [PubMed] [Google Scholar]
- 46.Jodal M. Neuronal influence on intestinal transport. J Intern Med Suppl 1990;732:125–30. [DOI] [PubMed] [Google Scholar]
- 47.Theodorou V, Marche P, Fioramonti J, et al. Sensitive innervation and mast cells are involved in E-coli enterotoxin (E. coli-Sta)-induced diarrhea in mice. Neurogastroenterol Motil 1996;8:193. [Google Scholar]
- 48.Castagliuolo I, LaMont JT, Letourneau R, et al. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 1994;107:657–65. [DOI] [PubMed] [Google Scholar]
- 49.Lundgren O, Peregrin AT, Persson K, et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 2000;287:491–5. [DOI] [PubMed] [Google Scholar]
- 50.Van Thiel DH. Somatostatin—Its uses in gastroenterology. Dig Dis Sci 1989;34:4S. [Google Scholar]
- 51.Farthing MJ. The role of somatostatin analogues in the treatment of refractory diarrhoea. Digestion 1996;1:107–13. [DOI] [PubMed] [Google Scholar]
- 52.Schusdziarra V. Physiological significance of gastrointestinal somatostatin. Horm Res 1988;29:75–8. [DOI] [PubMed] [Google Scholar]
- 53.Costa M, Furness JB. Somatostatin is present in a subpopulation of noradrenergic nerve fibers supplying the intestin. Neuroscience 1984;13:911–19. [DOI] [PubMed] [Google Scholar]
- 54.Warhust G, Higgs WB, Fakhoury H, et al. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 1996;111:325–33. [DOI] [PubMed] [Google Scholar]
- 55.Theodorou V, Chovet M, Fioramonti J, et al. Comparative antisecretory effect of loperamide, somatostatin and the sigma ligand, igmesine, on IL-1-induced colonic hypersecretion in rats. Gastroenterology 1998;114:A423. [Google Scholar]
- 56.Eklund S, Sjöqvist A, Fahrenkrug J, et al. Somatostatin and methionine-enkephalin inhibit cholera toxin-induced jejunal net fluid secretion and release of vasoactive intestinal polypeptide in the cat in vivo. Acta Physiol Scand 1988;133:551–7. [DOI] [PubMed] [Google Scholar]
- 57.Campbell BG, Scherz MW, Keanna JFW, et al. Sigma receptors regulate contractions of the guinea pig ileum longitudinal muscle/myenteric plexus preparation elicited by both electrical stimulation and exogenous serotonin. J Neurosci 1989;9:3380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell BG, Bobker DH, Leslie FM, et al. Both the sigma-receptor-specific ligand (+) 3PPP and the PCP receptor-specific ligand TCP, act in the mouse vas deferens via augmentation of electrically evoked norepinephrine release. Eur J Pharmacol 1987;138:447–9. [DOI] [PubMed] [Google Scholar]