Abstract
Aims: Dyschesia can be provoked by inappropriate defecation movements. The aim of this prospective study was to demonstrate dysfunction of the anal sphincter and/or the musculus (m.) puborectalis in patients with dyschesia using anorectal endosonography.
Methods: Twenty consecutive patients with a medical history of dyschesia and a control group of 20 healthy subjects underwent linear anorectal endosonography (Toshiba models IUV 5060 and PVL-625 RT). In both groups, the dimensions of the anal sphincter and the m. puborectalis were measured at rest, and during voluntary squeezing and straining. Statistical analysis was performed within and between the two groups.
Results: The anal sphincter became paradoxically shorter and/or thicker during straining (versus the resting state) in 85% of patients but in only 35% of control subjects. Changes in sphincter length were statistically significantly different (p<0.01, χ2 test) in patients compared with control subjects. The m. puborectalis became paradoxically shorter and/or thicker during straining in 80% of patients but in only 30% of controls. Both the changes in length and thickness of the m. puborectalis were significantly different (p<0.01, χ2 test) in patients versus control subjects.
Conclusions: Linear anorectal endosonography demonstrated incomplete or even absent relaxation of the anal sphincter and the m. puborectalis during a defecation movement in the majority of our patients with dyschesia. This study highlights the value of this elegant ultrasonographic technique in the diagnosis of “pelvic floor dyssynergia” or “anismus”.
Keywords: pelvic floor dyssynergia, anorectal endosonography, anismus, dyschesia, constipation
Constipation is one of the most common gastrointestinal problems in Western Europe. Among other criteria, constipation is defined as less than three bowel movements per week for at least 12 weeks over one year.1 Dyschesia is an entity characterised mainly by difficult evacuation of stools, forced straining during defecation, and a sensation of incomplete evacuation after defecation. Although not specific for dyssynergia, a need for manual disimpaction or support of the pelvic floor can exist during defecation. Defecation frequency can be normal.
The aetiology of dyschesia can be divided into two groups. Organic aetiological factors (tumours, etc.) need to be differentiated from functional disorders. The most prevalent functional cause of dyschesia is pelvic floor dyssynergia where the patient does not sufficiently relax and sometimes even paradoxically contracts the pelvic floor muscles (anal sphincter and musculus (m.) puborectalis) during straining to defecate.2–8
To demonstrate insufficient relaxation of the anal sphincter and m. puborectalis during defecation, a manometric and/or electromyographic study of these pelvic floor muscles can be performed.
Anorectal endosonography was introduced by John Wild in 1949.9 The technique has continuously been improved since then. Flexible as well as rigid echoprobes can now be used to visualise the rectal wall and other pelvic floor structures. Two types of acoustic windows are used. Accurate imaging of rectal wall lesions10 and mapping of anal sphincter defects11 are possible with the axial window. The linear window not only allows good visualisation of the entire anal sphincter12 but also of the m. puborectalis.13 Moreover, a dynamic study of the pelvic floor muscles14 and surrounding structures14,15 is possible.
The aim of this prospective study was to examine the rectal wall, anal sphincter, and m. puborectalis in patients with a history of dyschesia using linear anorectal endosonography. The results were compared with data obtained from a comparable healthy control group. Dynamic ultrasound examination should be able to demonstrate dysfunction of the anal sphincter and/or m. puborectalis during straining to defecate in patients with dyschesia.
METHODS
Subjects
In the first part of the study, 20 healthy individuals without constipation were examined (19 women, one man; mean age 53.1 years (range 18–82)). All individuals without bowel complaints underwent a general internal examination. In the second part of the study, 20 consecutive patients with a history of dyschesia (excessive straining and a sensation of incomplete evacuation of stools) were examined (19 women, one man; mean age 52.9 years (range 21–81)). Organic pathology of the colon or anorectum was excluded by proctological examination, sigmoidoscopy and barium enema, or colonoscopy. To assess the agreement between both populations, a non-paired t test (age) and a χ2 test (sex) were used.
This study was approved by the Ethics Commission of the University Hospital of Antwerp.
Technique
Ultrasound examinations were performed with a rigid linear endorectal probe (model IUV 5060 or model PVL-625 RT; Toshiba, Japan) by the same investigator (MVO). A water filled balloon at the top of the probe serves as an acoustic window for the ultrasound waves. The frequency of the ultrasound waves is 5–7 MHz. After placing the patient in the left lateral decubitus position, the probe is introduced via the anus. By rotating the probe through 360°, the individual rectal wall layers, anal sphincter, m. puborectalis, and perirectal tissues (urinary bladder, cervix, and vagina in women; urinary bladder, prostate, and seminal vesicles in men) are clearly visualised.
Variables examined
Anal sphincter and m. puborectalis
The length and thickness of these muscles were examined at rest, during voluntary contraction (“squeezing”), and during a defecation movement (“straining”). The internal component of the anal sphincter is quite homogeneous and echo poor; the external component is more echo rich. The length and thickness of the anal sphincter were easily measured. Length was defined as the largest craniocaudal diameter (in mm). The cranial margin was defined as the transition from the internal muscular layer of the rectum to the internal anal sphincter, and the caudal margin as the most distal portion of the echo rich external sphincter. The thickness of the anal sphincter (internal+external) was defined as the largest diameter, measured from the most medial part (near to the anal canal lumen) to the most lateral part of the anal sphincter (in mm). The m. puborectalis was defined as the central part of the m. levator ani, originating from the pubic bone and joining the external component of the anal sphincter. A previous study in healthy individuals showed a decrease in length and an increase in thickness of the anal sphincter during voluntary contraction.14 During straining, the anal sphincter increased in length and decreased in thickness. The puborectalis muscle also relaxed, resulting in an increased length and decreased thickness.
Morphological anomalies
Morphological anomalies of the rectal wall, anal sphincter, m. puborectalis, and perirectal tissues were detected at rest and during straining. A rectocele was defined as ventral displacement of the rectal wall over a distance of at least 1 cm during straining to defecate, in comparison with the resting state.
All variables were examined in both populations.
Statistics
All data are expressed as mean (SEM), n being the number of examined individuals. Two way analysis of variance (ANOVA) was used for quantitative evaluation of the dimensions of the different pelvic floor muscles (length and thickness of the anal sphincter and of the m. puborectalis). Two different factors were taken into consideration. The within factor consisted of different measurements in each examined individual: the dimensions of a muscle at rest, during voluntary contraction, and during straining. The between factor was the presence or absence of dyschesia. Statistical significance of the within factor was further studied by a one way ANOVA test followed by a Student-Newman-Keuls correction to adjust the significance level to avoid a type I error. Statistical significance of the between factor was studied using an unpaired Student’s t test.
Changes in length and thickness of both the anal sphincter and m. puborectalis during straining versus the resting state were evaluated in a qualitative way using a χ2 test.
To evaluate differences in the occurrence of a rectocele during straining between both groups, a χ2 test was used. p values <0.05 were considered significant.
RESULTS
Anal sphincter at rest and under dynamic conditions
In all individuals, with or without dyschesia, the anal sphincter was clearly delineated and was easily measured in a standardised way (fig 1 ▶).
Figure 1.
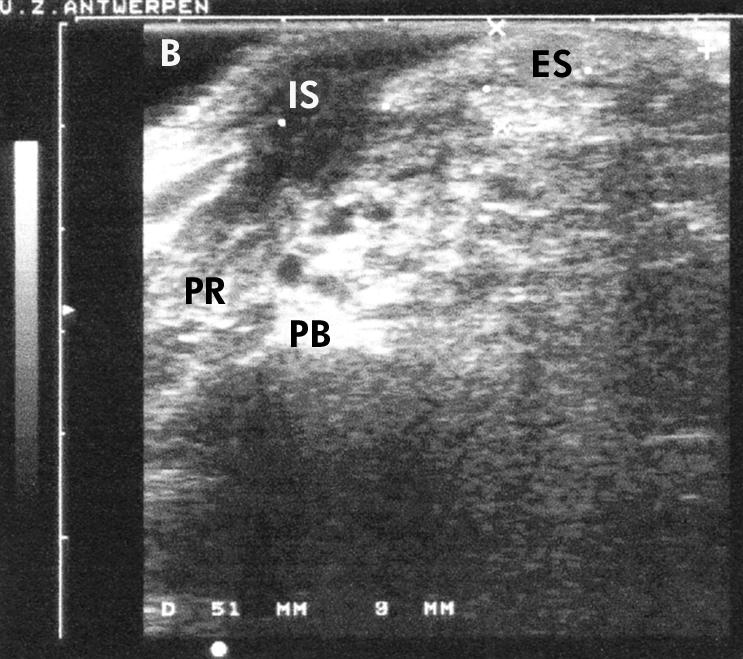
Anorectal linear endosonographic view of the anal sphincter, with an echo poor internal component (IS) and an echo rich external component (ES) in a healthy control subject. Measurements were expressed in millimetres (length=51 mm, thickness=9 mm). Note the musculus puborectalis (PR), water filled balloon (B), and pubic bone (PB).
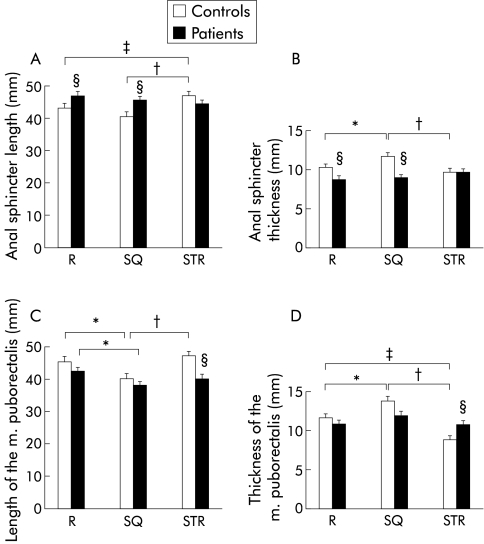
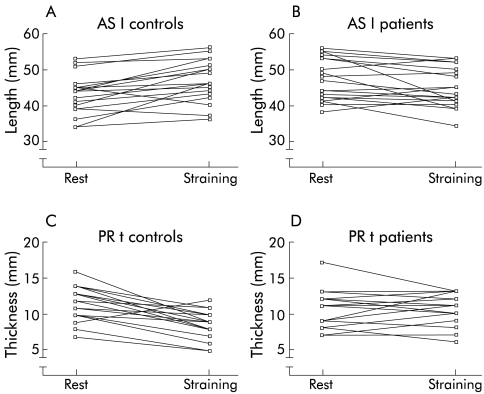
During voluntary contraction (squeezing), the length of the anal sphincter decreased in comparison with the resting state in both healthy individuals and patients. During straining, the length of the anal sphincter increased significantly in healthy individuals in comparison with dimensions at rest and during contraction (fig 2A ▶). In contrast, in constipated patients there was no significant difference between the length of the anal sphincter at rest, during voluntary contraction, and during a defecation movement (fig 2A ▶). To demonstrate the evolution of sphincter length for individual control subjects (fig 3A ▶) and patients (fig 3B ▶), data are shown on an X-Y plot during straining versus the resting state.
Figure 2.
Length (A) and thickness (B) of the anal sphincter, and length (C) and thickness (D) of the musculus (m.) puborectalis in patients with dyschesia compared with healthy controls, at rest (R), during voluntary contraction (SQ), and during straining (STR). Values are mean (SEM); n=20. Data were analysed using two way ANOVA. Significant differences: *p<0.05 between squeezing and rest; †p<0.05 between straining and squeezing; ‡p<0.05 between straining and rest; §p<0.05 between patients and controls.
Figure 3.
X-Y plot of the evolution of the length of the anal sphincter (AS l; A, B) and thickness of the musculus puborectalis (PR t; C, D) during straining versus the resting state in individual control subjects and patients.
During squeezing, the thickness of the anal sphincter increased in comparison with the resting state in both groups. Only in the control group was the thickness of the sphincter during contraction significantly different from the thickness at rest and during straining to defecate. In contrast, in patients with dyschesia, there was no significant difference between the thickness of the anal sphincter at rest, during voluntary contraction, or during straining (fig 2B ▶).
In our study, the anal sphincter increased in length in 18/20 subjects in the control group but in only 5/20 patients with dyschesia during straining versus at rest (table 1 ▶). Moreover, in 12/20 patients with dyschesia, the anal sphincter widened paradoxically during a defecation movement compared with at rest. Overall, when we combined the evolution of both parameters (length and thickness), the anal sphincter became paradoxically shorter and/or thicker during straining in 17/20 (85%) patients with dyschesia compared with only 7/20 (35%) control subjects.
Table 1.
Changes in length and thickness of the anal sphincter during straining versus the resting state. Number of patients with dyschesia and controls with a normal straining movement (increase in length, decrease in thickness) versus an abnormal straining movement (paradoxical decrease in length, increase in thickness) or absence of movement during defecation in comparison with the resting state
| Normal (↑) | Abnormal (↓) | No movement | p Value | |
| Length | ||||
| Controls (n=20) | 18 | 2 | 0 | |
| Patients (n=20) | 5 | 14 | 1 | <0.01 |
| Normal (↓) | Abnormal (↑) | No movement | p Value | |
| Statistical analysis by χ2 test. | ||||
| Thickness | ||||
| Controls (n=20) | 9 | 5 | 6 | |
| Patients (n=20) | 4 | 12 | 4 | NS |
M. puborectalis at rest and under dynamic conditions
In all individuals, with or without dyschesia, the m. puborectalis (left and right) was clearly delineated by rotating the probe laterally and was easily measured in a standardised way.
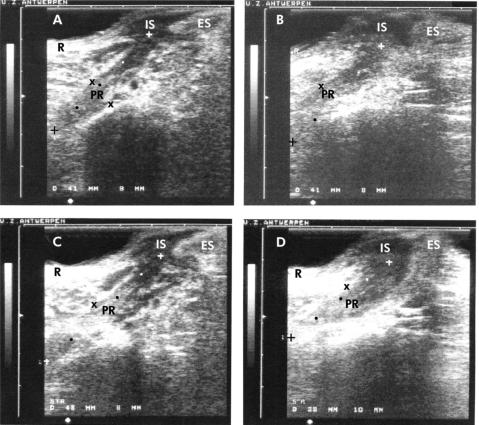
A linear endosonographic view of the m. puborectalis is shown in fig 4 ▶.
Figure 4.
Anorectal linear endosonographic view of the musculus (m.) puborectalis (PR) in a healthy control subject (A, at rest; C, during straining) and in a patient with dyschesia (B, at rest; D, during straining). Measurements are expressed in millimetres. An increase in length (from 41 mm to 48 mm) and decrease in thickness (from 9 mm to 8 mm) of the m. puborectalis was seen during straining in comparison with the resting state in the control subject. In the patient with dyschesia, a decrease in length (from 41 mm to 38 mm) and increase in thickness (from 8 mm to 10 mm) of the m. puborectalis was demonstrated during straining versus the resting state. Note the rectal wall (R) and the anal sphincter, with internal (IS) and external (ES) components.
In controls and in constipated patients, the length of the m. puborectalis decreased significantly during squeezing compared with the resting state. The length of the m. puborectalis during squeezing differed significantly from the length during straining in the control group in contrast with patients. Comparing the data between patients and controls, a significant difference in the length of the m. puborectalis was clearly demonstrated during straining (fig 2C ▶).
Figure 2D ▶ illustrates the data for the thickness of the m. puborectalis at rest, during voluntary contraction, and during a defecation movement within and between both groups. Widening of the m. puborectalis was seen in both populations during squeezing. The thickness of the m. puborectalis during squeezing was significantly different from the thickness at rest and during straining in the group of healthy individuals in contrast with the patients. In the control group, there was also a significant difference between the thickness of the m. puborectalis during straining versus at rest. In contrast, there was no significant difference between the thickness of the m. puborectalis at rest, during voluntary contraction, or during straining in patients with dyschesia. Comparing the data between the two populations, a significant difference in the thickness of the m. puborectalis was clearly demonstrated during straining. To demonstrate the evolution of the thickness of the m. puborectalis for individual control subjects (fig 3C ▶) and patients (fig 3D ▶), the data are shown graphically on an X-Y plot during straining versus the resting state.
In our study, the m. puborectalis increased in length in 12/20 healthy controls but in only 4/20 patients with dyschesia during straining versus the resting state (table 2 ▶). In 19/20 controls versus 10/20 patients with dyschesia, the m. puborectalis narrowed during straining compared with the resting state. Overall, when we combined the evolution of both parameters, the m. puborectalis became paradoxically shorter and/or thicker during straining in 16/20 (80%) patients with dyschesia compared with only 6/20 (30%) control subjects.
Table 2.
Changes in length and thickness of the musculus puborectalis during straining versus the resting state. Number of patients with dyschesia and controls with a normal straining movement (increase in length, decrease in thickness) versus an abnormal straining movement (paradoxical decrease in length, increase in thickness) or absence of movement during defecation in comparison with the resting state
| Normal (↑) | Abnormal (↓) | No movement | p Value | |
| Length | ||||
| Controls (n=20) | 12 | 5 | 3 | |
| Patients (n=20) | 4 | 15 | 1 | < 0.01 |
| Normal (↓) | Abnormal (↑) | No movement | p Value | |
| Statistical analysis by χ2 test. | ||||
| Thickness | ||||
| Controls (n=20) | 19 | 1 | 0 | |
| Patients (n=20) | 10 | 7 | 3 | < 0.01 |
Morphological anomalies
In our control group (n=20), one individual had an anomaly of the m. puborectalis, showing an extra branch.
In our constipated patients (n=20), noticeable ventral displacement of the rectum (rectocele) of at least 1 cm was seen in 13 patients, and in none of the control group (p<0.0001). Intussusception of the distal rectum into the proximal part of the anal canal was seen during straining in three patients. In two patients the characteristic five layer structure of the rectal wall, according to Boscaini and colleagues16 and Hildebrandt and colleagues,10 was disrupted. In these individuals, the thickness of the rectal wall exceeded the maximum normal thickness of 4 mm and the mucosa exceeded the maximum normal thickness of 1 mm, with an irregular and disrupted muscularis propria.
DISCUSSION
Dyschesia can be provoked by inappropriate function of the pelvic floor muscles. If the m. puborectalis and anal sphincter do not relax sufficiently during straining, the anorectal angle will not increase, preventing the descent of faeces from the rectum into the anal canal. The pelvic floor will not be displaced distally during straining. This leads to dyschesia with forced straining and a sensation of incomplete evacuation of stools. After a period of time, a persistent inappropriate defecation movement can lead to the formation of a rectocele or even intussusception of the distal rectum into the proximal anal canal. Paradoxical contraction of the pelvic floor muscles could eventually result in the development of a solitary rectal ulcer syndrome.13 The reason for the paradoxical movements of the pelvic floor during straining is still unclear.
Anal sphincter and m. puborectalis during contraction
During a normal voluntary contraction, a decrease in length and increase in thickness of the anal sphincter and of the m. puborectalis is observed both in controls and in patients. As no one in the control group or in the patient population suffered from faecal incontinence, this will not be discussed further.
Anal sphincter during straining
During straining to defecate, an increase in length and decrease in thickness of the anal sphincter is expected, in comparison with the dimensions of this muscle at rest.14 In 85% of our constipated patients however, a paradoxical shortening and/or increase in thickness was seen during straining versus the resting state. This paradoxical movement was observed in only 35% of healthy controls. This implies that 85% of our patients with dyschesia did not sufficiently relax, and even contracted their anal sphincter during a defecation movement. This paradoxical contraction of the sphincter apparently plays a role in the pathophysiology of dyschesia, obstructing evacuation of the anorectal contents.
M. puborectalis during straining
In healthy controls, elongation of the m. puborectalis is expected during straining to defecate.13 A paradoxical decrease in length and/or increase in thickness however was observed in most of our patients (80%) during straining compared with the dimensions at rest. This paradoxical contraction was observed in only 30% of healthy controls. Moreover, the dimensions of the m. puborectalis during straining were not significantly different from the dimensions during squeezing in our constipated patients while the squeezing dimensions were significantly different from the dimensions of this muscle at rest.
These data indicate that not only the anal sphincter but also the m. puborectalis is often contracted instead of relaxed during a straining effort. This paradoxical contraction plays an apparent role in the development of dyschesia. Direct visualisation and measurement of these functionally important pelvic floor muscles at rest and during straining facilitated the diagnosis of “anismus” or “pelvic floor dyssynergia” in the majority of our patient population.
Morphological anomalies
The occurrence of morphological anomalies sometimes indicated the presence of an abnormal defecation technique. The formation of a rectocele was seen during straining in 65% of our constipated patients. A rectocele is a frequent finding in women with dyschesia. The debate on whether the rectocele is the cause or consequence of dyschesia cannot be answered by our study, as has already been demonstrated by others.6 Intussusception of the distal rectum into the proximal anal canal was demonstrated during straining in three of our patients (15%). None of these morphological anomalies was demonstrated during straining in our control population.
Pelvic floor dyssynergia
The evolution of anal sphincter length and m. puborectalis length and thickness during straining versus the resting state appear to be reliable criteria for diagnosing a non-relaxing pelvic floor syndrome or pelvic floor dyssynergia. If we take into account two of these three criteria (anal sphincter length, m. puborectalis length, and thickness), whatever the combination, this diagnosis can be made in 12 patients (60%) in comparison with one control subject (5%). In this way, paradoxical contraction of the anal sphincter and of the m. puborectalis was clearly visualised by linear anorectal endosonography in more than half of our patients with dyschesia. Although our study was not double blind, our data confirm the results of other studies regarding the pathophysiology of dyschesia.2–8
The true incidence of pelvic floor dyssynergia as a cause of dyschesia is still not known. Most authors recognise the existence of an abnormal defecation technique in patients with dyschesia2–8 although some authors doubt the existence of a “non-relaxing pelvic floor syndrome”.17,18
Diagnostic armamentarium
To examine the pelvic floor, anorectal manometry, defecography, electromyography of the anal sphincter and pelvic floor muscles, and a balloon expulsion test can also be performed. These examinations cannot be replaced by anorectal endosonography as all of these techniques have different specific features. None of these techniques however is absolute in the diagnosis of pelvic floor dyssynergia.
Anorectal manometry shows incomplete or even absent relaxation of the external anal sphincter during straining in patients with dyschesia.7 However, structural anomalies cannot be evaluated. Anorectal endosonography has previously been compared with anorectal manometry in patients with defecation disorders. A correlation between manometric maximum squeezing pressure and ultrasonographic thickness of the external anal sphincter has been demonstrated by Schäfer and colleagues.19,20 In these studies however dynamic evaluation of the pelvic floor by means of ultrasonography was not performed. Moreover, the anal sphincter was measured in only one way (thickness) and with a radial probe.
Defecography allows structural and functional evaluation of the pelvic floor. Morphological anomalies (mucosal prolapse, intussusception, rectocele, etc) can be visualised directly during straining in patients with defecation disorders. Pelvic floor dyssynergia is suspected when a marked impression of the m. puborectalis on the distal rectal wall is detected, when evacuation time is prolonged, rectal emptying is incomplete, and normal perineal descent is impaired.21 Measurement of the anorectal angle with this technique does not seem to be reliable.21–24 Comparison between defecography and linear anorectal endosonography regarding perineal insufficiency and rectocele formation during straining has recently been made by Barthet and colleagues.15 A good correlation between the two techniques was demonstrated. Our ultrasonographic study also demonstrated several morphological anomalies indicating an abnormal defecation technique. However, defecography has several disadvantages. Direct visualisation of the m. puborectalis is not possible and the examination can be embarrassing for the patient, interfering with the interpretation of the results. Moreover, pelvic irradiation of defecography can be avoided by ultrasonography.
Electromyography shows an incomplete decrease in electrical activity of the external anal sphincter and the m. puborectalis in patients with dyschesia during straining.3,18 The presence of structural anomalies during straining can not be assessed electromyographically.
Future comparative studies of the different examination techniques, including linear anorectal endosonography, need to be performed to assess the gold standard in the diagnosis of pelvic floor dyssynergia. Because of the different features of all of these tests they will probably remain complementary in the evaluation of a patient with dyschesia. In our opinion, linear anorectal endosonography deserves its place in the evaluation of these patients. The technique is safe and easy to perform. Moreover, dynamic evaluation of the pelvic floor can be made, with accurate measurements of the anal sphincter and m. puborectalis.
CONCLUSIONS
With linear anorectal endosonography, the anal sphincter, m. puborectalis, and other structures of the pelvic floor can clearly be visualised. Accurate measurement of the length and thickness of the anal sphincter and the m. puborectalis at rest and under dynamic conditions is possible. With dynamic ultrasound examination, incomplete or even absent relaxation of the anal sphincter and of the m. puborectalis was seen in more than 50% of our patients with dyschesia. Hence our study highlights the value of this technique in the evaluation of patients with dyschesia and in the diagnosis of non-relaxing pelvic floor syndrome or pelvic floor dyssynergia.
Acknowledgments
Special thanks to T Moreels, MD, for statistical analysis and to engineer D Callens for image processing.
REFERENCES
- 1.Rome II. A multinational consensus document on functional gastrointestinal disorders. Gut 1999;45(suppl II):II 1–81.10369691 [Google Scholar]
- 2.Wasserman IF. Puborectalis syndrome (rectal stenosis due to anorectal spasm). Dis Colon Rectum 1964;7:87–98. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull GK, Lennard-Jones JE, Bartram CI. Failure of rectal expulsion as a cause of constipation: why fibre and laxatives sometimes fail. Lancet 1986;1:767–9. [DOI] [PubMed] [Google Scholar]
- 4.Bartolo D, Duthie G. Pelvic floor incoordination. In: Kamm M, Lennard-Jones, eds. Constipation. Hampshire, UK: Wrightson Biomedical Publishing Ltd, 1994:77–85.
- 5.Papachrysostomou M, Smith AN. Effects of biofeedback on obstructive defecation—reconditioning of the defecation reflex? Gut 1994;35:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pucciani F, Rottoli ML, Bologna A, et al. Anterior rectocele and anorectal dysfunction. Int J Colorect Dis 1996;11:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Sutphen J, Borowitz S, Ling W, et al. Anorectal manometric examination in encopretic-constipated children. Dis Colon Rectum 1997;40:1051–5. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol 1998;93:1042–50. [DOI] [PubMed] [Google Scholar]
- 9.Wild JJ. The use of ultrasonic pulses for the measurement of biologic tissues and the detection of tissue density changes. Surgery 1950;27:183–8. [PubMed] [Google Scholar]
- 10.Hildebrandt U, Feifel G, Ecker K. Rectal endosonography. Bailliére’s Clin Gastroenterol 1989;3:531–41. [DOI] [PubMed] [Google Scholar]
- 11.Sultan AH, Kamm MA, Hudson CN, et al. Anal sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905–11. [DOI] [PubMed] [Google Scholar]
- 12.Van Outryve MJ, Pelckmans PA, Fierens H, et al. Transrectal ultrasonographic examination of the anal sphincter. Acta Gastroenterol Belg 1994;57:26–7. [PubMed] [Google Scholar]
- 13.Van Outryve MJ, Pelckmans PA, Fierens H, et al. Transrectal ultrasound study of the pathogenesis of solitary rectal ulcer syndrome. Gut 1993;34:1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Outryve MJ, Pelckmans PA, Michielsen PP, et al. Value of transrectal ultrasonography in Crohn’s disease. Gastroenterology 1991;101:1171–7. [DOI] [PubMed] [Google Scholar]
- 15.Barthet M, Portier F, Heyries L, et al. Dynamic anal endosonography may challenge defecography for assessing dynamic anorectal disorders: results of a prospective pilot study. Endoscopy 2000;32:300–5. [DOI] [PubMed] [Google Scholar]
- 16.Boscaini M, Moscini PL, Montori A. Transrectal ultrasonography: interpretation of normal intestinal wall structure for the preoperative staging of rectal cancer. Scand J Gastroenterol 1986;21(suppl 123):87–98. [DOI] [PubMed] [Google Scholar]
- 17.Jones PN, Lubowski DZ, Swash M, et al. Is paradoxical contraction of puborectalis muscle of functional importance? Dis Colon Rectum 1987;30:667–70. [DOI] [PubMed] [Google Scholar]
- 18.Schouten WR, Briel JW, Auwerda JJ, et al. Anismus: fact or fiction? Dis Colon Rectum 1997;40:1033–41. [DOI] [PubMed] [Google Scholar]
- 19.Schäfer A, Enck P, Heyer T, et al. Endosonography of the anal sphincters: incontinent and continent patients and healthy controls. Z Gastroenterol 1994;32:328–31. [PubMed] [Google Scholar]
- 20.Schäfer R, Heyer T, Gantke B, et al. Anal endosonography and manometry. Comparison in patients with defecation problems. Dis Colon Rectum 1997;40:293–7. [DOI] [PubMed] [Google Scholar]
- 21.Halligan S, Bartram CI, Park HJ, et al. Proctographic features of anismus. Radiology 1995;197:679–82. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen J, Stein P, King DW, et al. The anorectal angle is not a reliable parameter on defaecating proctography. Aust N Z J Surg 1993;63:105–8. [DOI] [PubMed] [Google Scholar]
- 23.Penninckx F, Debruyne C, Lestar B, et al. Intraobserver variation in the radiological measurement of the anorectal angle. Gastrointest Radiol 1991;16:73–6. [DOI] [PubMed] [Google Scholar]
- 24.Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: results and implications. Gut 1989;30:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]