Abstract
Background and aims: Interferon (IFN) induced hepatitis B e antigen (HBeAg) seroconversion is durable in 80–90% of chronic hepatitis B patients. Preliminary reports on the durability of HBeAg seroconversion following lamivudine are contradictory. We investigated the durability of response following IFN, lamivudine, or IFN-lamivudine combination therapy in a meta-analysis of individual patient data.
Patients and methods: Twenty four centres included 130 patients in total with an HBeAg seroconversion (HBeAg negative, antibodies to hepatitis B e antigen positive) at the end of antiviral therapy: 59 with lamivudine, 49 with interferon, and 22 with combination therapy. Relapse was defined as confirmed reappearance of HBeAg.
Results: The three year cumulative HBeAg relapse rate by the Kaplan-Meier method was 54% for lamivudine, 32% for IFN, and 23% for combination therapy (p=0.01). Cox regression analysis identified pretreatment hepatitis B virus (HBV) DNA, alanine aminotransferase (ALT), sex, and therapy as independent predictive factors of post-treatment relapse; Asian race, previous therapy, centre, and type of study were not predictive of relapse. The relative HBeAg relapse risk of lamivudine compared with IFN therapy was 4.6 and that of combination therapy to IFN therapy 0.7 (poverall=0.01).
Conclusions: The durability of HBeAg seroconversion following lamivudine treatment was significantly lower than that following IFN or IFN-lamivudine combination therapy. The risk of relapse after HBeAg seroconversion was also related to pretreatment levels of serum ALT and HBV DNA, but independent of Asian race.
Keywords: chronic hepatitis B, interferon α, lamivudine, combination therapy, HBeAg seroconversion
Loss of hepatitis B e antigen (HBeAg), either spontaneously or following alpha interferon (IFN) therapy, significantly improves the clinical outcome and survival in chronic hepatitis B virus (HBV) patients.1–3 Therefore, HBeAg loss with seroconversion to antibodies to hepatitis B e antigen (anti-HBe) has remained a major end point of antiviral therapy in chronic HBV infection.
Monotherapy of 16–26 weeks of IFN is associated with loss of serum HBeAg in 20–40% of patients, depending on baseline alanine aminotransferase (ALT) levels.4 Several long term follow up studies have been carried out in IFN responders showing that IFN induced HBeAg seroconversion is sustained in approximately 80–90% of patients.1,2,5–10 IFN responders are more likely to clear hepatitis B surface antigen (HBsAg) (23−86%) from serum than spontaneous seroconverters during a median follow up of 3.8–9 years.1,2,5,6,10
HBeAg seroconversion is observed in 15–30% of patients after 1–2 years of lamivudine therapy.11–14 The clinical implication of lamivudine induced HBeAg seroconversion is suggested to be comparable with that of seroconversion following IFN therapy.12,15,16 A relapse of HBeAg was observed in 19% (8/42 ) of patients with a response after lamivudine.16 In contrast, a single centre cohort study showed a considerably higher percentage of relapse, increasing to 50% (16/32) at two years.17
Combination therapy of 16–26 weeks results in HBeAg seroconversion in 29% of treatment naive patients and in 12% of previous IFN non-responders.13,18 Reports on durability of seroconversion after IFN-lamivudine combination therapy reflect only the first 16 weeks after therapy; similar relapse rates are reported for responders after combination therapy (22%) compared with lamivudine (23%) and IFN (25%) alone.19
Large comparative long term post-treatment studies are lacking. To investigate the post-treatment durability of HBeAg seroconversion following lamivudine, IFN monotherapy, or IFN-lamivudine combination therapy, we performed a multicentre meta-analysis of individual patient data. As such a study does not include randomisation, we have taken strict precautions1 to minimise differences in outcome due to age, sex, race, previous therapy, baseline ALT and HBV DNA, and treatment centre. Multivariate analysis stratified for centre was performed to obtain the current best estimate of the durability of HBeAg seroconversion following the three therapies; in addition, factors predictive of relapse were identified.
PATIENTS AND METHODS
Long term follow up data of chronic HBV patients with HBeAg seroconversion after antiviral therapy were collected. We contacted 53 centres that had patients with HBeAg seroconversion in randomised controlled trials in which we participated ourselves.13,18,20,21 In addition, five centres submitted data from patients treated in cohort studies between 1998 and 2000. Patients who were HBV DNA and HBeAg positive before the start of antiviral therapy were eligible if they seroconverted after receiving lamivudine in a dose of at least 100 mg daily for 24 weeks or more, IFN monotherapy 30 million units per week for at least 16 weeks, or IFN-lamivudine combination therapy for at least 16 weeks.
HBeAg seroconversion was defined as loss of HBeAg, appearance of anti-HBe, and HBV DNA negativity (hybridisation methodology) on two occasions at least one month apart, at the end of lamivudine monotherapy, or within 12 months of initiation of IFN therapy in case of IFN mono or combination therapy.9 Patients were excluded if at baseline there were coinfections (hepatitis C virus, hepatitis D virus, or human immunodeficiency virus), signs of decompensated cirrhosis (defined as jaundice, variceal bleeding, ascites, or encephalopathy), or other causes of liver disease.
Virological and biochemical measurements
We collected follow up data on HBeAg, anti-HBe, HBV DNA, HBsAg, and ALT, one month before the end of therapy, at the end of therapy, three, six, and 12 months after the end of therapy, and yearly thereafter.
Serum HBeAg, anti-HBe, and HBsAg were measured using the routine commercially available immunoassays ImX (Abbott, Chicago, Illinois, USA), Kodak Amerlite (Kodak Clinical and Diagnostics, Amersham, UK), and DiaSorin (Vercelli, Italy). Depending on the centre and date of measurement, serum HBV DNA was measured using the solution hybridisation assay by Abbott (detection limit 2×107 g eq/ml), bDNA assay (Chiron, Emeryville, California, USA; limit of detection 7×105 g eq/ml), or a hybrid capture tube assay (Digene, Murex, UK; detection limit 1.5×106 g eq/ml); all HBV DNA results were convertedto Eurohep genome equivalents.22 ALT (IU/l) was expressed as times the upper limit of normal (ULN).
Definition of relapse
Relapse was defined as reappearance of HBeAg in serum, confirmed by HBV DNA positivity in the same sample or by HBeAg positivity in a consecutive sample. Relapse of HBV DNA was defined as reappearance of HBV DNA in serum above the cut off for the method employed.
Statistics
Results are presented as mean (SD). The cumulative relapse rate for the total study population was calculated by the Kaplan-Meier method with the log rank test for statistical comparison between groups. To adjust for baseline differences in patient viral and host characteristics, multivariate analysis was performed by Cox regression analysis with the likelihood ratio test for statistical significance, as described by Krogsgaard and colleagues.4 To account for a possible centre effect, Cox regression was stratified by centre; centres with less than four patients were clustered in one stratum. Furthermore, interactions between therapy, Asian race, baseline ALT level, baseline HBV DNA, sex, and previous therapy were investigated. Sensitivity analysis was performed excluding one centre at a time. The level of significance was set to p<0.05.
To determine whether the duration of lamivudine therapy influences relapse rates, a Cox regression analysis stratified for centre was performed on the subset of patients on lamivudine therapy (n=59) divided into two categories (<24–48 weeks, n=26; >48 weeks, n=33).
RESULTS
Worldwide, 24 centres from 14 countries responded to our request and collected follow up data of all of their patients in the trials; 130 patients with chronic hepatitis B who had responded to antiviral therapy with HBeAg seroconversion were included. Baseline characteristics are presented in table 1 ▶. Fifty nine patients had received lamivudine, 49 IFN, and 22 combination therapy; 71 patients received the treatment as a participant in an international randomised controlled trial. Follow up until time of relapse or until the last observation ranged from four weeks to seven years for both HBeAg and HBV DNA. At the start of follow up, groups were comparable regarding sex, age, and pretreatment serum levels of HBV DNA and ALT. There were differences in the distribution of race, previous antiviraltherapy, and participation in a randomised controlled trial between the three groups.
Table 1.
Baseline characteristics of chronic hepatitis B e antigen (HBeAg) positive patients prior to successful antiviral therapy, by therapy group
| IFN (n=49) | LAM (n=59) | IFN+LAM (n=22) | |
| Sex, male | 36 (73%) | 42 (71%) | 14 (64%) |
| Age (y)* | 41 (16) | 37 (12) | 43 (15) |
| Ethnicity | |||
| Caucasian, other | 39 (80%) | 19 (33%) | 18 (82%) |
| Asian | 10 (20%) | 39 (66%) | 3 (14%) |
| Log HBV DNA*† | 8.4 (1.0) | 8.7 (0.8) | 8.5 (0.8) |
| ALT (×ULN)* | 4.3 (2.4) | 4.5 (4.2) | 3.3 (2.5) |
| Previous therapy | |||
| IFN | 9 (18%) | 26 (44%) | 8 (36%) |
| LAM | 0 (0%) | 4 (7%) | 1 (5%) |
| Type of study | |||
| RCT | 46 (94%) | 10 (17%) | 15 (68%) |
| Cohort | 3 (6%) | 49 (83%) | 7 (32%) |
Means (SD)
†Corrected for the HBV DNA test used: Abbott or bDNA pg/ml are converted to Eurohep genome equivalents/ml (g eq/ml).
IFN, interferon; LAM, lamivudine; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; ULN, upper limit of normal; RCT, randomised controlled trial.
Figure 1 ▶ shows the percentage of sustained HBeAg seroconversion and HBV DNA negativity for the total study population by Kaplan Meier analysis. A statistically significant higher relapse rate of HBeAg and/or HBV DNA positivity was observed after the end of lamivudine monotherapy compared with IFN monotherapy or combination therapy.
Figure 1.
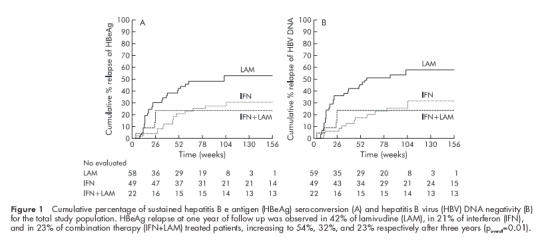
Cumulative percentage of sustained hepatitis B e antigen (HBeAg) seroconversion (A) and hepatitis B virus (HBV) DNA negativity (B) for the total study population. HBeAg relapse at one year of follow up was observed in 42% of lamivudine (LAM), in 21% of interferon (IFN), and in 23% of combination therapy (IFN+LAM) treated patients, increasing to 54%, 32%, and 23% respectively after three years (poverall=0.01).
The results of the univariate analysis, shown in table 2 ▶, demonstrate that lamivudine therapy, male sex, high pretreatment HBV DNA, and low ALT are potential predictive factors for relapse of HBeAg and HBV DNA in serum. Age, race, previous antiviral therapy, centre, and study (randomised controlled trial or cohort study) were not significantly related to outcome.
Table 2.
Relative risk (hazard rates) of relapse according to the univariate analysis (Cox regression) stratified by centre
| Variable | HBeAg relapse | HBV DNA relapse | ||
| Variable | Relative risk (95% CI) | p Value (log likelihood) | Relative risk (95% CI) | p Value (log likelihood) |
| Therapy | 0.0008 | 0.0005 | ||
| IFN | 1 | 1 | ||
| LAM | 7.4 (2.5–22.4) | 0.0003 | 7.1 (2.4–20.1) | 0.0002 |
| Combination | 1.3 (0.8–4.5) | 0.68 | 1.1 (0.3–3.8) | 0.87 |
| Age (y) | 1.00 (0.98–1.02) | 0.87 | 1.00 (0.98–1.03) | 0.70 |
| Sex | ||||
| Male | 1 | |||
| Female | 0.4 (0.2–0.9) | 0.02 | 0.5 (0.2–1.1) | 0.05 |
| Race | ||||
| Caucasian | 1 | 1 | ||
| Asian | 0.8 (0.3–2.3) | 0.75 | 1.0 (0.4–2.6) | 0.97 |
| Previous therapy | 0.24 | 0.05 | ||
| None | 1 | 1 | ||
| IFN | 1.3 (0.6–2.6) | 0.49 | 1.4 (0.7–2.7) | 0.37 |
| LAM | 3.9 (0.8–19.3) | 0.11 | 6.6 (1.4–31.3) | 0.02 |
| Study type | ||||
| RCT | 1 | 1 | ||
| Cohort | 1.3 (0.3–5.6) | 0.75 | 1.85 (0.5–6.8) | 0.38 |
| HBV DNA (log) | 1.51 (1.04–2.21) | 0.03 | 1.50 (1.03–2.19) | 0.03 |
| HBV DNA | ||||
| HBV DNA <108 | 1 | 1 | ||
| HBV DNA >108 | 2.2 (1.2–4.2) | 0.01 | 2.1 (1.1–3.8) | 0.02 |
| ALT (×ULN) | 0.04 | 0.07 | ||
| ≤2 | 1 | 1 | ||
| 2–5 | 0.4 (0.2–0.9) | 0.03 | 0.5 (0.2–1.0) | 0.04 |
| ≥5 | 0.4 (0.1–0.9) | 0.02 | 0.4 (0.2–1.0) | 0.04 |
| Duration of lamivudine therapy | 0.28 | 0.22 | ||
| 24–48 weeks | 1 | 1 | ||
| 48–72 weeks | 0.4 (0.2–1.2) | 0.11 | 0.4 (0.2–1.1) | 0.09 |
| >72 weeks | 0.5 (0.1–2.8) | 0.45 | 0.7 (0.2–2.8) | 0.57 |
IFN, interferon; LAM, lamivudine; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; ULN, upper limit of normal; RCT, randomised controlled trial
Multivariate Cox analysis stratified by centre showed that high pretreatment HBV DNA, low ALT, lamivudine therapy, and (male) sex were independent predictive factors of post-treatment relapse (table 3 ▶). There were no significant interactions. Sensitivity analysis excluding each centre one at a time gave similar relative risks as the outcome in table 3 ▶. Subgroup analysis for patients with or without previous antiviral therapy (IFN and/or lamivudine) showed equal relapse risks in both groups. In a further analysis, race remained included because of the unequal distribution across therapies. Patients with lamivudine induced HBeAg seroconversion showed a significantly higher risk of relapse compared with HBeAg seroconverters following IFN or combination therapy. The relative HBeAg relapse risk compared with IFN therapy was 4.6 (95% confidence interval (CI) 1.4–14.5) for lamivudine monotherapy and 0.7 (0.1–3.9) for combination therapy (poverall=0.01).The relative risk of an HBV DNA relapse in serum compared with IFN therapy was 3.9 (95% CI 1.3–12) for lamivudine monotherapy and 0.6 (0.13–2.9) for combination therapy (poverall=0.010). The risk of relapse over time for the three therapies corrected for pretreatment HBV DNA, sex, and any possible race effect can be calculated for a given patient. Figure 2 ▶ illustrates the risk of relapse over time for a male Caucasian patient with a mean pretreatment HBV DNA level of 4×108 g eq/ml for three different categories of baseline ALT levels.
Table 3.
Relative risks (hazard rates) of relapse according to the multivariate analysis (Cox regression stratified by centre and correcting for sex, HBV DNA, ALT, and race)
| Variable | HBeAg relapse | HBV DNA relapse | ||
| Variable | Relative risk (95% CI) | p Value (log likelihood) | Relative risk (95% CI) | p Value (log likelihood) |
| Therapy | 0.01 | 0.01 | ||
| IFN | 1 | 1 | ||
| LAM | 4.6 (1.4–14.5) | 0.01 | 3.9 (1.3–12.1) | 0.02 |
| Combination | 0.7 (0.1–3.9) | 0.72 | 0.6 (0.1–2.9) | 0.50 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.4 (0.2–0.9) | 0.04 | 0.5 (0.2–1.2) | 0.12 |
| Race | ||||
| Caucasian | 1 | 1 | ||
| Asian | 0.8 (0.3–2.4) | 0.74 | 1.1 (0.4–3.0) | 0.84 |
| Log HBV DNA | 1.61 (1.03–2.52) | 0.03 | 1.7 (1.1–2.6) | 0.02 |
| ALT (×ULN) | 0.03 | 0.05 | ||
| ≤2 | 1 | 1 | ||
| 2–5 | 0.4 (0.2–0.9) | 0.02 | 0.4 (0.2–0.9) | 0.03 |
| ≥5 | 0.3 (0.1–0.8) | 0.01 | 0.4 (0.2–0.9) | 0.03 |
IFN, interferon; LAM, lamivudine; HBV, hepatitis B virus; ALT, alanine aminotransferase; ULN, upper limit of normal.
Figure 2.
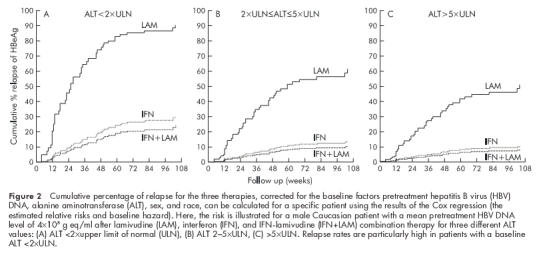
Cumulative percentage of relapse for the three therapies, corrected for the baseline factors pretreatment hepatitis B virus (HBV) DNA, alanine aminotransferase (ALT), sex, and race, can be calculated for a specific patient using the results of the Cox regression (the estimated relative risks and baseline hazard). Here, the risk is illustrated for a male Caucasian patient with a mean pretreatment HBV DNA level of 4×108 g eq/ml after lamivudine (LAM), interferon (IFN), and IFN-lamivudine (IFN+LAM) combination therapy for three different ALT values: (A) ALT <2×upper limit of normal (ULN), (B) ALT 2–5×ULN, (C) >5×ULN. Relapse rates are particularly high in patients with a baseline ALT <2×ULN.
The relative risk of relapse after lamivudine therapy was not significantly influenced by the duration of therapy (p=0.28) but a trend towards a diminished relapse rate with longer treatment was found. The relative risk of a HBeAg relapse compared with IFN therapy was 3.8 (95% CI 1.1–12.6) for lamivudine monotherapy of 48 or more weeks and 14.7 (95% CI 3.2–6.7) for lamivudine therapy of less than 48 weeks.
DISCUSSION
The durability of HBeAg seroconversion after lamivudine therapy was reported to be 80–90% in the first studies performed in Western countries.12,15,16,19 Recently, Song et al observed a relapse of viral activity in half of responding patients after withdrawal of lamivudine monotherapy in an Asian cohort study.17 Ethnicity and duration of therapy prior to seroconversion have been suggested to be predictive factors for post-treatment relapse. In this study, comparing long term post-treatment data in 130 responders after lamivudine, IFN, and IFN-lamivudine combination therapy, lamivudine induced HBeAg seroconversion was significantly less durable than HBeAg seroconversion following IFN containing therapies, independent of race. However, the pretreatment factors high serum HBV DNA and low serum ALT were associated with a higher relapse rate; duration of therapy less than 48 weeks may also be a factor although this was not significant in our study.
The US studies comprised a low number of patients with HBeAg seroconversion, 11 and five, respectively, with a follow up of 4–12 months. The studies reported by Schiff et al in two abstracts only included patients who remained HBeAg negative three months after the end of therapy, thereby excluding early relapsers.16 We have tried to increase the accuracy of the estimate of the durability of HBeAg seroconversion by including a large number of responders in the study, by prolonging the duration of follow up to three years, and by thorough statistical analysis. We corrected for differences in baseline characteristics by using multivariate analysis. The finding that our results were valid for each centre separately should markedly increase the confidence in the results.
However, factors that were not part of the multivariate analysis may still be of relevance. It is possible that patients undergoing relapse are more likely to return to their physician than patients with a sustained response. We collected data from more than 90% of responders of the centres that participated, minimising the likelihood of selection bias. Furthermore, this potential pitfall would affect all three therapies and should therefore not influence the relative risk.
The HBeAg relapse rate following IFN therapy in this study population (32% in three years) may appear high. However, when corrected for mean HBV DNA levels and stratified for ALT category, the post-treatment relapse rate following IFN (fig 2 ▶) was in accordance with the literature.1,2,5–10
Serum HBV DNA and ALT have been identified as predictors of response to antiviral therapy in chronic HBV infection.1,23 More recently, the degree of ALT elevation was found to be the most powerful predictor for HBeAg seroconversion.13,24 In this study, pretreatment HBV DNA levels were the major predictor for sustained response. In contrast with Song and colleagues,17 we also found a significant predictive value of pretreatment ALT levels for the durability of HBeAg seroconversion (higher baseline ALT—lower chance of relapse).
Differences in relapse following lamivudine and IFN therapy suggest a lack of an efficient immune control following HBeAg seroconversion in lamivudine treated patients. Resolution of acute hepatitis B is associated with a strong humoral and cellular immune response which is often maintained for years by persistence of minute amounts of HBV in liver or serum.25–27 In contrast with acute HBV infections, chronic HBV is generally associated with a weak and ineffective antiviral T cell response.26,28 Spontaneous exacerbations and antiviral therapy with IFN can elucidate a significant T helper cell response preceding HBeAgseroconversion.29–31 Although Boni et al reported a restored HBV specific T cell response following the strong decline in HBV DNA levels in lamivudine treated patients, HBcAg specific T cell response remained undetectable during lamivudine therapy in another study.31,32 The differences in relapse after lamivudine and IFN therapies in our study suggest induction of immune control as an essential element for long term durability of HBeAg seroconversion.
Acknowledgments
We thank the following investigators who submitted data for this International Meta-Analysis of Individual Patient Data project: GC Farrell (Australia), M Adler, P Michielsen, F Nevens (Belgium), B Willems (Canada), P Husa, J Svejda (Czech Republic), H Ring-Larsen (Denmark), MJP Arthur, M Bassendine, PR Mills, T Warnes, (UK), P Marcellin (France), GE Kitis (Greece), J Cianciara (Poland), M Buti, (Spain), G Norkrans (Sweden), and BCM Vroom (The Netherlands). We are grateful to Professor Th Stijnen (the Netherlands) for his critical evaluation of the statistical methods used and his assessment of its validity for this study. The study was supported by the Foundation for Liver Research, Rotterdam, the Netherlands
Abbreviations
IFN, interferon
HBV, hepatitis B virus
HBeAg, hepatitis B e antigen
anti-HBe, antibodies to hepatitis B e antigen
ALT, alanine aminotransferase
HBsAg, hepatitis B surface antigen
ULN, upper limit of normal
REFERENCES
- 1.Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996;334:1422–7. [DOI] [PubMed] [Google Scholar]
- 2.Lau DT, Everhart J, Kleiner DE, et al. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology 1997;113:1660–7. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Tai DI, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988;8:493–6. [DOI] [PubMed] [Google Scholar]
- 4.Krogsgaard K, Bindslev N, Christensen E, et al. The treatment effect of alpha interferon in chronic hepatitis B is independent of pre-treatment variables. Results based on individual patient data from 10 clinical controlled trials. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol 1994;21:646–55. [DOI] [PubMed] [Google Scholar]
- 5.Krogsgaard K. The long-term effect of treatment with interferon-alpha 2a in chronic hepatitis B. The Long-Term Follow-up Investigator Group. The European Study Group on Viral Hepatitis (EUROHEP). Executive Team on Anti-Viral Treatment. J Viral Hepat 1998;5:389–97. [DOI] [PubMed] [Google Scholar]
- 6.Carreno V, Castillo I, Molina J, et al. Long-term follow-up of hepatitis B chronic carriers who responded to interferon therapy. J Hepatol 1992;15:102–6. [DOI] [PubMed] [Google Scholar]
- 7.Fattovich G, Giustina G, Realdi G, et al. Long-term outcome of hepatitis B e antigen-positive patients with compensated cirrhosis treated with interferon-alpha. European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology 1997;26:1338–42. [DOI] [PubMed] [Google Scholar]
- 8.Lin SM, Sheen IS, Chien RN, et al. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection therapy. Hepatology 1999;29:971–5. [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, Chung HT, Liu VW, et al. Long-term follow-up of chronic hepatitis B patients treated with interferon alfa. Gastroenterology 1993;105:1833–8. [DOI] [PubMed] [Google Scholar]
- 10.Koorenman J, Baker B, Waggoner J, et al. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med 1991;114:629–34. [DOI] [PubMed] [Google Scholar]
- 11.Lai CL, Chien RN, Leung NWY, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998;339:61–8. [DOI] [PubMed] [Google Scholar]
- 12.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999;341:1256–63. [DOI] [PubMed] [Google Scholar]
- 13.Schalm SW, Heathcote J, Cianciara J, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut 2000;46:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liaw YF, Leung NW, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology 2000;119:172–80. [DOI] [PubMed] [Google Scholar]
- 15.Dienstag JL, Schiff ER, Mitchell M, et al. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology 1999;30:1082–7. [DOI] [PubMed] [Google Scholar]
- 16.Schiff ER, Cianciara J, Karayalcin S, et al and the International Lamivudine Investigator Group. Durable HBeAg and HBsAg seroconversion after lamivudine for chronic hepatitis B (CHB). J Hepatol 2000;2(suppl):99A. [Google Scholar]
- 17.Song BC, Suh DJ, Lee HC, et aL. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology 2000;32:803–6. [DOI] [PubMed] [Google Scholar]
- 18.Schiff ER, Karayalcin S, Grimm IS, et al. Lamivudine and lamivudine/interferon combination therapy in patients with hepatitis B who previously failed interferon: an international controlled trial. J Infect Dis 2002 (submitted).
- 19.Schiff E, Cianciara J, Willems B,et al. Durability of HBeAg responses to lamivudine and interferon in the early post-treatment period in adults with chronic hepatitis B. Hepatology 2000;32(suppl):377A. [Google Scholar]
- 20.Janssen HL, Gerken G, Carreno V, et al. Interferon alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology 1999;30:238–43. [DOI] [PubMed] [Google Scholar]
- 21.Mutimer D, Naoumov N, Honkoop P, et al. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol 1998;28:923–9. [DOI] [PubMed] [Google Scholar]
- 22.Heermann KH, Gerlich WH, Chudy M, et al. Quantitative detection of hepatitis B virus DNA in two international reference plasma preparations. Eurohep Pathobiology Group. J Clin Microbiol 1999;37:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brook MG, Karayiannis P, Thomas HC. Which patients with chronic hepatitis B virus infection will respond to alpha-interferon therapy? A statistical analysis of predictive factors. Hepatology 1989;10:761–3. [DOI] [PubMed] [Google Scholar]
- 24.Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology 1999;30:770–4. [DOI] [PubMed] [Google Scholar]
- 25.Penna A, Del Prete G, Cavalli A, et al. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapside-specific T cells in acute self-limited hepatitis B. Hepatology 1997;25:1022–7. [DOI] [PubMed] [Google Scholar]
- 26.Lohr HF, Krug S, Herr W, et al. Quantitative and functional analysis of core-specific T-helper cell and CTL activities in acute and chronic hepatitis B. Liver 1998;18:405–13. [DOI] [PubMed] [Google Scholar]
- 27.Rehermann B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients‘ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996;2:1104–8. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari C, Penna A, Bertoletti A, et al. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B infection. J Immunol 1990;145:3442–9. [PubMed] [Google Scholar]
- 29.Tsai SL, Chen PJ, Lai PM, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens.J Clin Invest 1992;89:87–96. [DOI] [PMC free article] [PubMed]
- 30.Jung MC, Diepolder HM, Spengler U, et al. Activation of a heterogenous hepatitis B (HB) core and e antigen specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol 1995;69:3358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinos G, Torre F, Chokshi S, et al. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology 1995;22:1040–9. [DOI] [PubMed] [Google Scholar]
- 32.Boni C, Bertoletti A, Penna A, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest 1998;102:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


