Abstract
Background and aim: Autoimmune hepatitis (AIH) has been reported to recur after orthotopic liver transplantation (OLT) in 10–35% of patients in small series with a short follow up. The aim of the present study was to examine the clinical and histological outcome more than 10 years after OLT for AIH.
Patients and methods: Seventeen women with a mean age of 30 (12) years at the time of OLT, selected from among 44 patients transplanted for AIH, were followed for more than 10 years. The criteria for definite AIH, as established by the International Autoimmune Hepatitis Group, were met in every case. Liver biopsies were performed 1, 2, 5, and 10 years after OLT, and when indicated by abnormal liver function tests. Specimens were examined for evidence of recurrent AIH, namely interface hepatitis, lobular activity, portal lymphoplasmocytic infiltration, and fibrosis. Other signs of recurrence included hypertransaminasaemia, serum autoantibodies, and the response to steroid reintroduction or significant steroid dose increments.
Results: AIH recurred in 7 (41%) of 17 patients. In four patients histological abnormalities were detected by means of protocol biopsies 1–5 years before the onset of biochemical abnormalities. Two patients developed severe recurrences after 10 and 15 years, respectively, and required treatment with steroids and tacrolimus. In the other three patients histological recurrence was detected 0.6–3 years post-OLT, concomitantly with biochemical abnormalities.
Conclusions: AIH recurred in 41% of patients followed for more than 10 years after OLT. As histological signs preceded biochemical abnormalities in four patients (23.5%), regular liver biopsy is warranted after OLT. Detection of isolated histological signs may call for closer follow up and/or a change in immunosuppressive therapy.
Keywords: autoimmune hepatitis, recurrence, orthotopic liver transplantation
The precise frequency of recurrent autoimmune hepatitis (AIH) after orthotopic liver transplantation (OLT) is not known as available reports lack long term follow up and use different definitions, based on biochemical abnormalities, autoantibody titres, histological lesions, and/or steroid dependency.1–5 The aim of this study was to document long term classical abnormalities and histological changes occurring in 17 patients who underwent OLT for AIH between 1985 and 1990.
PATIENTS AND METHODS
We reviewed the records of 17 patients who underwent liver transplantation for AIH at our centre between December 1985 and January 1990. The patients, who were all women and had a mean age at OLT of 30 (12) years, were selected from a group of 601 patients who underwent OLT during the same period, and from a subgroup of 44 patients who had undergone OLT for AIH in our centre. They were selected on the basis of more than 10 years of follow up. The clinical and laboratory characteristics of the 17 patients are summarised in table 1▶. The clinical, biochemical, and histological data of the remaining 27 patients who were followed for less than 10 years have also been reviewed.
Table 1 .
Clinical and biological features of 17 patients at the time of transplantation for autoimmune hepatitis
| Age (y) | 30 (12) [12–49] |
| Sex (F/M) | 17/0 |
| Indication for OLT | |
| Decompensated cirrhosis | n=14 |
| Fulminant/subfulminant hepatitis | n=3 |
| Serum markers at transplantation | |
| Prothrombin time (% of normal) | 38 (19.7) [9–82] |
| Total bilirubin (mM/l) | 143.6 (166.25) [22–50] |
| ALT (IU/l) | 246.0 (239.7) [22–605] |
| Gamma globulin level (g/l) | 23.2 (6.1) [14–33] |
| Autoantibodies | |
| ANA and/or SMA >1:80 | n=9* |
| LKM1 and/or LC1 >1:40 | n=4 |
| HLA | |
| DR3+ (1 homozygous and 2 heterozygous) |
3/17 |
| DR4+ (3 heterozygous) | 3/17 |
Values are mean (SD) [range] or number.
*One patient had no detectable autoantibodies but gamma globulin was >25 g/l; data not available in three cases.
OLT, orthotopic liver transplantation; ALT, alanine aminotransferase; ANA, antinuclear antibodies; SMA, smooth muscle antibodies; LKM, liver kidney microsome; LC1, liver cytosol type 1.
Indications for OLT were complications of end stage liver disease (n=14) and fulminant or subfulminant liver failure (n=3). The diagnosis of AIH in these patients was based on the presence of at least three of the following diagnostic criteria (International Autoimmune Hepatitis Group)6: (i) antinuclear antibodies (ANA), antismooth muscle antibodies (SMA), antiliver-kidney microsome type I (LKM1) antibodies, or antiliver cytosol antibodies (LC1) at significant titres7; (ii) a gamma globulin level above 20 g/l; (iii) concomitant non-hepatic autoimmune disorders such as autoimmune thyroiditis, Raynaud’s syndrome, and rheumatoid arthritis; (iv) HLA B8 DR3 or DR4 phenotype; and (v) liver biopsy showing features compatible with chronic hepatitis. Type 1 AIH was defined by the presence of ANA and/or SMA, and type II AIH by the presence of LKM1 and/or LC1.7
At the time of OLT, all patients were seronegative for hepatitis B (HBV) and C (HCV) virus infection. HBV infection was diagnosed using commercial kits for hepatitis B surface antigen, hepatitis B e antigen, and anti-hepatitis B e antibodies (ELISA; Murex Diagnostics Ltd, Kent, UK), and for anti-hepatitis B surface and anti-hepatitis B core antibodies (Hoechst Boehring Diagnostics, Marburg Germany). HCV infection was diagnosed using a commercial kit for anti-HCV (RIBA-2; Ortho Diagnostic, Raritan, NJ, USA) and by HCV RNA polymerase chain reaction (PCR) (amplification of the 5′ conserved region).
HLA class I typing was performed using a microlymphocytotoxicity test with automated reading and dual staining. Class II typing was done by DNA typing using PCR sequence specific oligonucleotide probe hybridisation.
A control population of 10 patients (six females and four males, mean age at OLT 30 (19.1) years) transplanted more than 10 years previously for neither an end stage autoimmune liver disease nor a viral disease, but for biliary atresia (n=5), metabolic diseases (Wilson’s disease and haemochromatosis in one case each), and alcoholic cirrhosis (n=3) were also studied.
Autoantibody detection
ANA, SMA, antimitochondrial antibodies (AMA), anti-LKM1, and anti-LC1 were detected in serum using an indirect immunofluorescence (IIF) method on unfixed cryostat sections of rat liver, kidney, and stomach.8 ANA was also screened by IIF on Hep-2 cell monolayers. Positivity cut offs were 1:80 for ANA and anti-SMA, and 1:40 for AMA, anti-LKM1, and anti-LC1.
Sera positive for both anti-LC1 and anti-LKM1 by IIF were confirmed by obtaining a line of identity with reference sera in the double immunodiffusion method, using liver cytosol or a microsomal fraction as antigen.8
Antisoluble liver antigen (SLA) antibodies were detected using an inhibition capture enzyme linked immunosorbent assay, as described elsewhere.9
Gamma globulin levels were determined by serum electrophoresis (Paragon Electrophoresis System; Beckman Coulter, Brea, California, USA) followed by densitometry (Appraise Densitometer; Beckman Coulter); values of 20 g/l or more were considered significant.
Histology
Biopsies were performed according to the protocol at set intervals (1, 2, 5, and 10 years post-OLT) or as clinically dictated. All liver biopsy specimens were stained with haematoxylin and eosin and were reviewed for this study by a pathologist blinded to all clinical information except for the pre-OLT diagnosis of AIH. Specimens with portal lymphoplasmocytic infiltration, lobular activity, and interface hepatitis were judged compatible with recurrent AIH. Portal fibrosis and necroinflammatory activity were graded according to the extent of piecemeal necrosis and lobular activity. All of these histological criteria were graded as absent, mild, moderate, or severe. Post-OLT biopsies (n=61) in the control group were reviewed by the same pathologist (MS) using the same histological criteria.
Post-graft immunosuppression was based on cyclosporin, azathioprine, and steroids. Cyclosporin doses were adjusted to maintain blood levels of 300–400 ng/ml during the first month, 200–300 ng/ml during the first three months, and 100–200 ng/ml thereafter. Cyclosporin concentrations were assayed with a monoclonal antibody based whole blood fluorescent polarisation immunoassay on a TDX analyser (Abbott Laboratories, Abbott Park, Illinois, USA). Prednisone was started at 20 mg/day and tapered to 10–5 mg/day by six months. Cyclosporin treated patients also received azathioprine 1 mg/kg/day. Single rejection episodes were usually treated with a bolus of methylprednisolone. After recurrent rejection episodes, patients on prednisone were maintained on prednisone 10 mg/day, and those on cyclosporin were switched to tacrolimus.
Diagnosis of recurrence was based on the following criteria: (1) hypertransaminasaemia, (2) reappearance or increase in autoantibody levels in serum, and (3) the need for steroid reintroduction or a significant steroid dose increase. Histological abnormalities compatible with autoimmune recurrence, and exclusion criteria such as acute rejection and HCV infection, were also taken into account.
RESULTS
Histological recurrence
Histological recurrence was diagnosed in seven of the 17 patients at a mean of 2.5 (1.7) years after OLT (table 2▶). In four of these seven patients (group 1), histological signs of recurrence were found on protocol biopsies between one and five years after OLT (mean 2.5 (1.7) years) (table 2▶). The signs consisted of portal plasmocytic infiltration in 4/4 patients (moderate or severe in three and mild in one); piecemeal necrosis in 3/4 patients; lobular activity in 4/4 patients; and fibrosis in 4/4 patients (severe in one, moderate in two, and mild in one). These features were not observed on post-OLT biopsies in the control group: in five patients a mild lymphocyte portal infiltrate was observed six months, one year, two years, five years, and 10 years post-OLT. This infiltrate did not affect the bile ducts, endothelium, or interface. Among the remaining 27 patients who had been transplanted less than 10 years previously, there were two patients who had histological recurrence three and five years post-OLT, respectively, and who were free of biochemical and immunological abnormalities at the time of biopsy.
Table 2 .
Features of histological recurrence on protocol biopsies (group 1) and at onset of biochemical abnormalities (group 2)
| Patient (OLT) | Interval after OLT (y) | Portal inflammation | Interface hepatitis | Lobular activity | Portal fibrosis | |
|---|---|---|---|---|---|---|
| Group 1 | 51 | 1 | Mild | None | Moderate | Mild |
| 155 | 2 | Moderate to severe | Mild | Moderate | Moderate | |
| 205 | 5 | Severe | Severe | Mild | Severe | |
| 421 | 2 | Dense | Moderate | Mild | Mild | |
| Group 2 | 86 | 0.6 | Moderate | None | Moderate | Mild |
| 145 | 3 | Moderate | Severe | Moderate | Severe | |
| 471 | 3 | Moderate | Moderate | Moderate | Moderate | |
OLT, orthotopic liver transplantation.
Figure 1▶ shows a typical example of histological recurrence two years after OLT (patient No OLT 205). In one patient (No OLT 421) who was infected by HCV during transplantation, the histological changes favoured recurrent AIH rather than hepatitis C; fibrosis was mild, piecemeal necrosis was moderate, lobular activity was mild, and the plasma cell infiltrate was severe. Steatosis was absent.
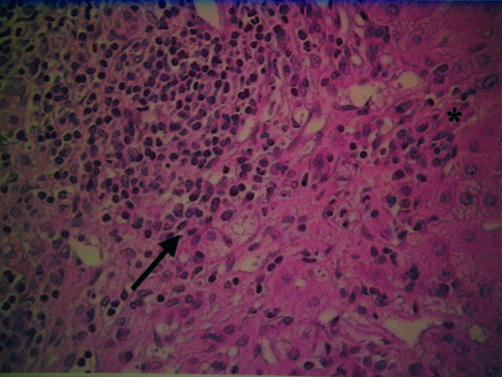
Figure 1 .
Recurrence of autoimmune hepatitis. The portal tract contains a moderate inflammatory infiltrate which is composed of predominant plasma cells (arrow) and lymphocytes, and which is responsible for piecemeal necrosis (*).
In three of seven patients (group 2), histological recurrence coincided with liver test abnormalities (table 2▶). Interestingly, all patients had moderate piecemeal necrosis and moderate plasmocytic infiltration. Fibrosis was moderate in two patients and mild in one.
All four patients in group 1 had normal liver test values at the time of histological recurrence. Autoantibody titres are given in table 3▶.
Table 3 .
Clinical and immunological status at the time of biological and histological recurrence in all patients who had recurrence of autoimmune hepatitis
| Patient (OLT) | Pre-OLT autoantibody titre | HLA DR status (donor/ recipient) |
Duration of follow up at the time of histological recurrence (y) | Cause of biopsy | AST/ALT (IU/l) (N<35/N<43) | HCV antibody/ HCV RNA | Autoantibody titre | Treatment |
|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||
| 51 | SMA=1:640 | 3,5/13,15 | 1 | Protocol | 20/25 | (−)/(−) | SMA=1:640 | 10 mg P, 1.2 ml×3 C, 50 mg A |
| 155 | SMA=1:640 | 5,7/1,4 | 2 | Protocol | 34/42 | (−)/(−) | SMA=1:640, anti-SLA+ | 10 mg P, (0.5 ml×2) C, 50 mg A |
| 205 | NA | 5,5/3,16 | 5 | Protocol | 48/40 | (−)/(−) | ANA=1:80, SMA=1:160, anti-SLA− | 10 mg P, 200 mg C, 50 mg A |
| 421 | SMA=1:1280 | 7,13/3,3 | 2 | Protocol | 54/90 | (+)/(+) | ANA=1:80, anti-SMA=1:80, anti-SLA− | 10 mg P, 0.5 ml×2 C, 50 mg A |
| Group 2 | ||||||||
| 86 | Anti-LKM=1:640 | 5,5/7,10 | 0.6 | Abnormal liver function tests | 160/405 | (+)/(+) | Anti-LKM−, ANA−, SMA− | 17.5 mg P, (1 ml×3) C, 50 mg A, 20 mg P, (1 ml×2) C |
| 145 | SMA=1:80 | 3 | Abnormal liver function tests | 235/275 | (−)/(−) | ANA=1:640 | 100 A, 10 mg P | |
| 471 | SMA=1:640 | /11,13 | 3 | Abnormal liver function tests | 101/185 | (−)/(−) | SMA=1:80, anti-SLA+ | (1.2×2 ml) C |
OLT, orthotopic liver tranplantation; NA, not available; AST/ALT, aspartate aminotransferase/alanine aminotransferase; HCV, hepatitis C virus; P, prednisone; C, cyclosporin; A, azathioprine; ANA, antinuclear antibodies; SMA, smooth muscle antibodies; SLA, soluble liver antigen; LKM, liver kidney microsome.
All four patients were receiving immunosuppressive therapy at the time of histological recurrence, consisting of prednisone, cyclosporin, azathioprine, and a mean steroid dose of 10 mg/day (table 3▶).
One of three patients in group 2 had no detectable serum autoantibodies (table 3▶). Two patients were taking prednisone (17.5 and 20 mg prednisone daily) as well as cyclosporin and azathioprine; the third patient was on a two drug immunosuppressive regimen (prednisone 10 mg/day and cyclosporin).
Outcome
Four patients in group 1 developed clinical and/or biochemical evidence of recurrence within 10 years after OLT (table 4▶). Two patients (Nos OLT 51 and OLT 421) developed severe recurrences 15 and 10 years post-OLT, respectively, with high transaminases levels, an increase in autoantibody titres, and marked portal inflammatory infiltration. Patient No OLT 421 developed jaundice and a prolonged prothrombin time. Patient No OLT 51 failed to respond to 1 g/day Solumedrol but clinical remission was obtained when this treatment was replaced by tacrolimus. Despite two intravenous boluses of 1 g Solumedrol, the patient’s clinical status and liver function deteriorated. Patient No OLT 421 developed jaundice and a prolonged prothrombin time; tacrolimus was prescribed but retransplantation was necessary 11 months later.
Table 4 .
Clinical recurrence and outcome in the four patients in whom histological recurrence was diagnosed in protocol biopsies
| Patient (OLT) | Delay post-OLT (y) | Liver enzyme titre (ALT (IU/l)) (N<43) | Autoantibody titre | Histological features | Treatment |
|---|---|---|---|---|---|
| 51 | 15 | 1000 | ANA=1:640, anti-SMA=1:160, anti-SLA− | Substantial portal inflammatory plasmocyte infiltration, moderate septal fibrosis | Switch to FK* |
| 155 | 10 | 80 | SMA=1:640, anti-SLA+ | Substantial plasmocyte infiltration in portal tract | Higher dose resumption of steroid therapy |
| 205 | 11 | 182 | SMA=1:640, anti-SLA− | Moderate plasmocyte infiltration in portal tract | Higher dose resumption of steroid therapy |
| 421 | 10 | 680 | SMA=1:320, anti-SLA− | Substantial portal inflammatory plasmocyte infiltration, scant areas of lobular necrosis | Switch to FK* and retransplantation |
FK, tacrolimus; OLT, orthotopic liver tranplantation; ALT, alanine aminotransferase; ANA, antinuclear antibodies; SMA, smooth muscle antibodies; SLA, soluble liver antigen.
Patients OLT 155 and OLT 205 developed clinical and biological recurrences 10 and 11 years post-OLT, respectively. Liver test results normalised when the steroid dose was increased. Interestingly, histological signs did not worsen between the first biopsy showing signs of recurrence and the onset of clinical signs.
In group 2 (table 5▶), patient No OLT 86, who was anti-HCV positive and had HCV viraemia, had normal liver test results until 13.5 years post-OLT when alanine aminotransferase levels rose to 1.5 times the normal value; transaminase activity normalised when the steroid dose was increased. Liver biopsy 15 years post-OLT showed moderate activity and fibrosis with rare fibrous bridges. Patient No OLT 145 was retransplanted three months after a histological diagnosis of recurrence because of deteriorating liver function. Thirteen years after the second OLT a significant increase in transaminase levels and ANA autoantibody titres occurred. Liver biopsy showed moderate activity with moderate fibrosis. Mycophenolate-mofetil was added to the immunosuppressive regimen (steroids and cyclosporin) and biochemical values normalised. The third patient (No OLT 471) had an episode of acute rejection five years post-OLT which was successfully treated with a methylprednisolone bolus. Two years later she became jaundiced and chronic rejection was diagnosed on the basis of ductopenia.
Table 5 .
Long term outcome of the three patients in whom histological recurrence was diagnosed after clinical deterioration
| Patient (OLT) | Duration of follow up (y) | HCV antibody/HCV RNA | Liver enzyme titre (ALT (IU/l)) (N<43) | Autoantibody titer | Histological features | Treatment |
|---|---|---|---|---|---|---|
| 86 | 13.5 | (+)/(+) | 58 | ANA=1:160, anti-SLA− | Moderate activity and mild fibrosis | Resumption of steroid therapy |
| 145 | 13 | (−)/(−) | 51 | ANA=1:640, anti-SLA− | Severe hepatitis pre-2nd OLT. Moderate activity and moderate fibrosis 13 y post 2nd OLT | Retransplantation, mycophenolate-mofetil |
| 471 | 11 | (−)/(−) | 191 | ANA−, SMA−, anti-SLA+ | Chronic rejection 2 years post OLT | Awaiting retransplantation |
OLT, orthotopic liver tranplantation; ALT, alanine aminotransferase; HCV, hepatitis C virus; ANA, antinuclear antibodies; SMA, smooth muscle antibodies; SLA, soluble liver antigen.
Association between recurrence and HLA phenotype
HLA typing results for patients with and without AIH recurrence are shown in table 6▶. Interestingly, in two of three cases of severe recurrence, the donor and recipient (No OLT 51 and No OLT 421) were both haplotype DR3; patient No OLT 421 was also A1, B8, DR3 homozygous.
Table 6 .
HLA DR status according to recurrence
| Recurrence |
No recurrence |
||
|---|---|---|---|
| Patient (OLT) | HLA DR status (donor/recipient) | Patient (OLT) | HLA DR status (donor/recipient) |
| 51 | 3,5/13,15 | 241 | 3,5/4,15 |
| 86 | 5,5/7,10 | 105 | 4,4/1,11 |
| 145 | 4,6/9,15 | 272 | 6,7/3,3 |
| 155 | 5,7/1,4 | 202 | 10,13/4,11 |
| 205* | 5,5/3,16 | 197 | 2,7/7,7 |
| 421* | 7,13/3,3 | 370 | 4,13/15,13 |
| 471 | /11,13 | 93 | 4,4/11,11 |
| 197 | 2,7/7,7 | ||
| 157 | 2,2/11,13 | ||
| 251 | 4,7/7,12 | ||
*Severe recurrence.
OLT, orthotopic liver tranplantation.
Association between recurrence and SLA autoantibodies
SLA autoantibodies were found in two of seven patients who had recurrences (28.6%) but not in patients who remained free of recurrence (tables 3, 4, 5, and 7▶▶▶▶). Interestingly, this was the only antibody detected in patient No OLT 471, 11 years after recurrence, anti-SMA having disappeared.
Table 7 .
Autoantibody titres before and after liver transplantation in patients without recurrent autoimmune hepatitis
| Patient (OLT) | Pre-OLT titre | Post-OLT titre, maximum level* |
|---|---|---|
| 241 | NA | Negative |
| 105 | SMA=1:180 | Negative |
| 272 | Anti-LC1=1: 320, anti-SLA− | LC1=1:320, anti-SLA− |
| 202 | Negative | SMA=1:80, anti-SLA− |
| 197 | NA | Negative |
| 370 | SMA=1:160 | SMA=1:160, anti-SLA− |
| 93 | Anti-LKM=1:1280, anti-SLA− | Anti-LKM=1:1280, anti-LC1=1:640, anti-SLA− |
| 157 | SMA=1:160 | Negative |
| 251 | Anti-LKM=1:1280, anti-SLA− | Anti-LKM=1:160, anti-SLA− |
| 391 | SMA=1:640 | Anti-SLA=1:60, anti-SLA− |
*Mean frequency of screening: yearly.
OLT, orthotopic liver tranplantation; NA, not available; SMA, smooth muscle antibodies; SLA, soluble liver antigen; LKM, liver kidney microsome; LC1, liver cytosol type 1.
Long term outcome of the 10 patients without AIH recurrence
Autoantibody titres from before OLT until the end of follow up are shown in table 7▶. Interestingly, except for two patients (Nos OLT 93 and OLT 272), autoantibody titres were low in the post-OLT period; high pre-OLT autoantibody titres always fell markedly after transplantation.
DISCUSSION
AIH has been reported to recur after liver transplantation in between 10% and 35% of patients but with a maximum follow up of five years.6,10,11 We report retrospective clinical, biological, and histological follow up data on 17 patients monitored for more than 10 years after OLT for AIH. Recurrence of AIH was observed in seven (41%) of the 17 patients.
Histological signs of recurrence were found in four patients by protocol biopsy, in the absence of clinical and immunological abnormalities. These histological signs included moderate to dense lymphoplasmocytic infiltration, lobular activity, and interface hepatitis. The mean interval between histological recurrence and onset of other signs was 9 (3.5) (range 6–14) years.
All four patients were asymptomatic at histological diagnosis. Mean time to histological diagnosis was 39 months after OLT, an interval similar to that previously reported by our team and other groups.5,10,11 Levels of serological markers (autoantibodies and gamma globulin) were not increased in comparison with the last titres analysed but serum autoantibodies (SMA in four patients, ANA in two, and anti-SLA in one) were present in all four patients.
This study underlines the fact that biological and histological recurrence may occur more than 10 years after OLT. Two severe cases in our series occurred 10 and 14 years after OLT. Both were successfully controlled with tacrolimus which has recently been shown to improve biochemical test values in patients with AIH treated for up to one year12 and to resolve severe recurrences of AIH.13 Our results extend this observation to patients with isolated histological signs of recurrence, and particularly those with the HLA A1B8DR3 phenotype. This HLA phenotype has frequently been implicated in other recent series3,4,11; interestingly, two of our patients who developed severe clinical recurrences were HLA DR3, and one of these was A1B8DR3; the other patient was A1B8DR4 and her donor was A1B8DR3. However, other studies have failed to identify the recipient and/or donor HLA DR3 phenotype as a risk factor for recurrent disease.14 This discrepancy may be explained by the small number of patients studied, and by the possible contribution of other genetic factors.15
Our results underline the importance of late protocol biopsies in this setting. Normal biochemical liver tests, gamma globulin levels, and the absence of anti-tissue autoantibodies may mask the recurrence of autoimmune disease. Interestingly, however, anti-SLA antibodies, which are specific markers of AIH,16 were detected in some patients with recurrence. In one case it was the only antibody detected. However, these results concern a small number of patients, and further investigations are necessary before recommending routine anti-SLA antibody screening for suspected recurrence.
The late recurrences observed in our series suggest that immunosuppressive therapy should be pursued for more than 10 years and that any dose reduction should only be undertaken with care.
Acknowledgments
The authors thank Professor Edward Krawitt for critical reading, and David Young and Ms Jerri Bram for reviewing the manuscript.
Abbreviations
AIH, autoimmune hepatitis
OLT, orthotopic liver transplantation
LT, liver transplantation
ANA, antinuclear antibodies
AMA, antimitochondrial antibodies
SMA, smooth muscle antibodies
SLA, soluble liver antigen
LKM, liver kidney microsome
LC1, liver cytosol type 1
HCV, hepatitis C virus
HBV, hepatitis B virus
PCR, polymerase chain reaction
IIF, indirect immunofluorescence
REFERENCES
- 1.Neuberger J, Portmann B, Calne R, et al. Recurrence of autoimmune chronic active hepatitis following orthotopic liver grafting. Transplantation 1984;37:363–5. [DOI] [PubMed] [Google Scholar]
- 2.Wright HL, Bou-Abboud CF, Hassanein T, et al. Disease recurrence and rejection following liver transplantation for autoimmune chronic active liver disease. Transplantation 1992;53:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prados E, Cuervas-Mons V, De La Mata M, et al. Outcome of autoimmune hepatitis after liver transplantation. Transplantation 1998;12:1645–50. [DOI] [PubMed] [Google Scholar]
- 4.Götz G, Neuhaus R, Bechstein WO, et al. Recurrence of autoimmune hepatitis after liver transplantation.Transpl Proc 1999;31:430–1. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Samuel D, Sebagh M, et al. Long-term follow-up after liver transplantation for autoimmune hepatitis: evidence of recurrence of primary disease. J Hepatol 1999;30:131–41. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929–38. [DOI] [PubMed] [Google Scholar]
- 7.Martini E, Abuaf N, Cavalli F, et al. Antibody to liver cytosol (anti LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology 1988;8:1662–6. [DOI] [PubMed] [Google Scholar]
- 8.Roitt M, Doniach D. Immunofluorescent test for the detection of antibodies. In: WHO Booklet of Immunological Studies. Geneva: World Health Organization, 1972:1–12.
- 9.Ballot E, Homberg JC, Johanet C. Antibodies to soluble liver antigen: an additional marker in type 1 auto-immune hepatitis. J Hepatol 2000;33:208–15. [DOI] [PubMed] [Google Scholar]
- 10.Reich DJ, Fiel I, Guarrera JV, et al. Liver transplantation for autoimmune hepatitis. Hepatology 2000;32:693–700. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Koch A, Czaja AJ, Carpenter HA, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl 2001;7:302–10. [DOI] [PubMed] [Google Scholar]
- 12.Van Thiel DH, Wright H, Caroll P, et al. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol 1995;90:771–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Hurtova M, Duclos-Vallée JC, Johanet C, et al. Successful tacrolimus therapy for a severe recurrence of type 1 autoimmune hepatitis in a liver graft recipient. Liver Transpl 2001;7:556–8. [DOI] [PubMed] [Google Scholar]
- 14.Milkiewicz P, Hübscher SG, Skiba G, et al. Recurrence of autoimmune hepatitis after liver transplantation. Transplantation 1999;68:253–6. [DOI] [PubMed] [Google Scholar]
- 15.Hübscher SG. Recurrent autoimmune hepatitis after liver transplantation: diagnostic criteria, risk factors, and outcome. Liver Transpl 2001;7:285–91. [DOI] [PubMed] [Google Scholar]
- 16.Kanzler S, Weidemann C, Gerken G, et al. Clinical significance of autoantibodies to soluble liver antigen in autoimmune hepatitis. J Hepatol 1999;31:635–40. [DOI] [PubMed] [Google Scholar]