Abstract
Background: Aldosterone has a rapid, non-genomic, inhibitory effect on macroscopic basolateral K+ conductance in the human colon, reducing its capacity for Cl− secretion. The molecular identity of the K+ channels constituting this aldosterone inhibitable K+ conductance is unclear.
Aim: To characterise the K+ channel inhibited by aldosterone present in the basolateral membrane of human colonic crypt cells.
Methods: Crypts were isolated from biopsies of healthy sigmoid colon obtained during colonoscopy. The effect of aldosterone on basolateral K+ channels, and the possible involvement of Na+:H+ exchange, were studied by patch clamp techniques. Total RNA from isolated crypts was subjected to reverse transcriptase-polymerase chain reaction (RT-PCR) using primers specific to intermediate conductance K+ channels (KCNN4) previously identified in other human tissues.
Results: In cell attached patches, 1 nmol/l aldosterone significantly decreased the activity of intermediate conductance (27 pS) K+ channels by 31%, 53%, and 54% after 1, 5 and 10, minutes, respectively. Increasing aldosterone concentration to 10 nmol/l produced a further 56% decrease in channel activity after five minutes. Aldosterone 1–10 nmol/l had no effect on channel activity in the presence of 20 μmol/l ethylisopropylamiloride, an inhibitor of Na+:H+ exchange. RT-PCR identified KCNN4 mRNA, which is likely to encode the 27 pS K+ channel inhibited by aldosterone.
Conclusion: Intermediate conductance K+ channels (KCNN4) present in the basolateral membranes of human colonic crypt cells are a target for the non-genomic inhibitory effect of aldosterone, which involves stimulation of Na+:H+ exchange, thereby reducing the capacity of the colon for Cl− secretion.
Keywords: aldosterone, human colon, KCNN4, potassium channels
The currently accepted model of electrogenic Cl− secretion in mammalian intestine requires activation of basolateral K+ channels to promote cell hyperpolarisation and recycling of K+ taken into the cell via basolateral Na+K+2Cl− cotransport and Na+K+ATPase.1 This maintains the electrochemical gradient for sustained Cl− exit via cAMP activated apical Cl− channels. The T84 human colonic adenocarcinoma cell line, widely used to study intestinal Cl− secretion, possesses two pharmacologically distinct basolateral K+ conductances at the macroscopic level, one activated by Ca2+ dependent and the other by cAMP dependent Cl− secretory agonists.2,3 Blockade of these basolateral K+ conductances inhibits the Cl− secretory process.3,4 Electrophysiological studies have revealed low conductance (1–3 pS) basolateral K+ channels in rat colonic crypt cells, which are activated during cAMP stimulated Cl− secretion and inhibited by the chromanol 293B.5,6 In T84 cells these low conductance K+ channels have been characterised as complexes of KCNQ1 and KCNE3.5 However, the basolateral membranes of human colonic crypt cells are dominated by intermediate conductance (∼25 pS) K+ channels, which are largely voltage independent and exhibit weak inward rectification, and are activated by the Cl− secretagogues carbachol (a Ca2+ mediated muscarinic agonist) and dibutyryl cAMP (a membrane permeant analogue of cAMP).7,8 The basic properties of these intermediate conductance K+ channels are similar to the IKCa channels (variously termed KCNN4 by the Human Genome Organisation, and hSK4, hIK1, hKCa4, and rSK4 by individual authors) recently cloned from cDNA libraries of human placenta,9 pancreas,10 lymph node11 and T lymphoblasts,12 and rat distal colon,13 but the molecular identity of the IKCa channel in the human colon is unclear.
New insights into the role of basolateral K+ channels in intestinal Cl− secretion have come from studies of the rapid non-genomic effects of aldosterone.14 In the mammalian colon, nanomolar concentrations of aldosterone stimulate Na+:H+ exchange, increase intracellular Ca2+ concentration, stimulate protein kinase C activity, inhibit the basolateral K+ conductance required for electrogenic Cl− secretion, and activate an ATP dependent basolateral K+ conductance involved in Na+ absorption.14–16 These findings have prompted the suggestion that the non-genomic effects of aldosterone lead to a rapid (within minutes) decrease in the capacity of the colon for Cl− secretion while simultaneously increasing its capacity for Na+ absorption.14
Having demonstrated an abundance of IKCa channels in the basolateral membrane of human colonic crypt cells,7,8 the first stage of our study was to determine whether these K+ channels constitute the macroscopic basolateral K+ conductance which is susceptible to non-genomic regulation by aldosterone and, if so, whether this effect is associated with a change in Na+:H+ exchange activity.14 The second stage was to determine whether or not the IKCa channels present in human colonic crypt cells equate with KCNN4 identified in other human tissues.9–12
METHODS
Isolation of human colonic crypts
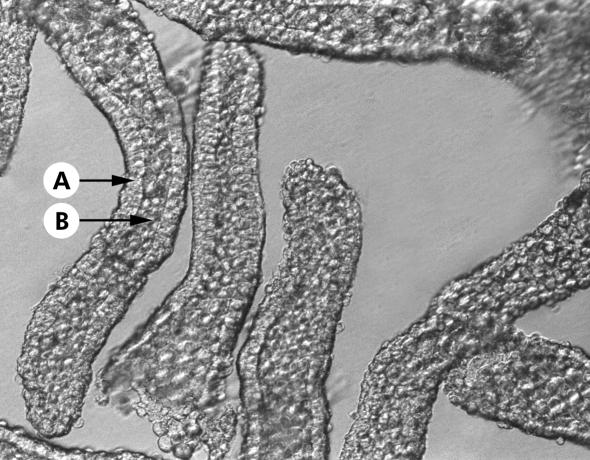
After obtaining written informed consent, 4–6 biopsies of normal appearing sigmoid colonic mucosa were obtained from patients undergoing colonoscopy or flexible sigmoidoscopy for investigation of altered bowel habit and/or abdominal pain. None of the patients was receiving medication at the time of the procedure. Patients undergoing colonoscopy had received Klean-Prep (Norgine, Harefield, UK) during the preceding 24 hours, and those undergoing flexible sigmoidoscopy received Fleet ready to use enema (De Witt, Runcorn, UK) before the procedure. Routine histology was normal in all cases. The study was approved by Leeds Health Authority Ethics Committee. Intact crypts were isolated by a modification of a previously described Ca2+ chelation method.8 Biopsies were incubated for 45 minutes at 37°C in a Ca2+ free solution containing (mmol/l): EDTA 10, NaCl 112, KCl 5, dithiothreitol 3, and HEPES 20, titrated to pH 7.1 with Trizma base. Biopsies were then gently stirred with a magnetic bar for 30 minutes at 37°C to liberate intact crypts. Crypts were collected by centrifugation, washed twice, and resuspended in a storage solution containing (mmol/l): K+ gluconate 100, KCl 30, NaCl 20, CaCl2 1.25, MgCl2 1, HEPES 10, glucose 5, Na+ pyruvate 5, and Na+ butyrate 5, supplemented with 1 g/l of bovine serum albumin, titrated to pH 7.4 with KOH, and kept on ice until required. Crypts isolated by this method appeared to consist entirely of epithelial cells (fig 1▶).
Figure 1 .
Photomicrograph of intact colonic crypts isolated by Ca2+ chelation and visualised using Hoffman modulated optics (×400). Note that the crypts were isolated devoid of non-epithelial cells originating from the lamina propria. The lumenal opening and base of each crypt can be clearly distinguished. Arrows indicate the continuous layer of crypt epithelial cells (A) and the crypt lumen (B).
Patch clamp studies
Single channel recordings were obtained from basolateral membrane patches of cells in the middle third of the crypts in the cell attached and excised inside out configurations. Experiments were carried out at 20–22°C rather than 37°C to maintain viability.17 For experiments performed in cell attached patches, the bath solution contained (mmol/l): NaCl 140, KCl 4.5, CaCl2 1.2, MgCl2 1.2, glucose 5, HEPES 10, and Na+ butyrate 5, titrated to pH 7.4 with NaOH. Experiments were also performed to study the pH sensitivity of the channels in excised inside out patches, in which the bath solution contained (mmol/l): KCl 145, CaCl2 0.1, MgCl2 1.2, glucose 5, HEPES 10, and Na+ butyrate 5, titrated to pH 6.8, 7.0, 7.2, 7.4, or 7.6 with KOH. Patch pipettes fabricated from borosilicate glass were filled with a solution containing (mmol/l): KCl 145, CaCl2 1.2, MgCl2 1.2, glucose 5, HEPES 10, and Na+ butyrate 5, and had resistances of 5–6 MΩ. For recordings using excised inside out patches, KCl solution was present in the patch pipettes and either NaCl or KCl solution was present in the bath. Single channel currents were recorded with a patch clamp amplifier (List model EPC7; Darmstadt, Germany) over a range of holding voltages referenced to the pipette interior. Currents were stored on videotape after pulse code modulation (Sony model PCM-701 ES, Japan). Stored currents were low pass filtered (750 Hz) and loaded into computer memory (Elonex Home PC 386S-200) via a DigiData 1200 interface system and pCLAMP software (Axon Instruments Inc., Union City, California, USA, version 5.6) for offline analysis. The single channel open probability (Po) of channels in the patch was calculated using an analysis program written in Quick Basic 4.0 (Microsoft, Reading, UK). Transitions between fully closed and fully open current levels occurred when the currents crossed a threshold set midway between these states. Po was calculated as:
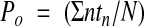 |
where N is the maximum number of channels seen to be open simultaneously during the 30 second recordings, n is the state of the channels (0 is closed, 1 is one channel open, etc), and tn is the time spent in state n.
The non-genomic effect of aldosterone was studied in cell attached patches by recording channel activity under basal conditions and at regular intervals after addition of 1 nmol/l aldosterone and then a further 9 nmol/l aldosterone (final bath concentration 10 nmol/l). The possible involvement of Na+:H+ exchange in the non-genomic effect was studied by repeating this protocol in the presence of 20 μmol/l ethylisopropylamiloride (EIPA), a Na+:H+ exchange inhibitor.
Reverse transcriptase-polymerase chain reaction (RT-PCR) studies
Colonic crypts were pelleted by centrifugation and total RNA was extracted using the guanidium thiocyanate-acid phenol-chloroform method of Chomczynski and Sacchi,18 and resuspended in RNase free water. Total RNA was reverse transcribed at 37°C using Superscript I reverse transcriptase with oligo (dT)12–18 primers according to the manufacturer’s instructions (Invitrogen, Paisley, UK). To control for possible genomic DNA contamination, reverse transcription was also performed in the absence of Superscript. Oligonucleotide primers were also designed to span intron/exon boundaries so as to generate a differently sized PCR product when amplified from genomic or complementary DNA (cDNA). cDNA was subsequently used as a template for two rounds of PCR with primers specific for human KCNN4 (GenBank accession No AF000972). Each PCR was performed with 2.5 U of Taq DNA polymerase (prepared inhouse) with the following cycling parameters: 30 seconds at 94°C, 45 seconds at 58°C, and 30 seconds at 72°C. The first 35 cycles generated PCR products of 830 base pairs (sense 5′-CAG AGA TGC TGT GGT TCG GGG-3′ and antisense 5′-CTT GTA GCA CTC GGG CAG CGG-3′). To increase the amplification, a second round of 35 cycles was performed which generated PCR products of 761 base pairs (sense 5′-TTT CCA CCT TCT TAC TCC TCT GCC-3′ and the above antisense primer). A human placental cDNA library amplified with sense and antisense primers to KCNN4 served as a positive control for PCR. PCR products were separated by agarose gel electrophoresis, visualised by UV illumination, and photographed using the Kodak digital science documentation and analysis system. Wide range DNA marker (Sigma, Poole, UK) was run with each gel for molecular weight comparison. PCR products were isolated from the gel (GeneClean protocol) and identified by automated fluorescence sequencing (Lark Technologies, Saffron Walden, UK). The resulting sequence was aligned with that in GenBank (AF000972) using the CLUSTAL X algorithm.
Statistical analysis
Results are expressed as mean (SEM). Statistical analyses were performed using the Student’s t test where p<0.05 was considered significant.
RESULTS
Properties of basolateral K+ channels in human colonic crypts
We have previously described Ba2+ blockable Ca2+ sensitive intermediate conductance K+ channels in the basolateral membrane of human colonic crypts which were present in approximately 75% of patches.7,8 In this study, similar channels were observed in 58/113 (51%) cell attached basolateral membrane patches in the mid third of isolated human colonic crypts. With NaCl solution in the bath and KCl solution in the pipette, inward currents were seen at the resting membrane potential (zero holding voltage) in cell attached basolateral membrane patches, and linear current-voltage relationships indicated a mean slope conductance and reversal potential of 27 (1) pS and 28 (6) mV, respectively (n=15), and channel activity was voltage independent between −80 mV and −20 mV. Curvilinear current-voltage relationships were seen after excision of the patches into the inside out configuration (n=11) from which the K+:Na+ permeability ratio (PK:PNa) and reversal potential were calculated (41 (3):1 and 74 (1) mV, respectively), using the Goldman-Hodgkin-Katz current and voltage equations.19,20 When inside out patches were exposed to KCl solution in the bath and pipette, the current-voltage relationships indicated inward rectification of the K+ channel, single channel conductance being greater at −60 mV than at +60 mV (29 (2) pS v 12 (2) pS; p<0.0008, n=7).
Non-genomic inhibition of basolateral K+ channels by aldosterone
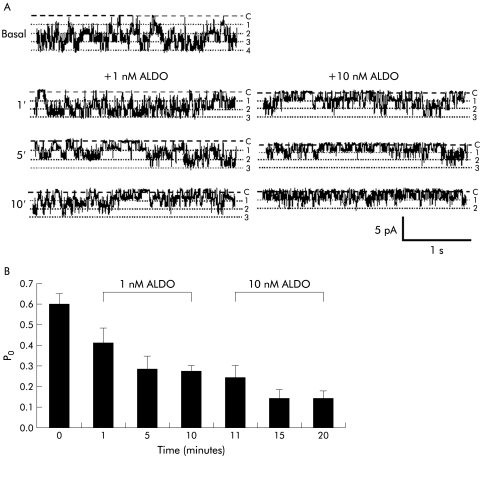
In cell attached basolateral membrane patches (holding voltage −40 mV), the addition of 1 nmol/l aldosterone to the bath solution rapidly decreased spontaneous 27 pS K+ channel activity, and this decreased further when the concentration of aldosterone was increased to 10 nmol/l (fig 2A▶). Data from 11 patches are summarised in fig 2B▶ which shows that adding 1 nmol/l aldosterone decreased Po from 0.59 (0.06) to 0.41 (0.07) (by 31%) after one minute (p<0.004), to 0.28 (0.07) (by 53%) after five minutes (p<0.0005 compared with basal values), and to 0.27 (0.04) (by 54%) after 10 minutes (p<0.0005 compared with basal values). In seven patches, increasing aldosterone concentration to 10 nmol/l decreased Po further from 0.32 (0.02) to 0.14 (0.05) (by a further 56%) after five minutes (p<0.01), a low level of activity which was maintained for an additional 10 minutes. In six cell attached patches not exposed to aldosterone, which served as time controls, channel activity did not change significantly during the 25 minute recording period (Po 0.58 (0.12) at time zero, Po 0.57 (0.16) after 25 minutes; NS).
Figure 2 .
Non-genomic inhibition of intermediate conductance basolateral K+ channels by aldosterone (ALDO). (A) Representative recordings from a cell attached patch containing multiple channels (holding voltage −40 mV, referenced to pipette interior; 140 mmol/l Na+ in bath, 145 mmol/l K+ in pipette), showing rapid onset of inhibition of K+ channel activity by 1 nmol/l aldosterone, with further inhibition after increasing the aldosterone concentration to 10 nmol/l. Dashed lines indicate zero current levels (C) and dotted lines open channel levels (1–4). (B) Summary of data from 7–11 cell attached patches.
Single channel current amplitude decreased significantly from 1.86 (0.30) pA to 1.46 (0.29) pA (p<0.015) 10 minutes after addition of 1 nmol/l aldosterone, suggesting depolarisation of the cell membrane. In contrast, in the time control studies, there was no change in current amplitude after 10 minutes (1.77 (0.27) pA v 1.71 (0.32) pA; NS). Cell membrane depolarisation induced by aldosterone was consistent with inhibition of basolateral K+ channels.
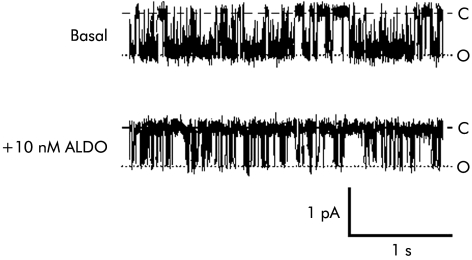
Close inspection of fig 2A▶ reveals that aldosterone decreased the apparent number of K+ channels in the patch. Thus the mineralocorticoid may stimulate recycling of channels into a pool of cytoplasmic vesicles rather than have a direct inhibitory effect on channels located in the basolateral membrane. However, as shown in fig 3▶, in five patches containing a single K+ channel, 10 nmol/l aldosterone decreased channel activity rather than abolishing it entirely, which is consistent with direct inhibition of the channels rather than stimulation of channel recycling.
Figure 3 .
Non-genomic inhibition of intermediate conductance basolateral K+ channels by aldosterone (ALDO). Representative recordings from a cell attached patch containing a single channel (holding voltage −40 mV, referenced to pipette interior; 140 mmol/l Na+ in bath, 145 mmol/l K+ in pipette) showing inhibition of K+ channel activity 10 minutes after addition of 10 nmol/l aldosterone. Dashed lines indicate zero current levels (C) and dotted lines open channel levels (O). The presence of a low residual level of channel activity post aldosterone suggests that channels are regulated in situ within the basolateral membrane rather than being endocytosed.
Effect of EIPA on K+ channel inhibition by aldosterone
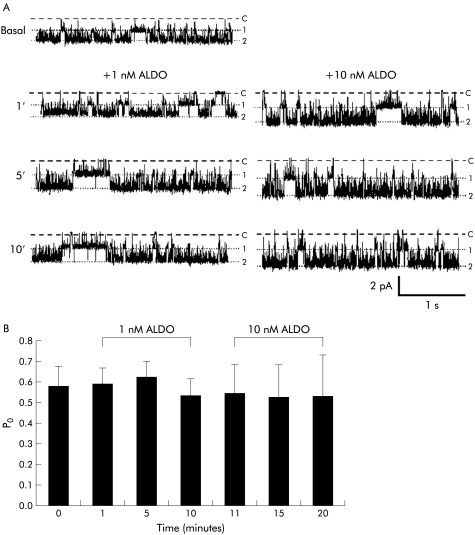
In a separate series of experiments, crypts were pretreated with 20 μmol/l EIPA, a specific Na+:H+ exchange inhibitor, which had no effect on channel activity in cell attached basolateral membrane patches after five minutes at a holding voltage of −40 mV (Po 0.53 (0.12) v Po 0.58 (0.10); n=6, NS). As shown in fig 4A▶, the subsequent addition of 1–10 nmol/l aldosterone had no effect on 27 pS K+ channel activity in the continuing presence of EIPA. Data from six experiments, summarised in fig 4B▶, indicate that channel activity was unchanged by 1 nmol/l aldosterone (Po 0.59 (0.08) after one minute, 0.63 (0.07) after five minutes, and 0.54 (0.08) after 10 minutes). In four patches, the inhibitory effect of 10 nmol/l aldosterone was also prevented by EIPA (Po 0.55 (0.14) after one minute, 0.54 (0.16) after five minutes, and 0.54 (0.20) after 10 minutes). Single channel current amplitude in the presence of EIPA was similar before and 10 minutes after addition of 1 nmol/l aldosterone (1.80 (0.38) pA and 2.04 (0.36) pA, respectively; NS), indicating that EIPA prevented the cell membrane depolarisation and decrease in current observed when aldosterone was added in the absence of EIPA (see above).
Figure 4 .
Ethylisopropylamiloride (EIPA) prevented non-genomic inhibition of intermediate conductance basolateral K+ channels by aldosterone (ALDO). (A) Representative recordings from a cell attached patch containing multiple channels (holding voltage −40 mV, referenced to pipette interior; 140 mmol/l Na+ in bath, 145 mmol/l K+ in pipette) showing failure of 1–10 nmol/l aldosterone to inhibit K+ channel activity in the presence of 20 μmol/l EIPA, a specific Na+:H+ exchange inhibitor. Dashed lines indicate zero current levels (C) and dotted lines open channel levels (1, 2). (B) Summary of data from six cell attached patches.
An additional set of experiments was performed to determine whether prolonged exposure to EIPA alone affected channel activity. In five cell attached patches, channel activity was similar before and 25 minutes after exposure to 20 μmol/l EIPA (Po 0.54 (0.07) and 0.55 (0.01), respectively; NS). Thus EIPA per se had no effect on the activity of the 27 pS K+ channel during the time course of these experiments.
pH sensitivity of basolateral K+ channels
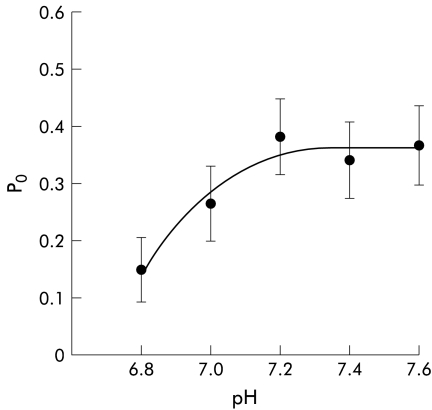
As shown above, EIPA prevented the non-genomic inhibitory effect of aldosterone on the 27 pS K+ channels, which suggests that aldosterone stimulates Na+:H+ exchange. This would have the effect of increasing intracellular pH, raising the possibility that cellular alkalinisation causes K+ channel inhibition. To investigate this further, recordings were obtained from excised inside out patches bathed in an “intracellular” high K+ solution at different values of pH between pH 6.8 to 7.6. Figure 5▶ shows that the 27 pS K+ channel was moderately pH sensitive, Po in eight patches increasing from 0.15 (0.06) at pH 6.8 to 0.37 (0.07) at pH 7.6 (p<0.04). These data indicate that alkalinisation alone causes stimulation rather than inhibition of the 27 pS K+ channel.
Figure 5 .
pH sensitivity of intermediate conductance basolateral K+ channels. Summary of data from eight excised inside out basolateral membrane patches, showing the pattern of channel activity when the pH of the bath solution was varied between 6.8 and 7.6 (holding voltage −40 mV, referenced to pipette interior; 145 mmol/l K+ in bath and pipette).
Detection of KCNN4 mRNA in human colonic crypt cells
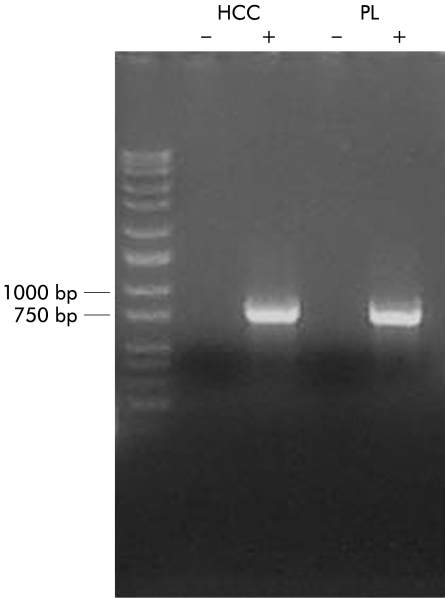
To investigate whether KCNN4 is expressed in colonic epithelial cells, total RNA was extracted from a human colonic crypt isolate which was devoid of all non-epithelial cell types such as fibroblasts, endothelial cells, and lamina propria cells (fig 1▶). Using primers specific to human KCNN4, RT-PCR (performed in triplicate) produced a single 761 base pair product (fig 6▶) which when sequenced showed 100% homology with human placental KCNN4 (GenBank accession No AF000972). These data confirm that KCNN4 mRNA is expressed in human colonic epithelial cells.
Figure 6 .
KCNN4 mRNA is present in human colonic epithelial cells. Reverse transcription of total RNA from colonic crypts (HCC) performed in the presence (+) and absence (−) of Superscript and amplified using specific primers for human KCNN4. A human placental cDNA library (PL) amplified with KCNN4 primers acted as a positive control. DNA molecular weight ladder is shown on the far left of the gel.
DISCUSSION
Mammalian distal colon is a major target epithelium for the genomic effects of aldosterone, which include stimulation of electrogenic Na+ absorption and an electrogenic K+ secretory process.21 These changes appear within hours of type 1 mineralocorticoid receptor activation although the full transport effects of sustained aldosterone stimulation occur after 2–3 days.22 Aldosterone also has non-genomic effects on colonic ion transport, increasing intracellular Ca2+ and stimulating protein kinase C activity in rat and human colonic epithelia.14–16 These effects are associated with an increase in Na+:H+ exchange that leads to intracellular alkalinisation.14 It has been suggested that the non-genomic effects of aldosterone inhibit the macroscopic, Ca2+ sensitive, basolateral K+ conductance involved in Cl− secretion,14 although this K+ conductance was not characterised at the single channel level. The present study provides the first indication that 27 pS basolateral K+ channels in human colonic crypt cells constitute the aldosterone inhibitable macroscopic basolateral K+ conductance in this epithelium. Aldosterone at low concentration (1 nmol/l) produced significant and sustained channel inhibition after one minute, which was even more marked at higher concentrations (10 nmol/l) (fig 2▶). This effect was too rapid to be accounted for by changes in gene transcription or translation of mRNA. It is also unlikely to have reflected stimulation of K+ channel recycling because limited channel activity persisted (rather than disappearing entirely) in patches containing a single K+ channel after addition of aldosterone (fig 3▶).
The non-genomic inhibitory effect of aldosterone on colonic Cl− secretion seems to be a direct consequence of downregulation of Ca2+ sensitive basolateral K+ channels,14 which are likely to be the inwardly rectifying 27 pS K+ channels we have identified in human colonic crypt cells. Similar basolateral K+ channels exist in T84 cells and rat colonic crypts, where they play a critical role in the Cl− secretory process.23,24 Their inhibition leads to a reduction in K+ recycling across the basolateral membrane (thus decreasing cellular Cl− uptake by Na+-K+-2Cl− cotransport), and failure to maintain the cell hyperpolarisation necessary for sustained Cl− exit via apical Cl− channels. The rapid inhibitory effect of aldosterone on Ca2+ dependent macroscopic basolateral K+ conductance in the rat and human colon has been linked to stimulation of protein kinase C sensitive Na+:H+ exchange and a rise in cytosolic pH.14 Aldosterone has a variety of non-genomic effects on signalling pathways in non-epithelial cells, changing intracellular ion concentrations, cell volume, and Na+:H+ exchange in human mononuclear leucocytes, 25,26 Na+:H+ exchange in vascular smooth muscle cells,27 increasing intracellular Ca2+ in vascular smooth muscle cells and porcine aortic endothelial cells,28,29 and enhancing phosphoinositide hydrolysis in human mononuclear leucocytes and vascular smooth muscle cells.30,31 In the present study, the presence of EIPA prevented the inhibitory effect of aldosterone on the 27 pS K+ channels, which suggests that aldosterone stimulated Na+:H+ exchange and caused intracellular pH to rise. Aldosterone has been shown to produce a rapid increase in intracellular pH from 7.11 to 7.32 in isolated human colonic crypts, the implication being that cellular alkalinisation is linked in some way to inhibition of the Ca2+ dependent macroscopic basolateral K+ conductance,14 which we believe is equivalent to 27 pS K+ channels. However, our studies with excised inside out patches indicate that alkalinisation alone stimulates these K+ channels (fig 5▶). Thus it seems that intracellular alkalinisation per se does not mediate the non-genomic inhibition of aldosterone of 27 pS basolateral K+ channels although it is possible that another pH sensitive signalling pathway may be involved.
The second aspect of this study was the molecular characterisation of 27 pS basolateral K+ channels. The biophysical and pharmacological properties of these K+ channels in human and rat colonic crypts, and in T84 cells, are similar to the IKCa channel cloned from cDNA libraries of a variety of tissues.9–12 The IKCa channel shows inward rectification, is extremely sensitive to Ca2+, binds calmodulin to the C terminus of each of its four subunits, and has minimal voltage sensitivity.9–12,32 In Cl− secreting epithelia such as the lung and colon, the role of IKCa channels is to hyperpolarise the cells and maintain the drive for electrogenic Cl− secretion.8,33,34 Using specific primers for human KCNN4, RT-PCR confirmed the presence of KCNN4 mRNA in human colonic cells (fig 6▶). KCNN4 mRNA has previously been identified by RT-PCR in mucosal scrapings of rat and human colon and in malignantly transformed T84 cells.13 The crypt isolation method we used has the advantage of providing non-malignant human colonic epithelial cells devoid of red blood cells, lymphoid cells, fibroblasts, endothelial cells, and smooth muscle cells (fig 1▶), all of which express IKCa (KCNN4) channels.11,35–38 It therefore seems reasonable to assume that the KCNN4 mRNA originated from colonic crypt cells. We cannot exclude the possibility that apical IKCa channels may also be present in human colonic crypt cells (adherent mucus prevented single channel recording from the apical membrane in crypts opened longitudinally) but we have only ever seen high conductance (220 pS) K+ channels in the apical membrane of surface cells around the luminal openings of human colonic crypts39 (and unpublished data). Thus we take the view that KCNN4 mRNA encodes 27 pS basolateral K+ channels in human colonic crypt cells, which are identical to the IKCa channels found in many other epithelial and non-epithelial cell types.
In summary, nanomolar concentrations of aldosterone exert a non-genomic inhibitory effect on 27 pS K+ channels in the basolateral membranes of human colonic crypt cells, thereby reducing the capacity of the colon for Cl− secretion. Although aldosterone has an associated non-genomic stimulatory effect on Na+:H+ exchange (which increases intracellular pH), our data suggest that alkalinisation in isolation enhances rather than inhibits the activity of these K+ channels, and the exact role of Na+:H+ exchange remains unclear. Nevertheless, these channels have similar biophysical properties to KCNN4 channels present in a variety of other human tissues. Having identified KCNN4 mRNA transcripts in these colonic crypt cells, we conclude that KCNN4 encodes 27 pS K+ channels residing in the basolateral (but not the apical) membrane domain.
Acknowledgments
KAB was supported by a Medical Research Council PhD Studentship. This work was supported by the Wellcome Trust and the Medical Research Council. The human placental cDNA library was a gift from Dr A Sivaprasadarao, Department of Biomedical Sciences, University of Leeds.
Abbreviations
EIPA, ethylisopropylamiloride
RT-PCR, reverse transcriptase-polymerase chain reaction
REFERENCES
- 1.Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract, vol 2, 3rd edn. New York: Raven, 1994:2133–71.
- 2.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 1986;77:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandel KG, McRoberts JA, Beuerlein G, et al. Ba2+ inhibition of VIP-stimulated and A23187-stimulated Cl− secretion by T84 cell monolayers. Am J Physiol 1986;250:C486–94. [DOI] [PubMed] [Google Scholar]
- 4.Devor DC, Singh AK, Gerlach AC, et al. Inhibition of intestinal Cl− secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol 1997;273:C531–40. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder BC, Waldegger S, Fehr S, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 2000;403:196–9. [DOI] [PubMed] [Google Scholar]
- 6.Warth R, Riedemann N, Bleich M, et al. The cAMP regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflügers Arch 1996;432:81–8. [DOI] [PubMed] [Google Scholar]
- 7.Lomax RB, Warhurst G, Sandle GI. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 1996;38:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandle GI, McNicholas CM, Lomax RB. Potassium channels in colonic crypts. Lancet 1994;343:23–5. [DOI] [PubMed] [Google Scholar]
- 9.Joiner WJ, Wang LY, Tang MD, et al. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A 1997;94:11013–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii TM, Silvia C, Hirschberg B, et al. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A 1997;94:11651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logsdon NJ, Kang J, Togo JA, et al. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem 1997;272:32723–6. [DOI] [PubMed] [Google Scholar]
- 12.Khanna R, Chang MC, Joiner WJ, et al. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. J Biol Chem 1999;274:14838–49. [DOI] [PubMed] [Google Scholar]
- 13.Warth R, Hamm K, Bleich M, et al. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Arch 1999;438:437–44. [DOI] [PubMed] [Google Scholar]
- 14.Maguire D, MacNamara B, Cuffe JE, et al. Rapid responses to aldosterone in human distal colon. Steroids 1999;64:51–63. [DOI] [PubMed] [Google Scholar]
- 15.Doolan CM, Harvey BJ. Rapid effects of steroid hormones on free intracellular calcium in T84 colonic epithelial cells. Am J Physiol 1996;271:C1935–41. [DOI] [PubMed] [Google Scholar]
- 16.Doolan CM, O’Sullivan GC, Harvey BJ. Rapid effects of corticosteroids on cytosolic protein kinase C and intracellular calcium concentration in human distal colon. Mol Cell Endocrinol 1998;138:71–9. [DOI] [PubMed] [Google Scholar]
- 17.Strater J, Wedding U, Barth TFE, et al. Rapid onset of apoptosis in vitro follows disruption of β1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 1996;110:1776–84. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 19.Goldman DE. Potential, impedence and rectification in membranes. J Gen Physiol 1943;27:37–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgkin AL, Katz B. Effect of sodium on the electrical activity of the giant axon of the squid. J Physiol (Lond) 1949;108:37–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder HJ, McGlone F, Sandle GI. Effects of corticosteroid hormones on the electrophysiology of rat distal colon—implications for Na+ and K+ transport. J Physiol (Lond) 1989;410:425–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halevy J, Budinger ME, Hayslett JP, et al. Role of aldosterone in the regulation of sodium and chloride transport in the distal colon of sodium-depleted rats. Gastroenterology 1986;91:1227–33. [DOI] [PubMed] [Google Scholar]
- 23.Devor DC, Frizzell RA. Calcium-mediated agonists activate an inwardly rectified K+ channel in colonic secretory cells. Am J Physiol 1993;265:C1271–80. [DOI] [PubMed] [Google Scholar]
- 24.Bleich M, Riedemann N, Warth R, et al. Ca2+ regulated K+ and non-selective cation channels in the basolateral membrane of rat colonic crypt base cells. Pflügers Arch 1996;432:1011–22. [DOI] [PubMed] [Google Scholar]
- 25.Wehling M, Armanini D, Strasser T, et al. Effect of aldosterone on sodium and potassium concentrations in human mononuclear leukocytes. Am J Physiol 1987;252:E505–8. [DOI] [PubMed] [Google Scholar]
- 26.Wehling M, Käsmayr J, Theisen K. Fast effects of aldosterone on electrolytes in human lymphocytes are mediated by the sodium-proton-exchanger of the cell membrane. Biochem Biophys Res Commun 1989;164:961–7. [DOI] [PubMed] [Google Scholar]
- 27.Christ M, Douwes K, Eisen C, et al. Rapid nongenomic effects of aldosterone on sodium-transport in rat vascular smooth-muscle cells: Involvment of the Na+/H+-antiport. Hypertension 1995;25:117–23. [DOI] [PubMed] [Google Scholar]
- 28.Wehling M, Neylon C, Fullerton M, et al. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res 1995;76:973–9. [DOI] [PubMed] [Google Scholar]
- 29.Wehling M, Ulsenheimer A, Schneider M, et al. Rapid effects of aldosterone on free intracellular calcium in vascular smooth muscle and endothelial cells: subcellular localization of calcium elevations by single cell imaging. Biochem Biophys Res Commun 1994;204:475–81. [DOI] [PubMed] [Google Scholar]
- 30.Christ M, Eisen C, Aktas J, et al. The inositol-1,4,5-trisphosphate system is involved in rapid effects of aldosterone in human mononuclear leukocytes. J Clin Endocrinol Metab 1993;77:1452–7. [DOI] [PubMed] [Google Scholar]
- 31.Christ M, Meyer C, Sippel K, et al. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase Cα. Biochem Biophys Res Commun 1995;213:123–9. [DOI] [PubMed] [Google Scholar]
- 32.Fanger CM, Ghanshani S, Logsdon NJ, et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem 1999;274:5746–54. [DOI] [PubMed] [Google Scholar]
- 33.McCann JD, Matsuda J, Garcia M, et al. Basolateral K+ channels in airway epithelia. 1. Regulation by Ca2+ and block by charybdotoxin. Am J Physiol 1990;258:L334–42. [DOI] [PubMed] [Google Scholar]
- 34.Welsh MJ, McCann JD. Intracellular calcium regulates basolateral potassium channels in a chloride-secreting epithelium. Proc Natl Acad Sci U S A 1985;82:8823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn PM. The action of blocking agents applied to the inner face of Ca2+-activated K+ channels from human erythrocytes. J Membrane Biol 1998;165:133–43. [DOI] [PubMed] [Google Scholar]
- 36.Kohler R, Degenhardt C, Kuhn M, et al. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery—A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res 2000;87:496–503. [DOI] [PubMed] [Google Scholar]
- 37.Neylon CB, Lang RJ, Fu Y, et al. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle. Circ Res 1999;85:33–43. [DOI] [PubMed] [Google Scholar]
- 38.Pena TL, Chen SH, Konieczny SF, et al. Ras/MEK/ERK up-regulation of the fibroblast K-Ca channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J Biol Chem 2000;275:13677–82. [DOI] [PubMed] [Google Scholar]
- 39.Mathialahan T, Perry MD, Sandle GI. Does bisacodyl decrease hyperkalaemia in end-stage renal failure by stimulating colonic apical K+ channels? Gastroenterology 2000;118:A607. [Google Scholar]