Abstract
Background: The purpose of this study was to assess the feasibility and usefulness of a new magnetic resonance (MR) colonography technique for the detection of colorectal pathology in comparison with conventional colonoscopy as the standard of reference.
Patients and methods: A total of 122 subjects with suspected colorectal disease underwent “dark lumen” MR colonography. A contrast enhanced T1w three dimensional VIBE sequence was collected after rectal administration of water. The presence of colorectal masses and inflammatory lesions were documented. Results were compared with those of a subsequently performed colonoscopy.
Results: MR colonography was found to be accurate regarding detection of clinically relevant colonic lesions exceeding 5 mm in size, with sensitivity and specificity values of 93%/100%.
Conclusion: Dark lumen MR colonography can be considered as a promising alternative method for the detection of colorectal disease. In addition, it allows assessment of extraluminal organs.
Keywords: colorectal masses, conventional colonoscopy, inflammatory bowel disease, magnetic resonance imaging, virtual colonoscopy
Conventional colonoscopy represents the gold standard for the detection of colorectal pathologies.1 Poor patient acceptance due to invasiveness and procedure related discomfort and high cost limit the utility of the technique for colorectal screening.2,3 Virtual colonography based on the acquisition of three dimensional computed tomography (CT) or MR imaging data sets can overcome this limitation. Initial studies documented high diagnostic accuracies for both CT and MR colonography.4,5 Reflecting their non-invasive character, these techniques are preferred over conventional endoscopy by the majority of patients.2,3 Exposure to ionising radiation casts a shadow over the future of CT as a screening examination.6,7 Hence efforts should be focussed on MR colonography (MRC).
To date, most approaches to MRC have been based on administration of a rectal enema containing paramagnetic contrast.8–11 On T1w data sets, polypoid colonic masses appear as dark filling defects within the bright colonic lumen—an appearance which renders differentiation of polyps from residual faecal material and/or small pockets of air very difficult. Furthermore, the technique requires three dimensional data acquisitions in both the prone and supine patient positions to compensate for the presence of residual air.
Recently “dark lumen” MRC has been introduced.12 The technique is based on the acquisition of a T1w sequence collected following administration of a water enema and intravenous administration of paramagnetic contrast. The colonic wall as well as masses arising from it enhance brightly and are thus easily delineated against the background of a dark water filled colonic lumen. In a preliminary trial involving 12 patients,12 dark lumen MRC was found to be highly accurate regarding the detection of colorectal masses.
The goal of this study was to assess the diagnostic accuracy of dark lumen MRC for the detection of a variety of colorectal pathologies in a larger patient cohort using conventional endoscopy as the gold standard.
METHODS
The study was conducted in accordance with all guidelines set forth by the approving institutional review board. Informed consent was obtained prior to each examination. Exclusion criteria included contraindications to MR imaging, such as the presence of a pacemaker, metallic implants in the central nervous system, or claustrophobia.
Subjects
Over a nine month period, MRC was performed on 122 subjects (56 men, 66 women; age range 17–90 years (mean 60.2)). Patients had been referred for conventional colonoscopy for various indications, including abdominal pain (n = 29), suspected Crohn’s disease or ulcerative colitis (n = 29), a positive faecal occult blood test (n = 18), a positive family history of colorectal cancer (n = 11), elevated hepatic enzymes (n = 7), immunosupression (n = 7), chronic diarrhoea (n = 4), a previous history of colorectal cancer (n = 5), and other (n = 12).
MR imaging
Following a standard preparation for bowel cleansing (oral ingestion of 3000 ml Golytely; Braintree Laboratories, Braintree, Massachusetts, USA) MR examinations were performed on a 1.5 T MR system (Magnetom Sonata; Siemens Medical Solutions, Erlangen, Germany) in the prone position. A combination of two surface coils were used in conjunction with the built-in spine array coil for signal reception to permit coverage of the entire colon. To minimise bowel peristalsis, 40 mg of scopolamine (Buscopan; Boehringer Ingelheim, Germany) were injected intravenously. No patient presented any contraindications to administration of scopolamine, such as the presence of glaucoma or severe cardiac arrhythmia.
Following placement of a rectal enema tube (E-Z-Em; Westbury, New York, USA), the colon was filled with approximately 2000 ml of warm tap water using hydrostatic pressure (1–1.5 m water column). The filling process was monitored with a real time fluoroscopic sequence (TR/TE 2.4/1.2 ms, flip 60°) permitting the acquisition of one image per second.
Following bowel distension, the first T1w three dimensional gradient echo data set was collected in the coronal plane. Sequence parameters included: TR/TE 3.1/1.1 ms, flip angle 12°, field of view 450×450 mm, matrix 168×256, and an effective slice thickness of 4.0 mm. Subsequently paramagnetic contrast (Gd-BOPTA; Multihance, Bracco, Italy) was administered intravenously at a dose of 0.2 mmol/kg and a flow rate of 3.5 ml/s. Following a delay of 75 seconds, the three dimensional acquisition was repeated with identical imaging parameters. The three dimensional data were collected breathheld over 22 seconds.
Conventional colonoscopy
After completion of MRC, conventional colonoscopy was performed using standard equipment (model CFQ 140; Olympus). The attending gastroenterologist was unaware of the MR findings. When necessary, sedatives (midazolam, Dormicum; Roche, Germany) or analgesics (pethidine, Dolantin; Hoechst, Germany) were administered. Location and size of colorectal masses were recorded. All polyps were removed. Suspicious cancers and inflammatory lesions were biopsied. All polyps and biopsy materials were analysed histopathologically. In nine patients conventional colonoscopy was incomplete. Causes included severe abdominal pain (n = 2), elongation of the sigmoid colon (n = 3), and non-passable stenosis (n = 4).
Data analysis
For each subject, both native and contrast enhanced three dimensional MR imaging data sets were transferred to a post processing workstation (Virtuoso; Siemens Medical Solutions). The data sets were assessed by two experienced radiologists in the multiplanar reformation mode, which permitted scrolling through the three dimensional data sets in all three orthogonal planes. For the purposes of analysis, the colon was divided into six segments: rectum (s1), sigmoid colon (s2), descending colon (s3), transverse colon (s4), ascending colon (s5), and caecum (s6).
MRC quality was assessed both qualitatively and quantitatively. Distension of each colonic segment was classified as: 1 = well distended, 2 = moderately distended, and 3 = poorly distended. Furthermore, each segment was evaluated for the presence of artefacts: 1 = no artefacts,; 2 = moderate artefacts, diagnostic image quality; 3 = extensive artefacts, non-diagnostic image quality. For quantitative analysis, regions of interest (ROI) were placed in the lumen and adjacent normal wall of all segments. Image noise, defined as the standard deviation of signal intensities measured in an ROI placed outside the body, was determined. Based on these measurements contrast to noise ratios (CNR) for representative parts of all bowel segments were calculated: CNR = (SI (colonic wall/colonic lesion)−SI (lumen))/noise. Localisation and size of all detected endoluminal masses were recorded.
Colorectal lesions were classified based on size: (a) <5 mm, (b) 5–10 mm, and (c) >10 mm in diameter. All MR data sets were assessed for the presence of inflammatory bowel disease. Criteria used included bowel wall thickening, increased contrast uptake of segmental parts of the colon, and loss of haustral folds. CNR values of all colorectal masses as well as colonic wall segments affected by inflammatory disease were determined in the same manner as described previously. Using conventional colonoscopy as the standard of reference, MRC accuracy was assessed by calculating point estimates for sensitivity and specificity.
RESULTS
Image quality
Image quality was “diagnostic” in 120 of 122 MRC examinations. Severe respiration induced motion artefacts rendered the interpretation of two MR data sets impossible. All other 120 MRC data sets were rated diagnostic, with a mean artefact value of 1.21. Best image quality was found in segment 1 (mean value 1.12) and poorest results were seen in segments 3 and 5 (average value 1.25). Artefacts were mainly related to moderate respiratory motion or wrap around. The mean distension value was 1.17, with best results in segments 1 and 6 (mean value 1.13) and poorest distension in segment 2 (mean value 1.27). Detailed data for all segments are listed in table 1 ▶.
Table 1.
Image quality of dark lumen MRC per bowel segment (S1 = rectum, S2 = sigmoid colon, S3 = descending colon, S4 = transverse colon, S5 = ascending colon, and S6 = caecum)
| Segment | |||||||
| S 1 | S 2 | S 3 | S 4 | S 5 | S 6 | Average | |
| Distension | 1.16 | 1.27 | 1.18 | 1.16 | 1.14 | 1.13 | 1.17 |
| Artefacts | 1.12 | 1.23 | 1.25 | 1.21 | 1.24 | 1.21 | 1.21 |
| CNR without IBD | 37.0 | 42.0 | 23.0 | 44.0 | 21.0 | 27.0 | 31.0 |
| CNR with IBD | 55 | 78 | 41 | 69 | 39 | 53 | 54 |
Mean values of bowel distension (1 = well distended, 2 = moderately distended, 3 = poorly distended) and evaluation of artefacts (1 = no artefacts, 2 = moderate artefacts but diagnostic image quality, 3 = extensive artefacts). Furthermore, the contrast to noise ratio (CNR) of the bowel wall in patients without and with inflammatory bowel disease (IBD) is shown.
Colorectal lesions
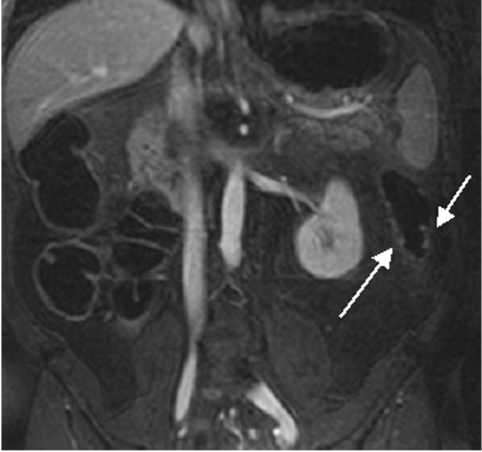
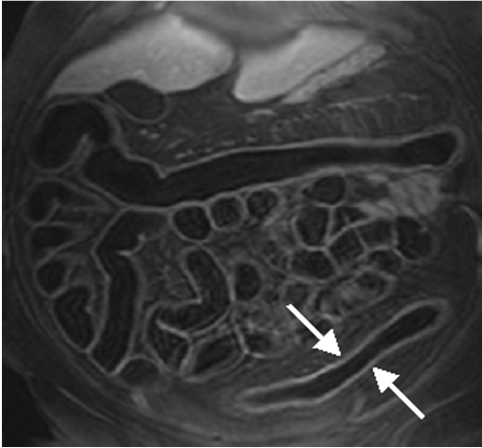
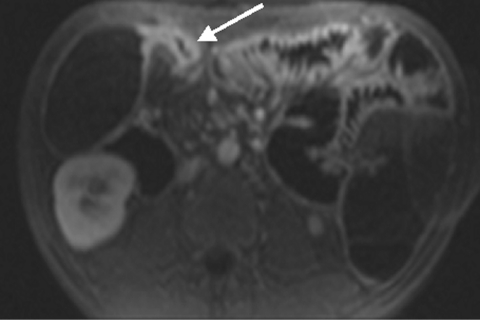
None of 30 polyps measuring <5 mm identified by conventional colonoscopy were detected on MRC images. In the size group 5–10 mm, MRC correctly detected 16 of 18 conventional colonoscopy documented lesions, and two polyps >10 mm were correctly seen on MRC images. Two of three patients with conventional colonoscopy documented polyposis coli were correctly diagnosed as such on MRC (fig 1 ▶). The third patient exhibited only very small polyps considered normal by MRC. All nine colorectal carcinomas with sizes exceeding 10 mm in all cases (range 13–22 mm) identified on conventional colonoscopy were seen on MRC images (fig 2 ▶). In one patient, a 10 mm intraluminal lipoma was detected by conventional colonoscopy, which was missed by MRC.
Figure 1.
Dark lumen magnetic resonance colonography of a 39 year old male patient with polyposis coli. Multiple small polyps can be detected in the descending colon (arrows).
Figure 2.
Dark lumen magnetic resonance colonography of a 56 year old female patient. A 22 mm sized protruding lesion in seen in the sigmoid colon (arrow). Conventional endoscopy confirmed the presence of a sigmoid carcinoma.
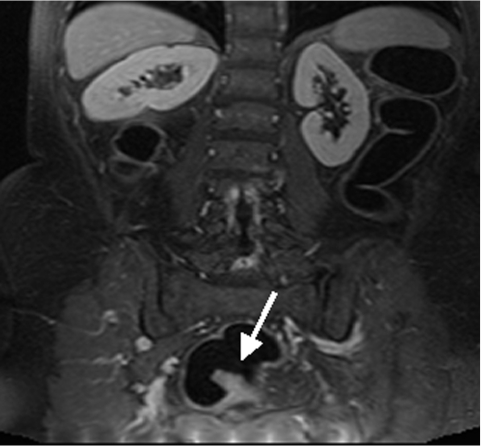
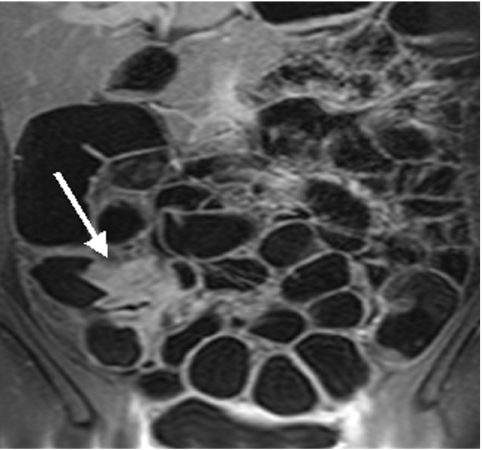
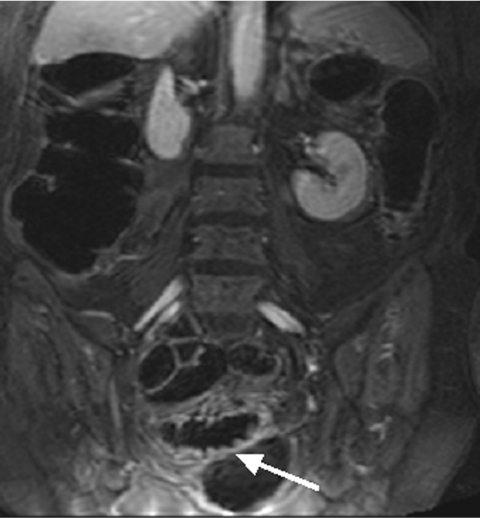
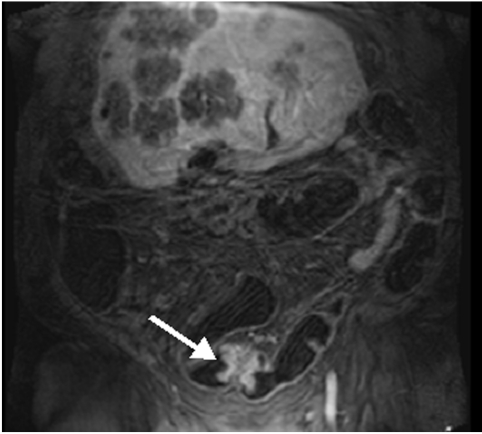
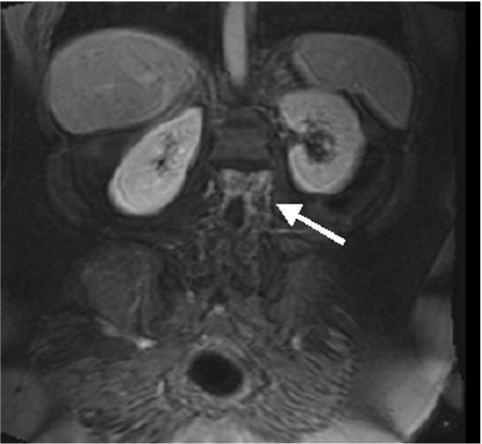
Conventional colonoscopy documented inflammatory wall alterations consistent with Crohn’s disease in 15 and ulcerative colitis in 13 patients. Of those, MRC correctly diagnosed inflammatory changes in 13 and 12 patients with Crohn’s disease and ulcerative colitis, respectively. Ulcerative colitis impressed as a loss of haustral markings and revealed increased contrast uptake of the affected bowel segments (figs 3 ▶, 4 ▶). Crohn’s disease revealed skip lesions characterised by a thickened colonic wall and increased contrast uptake. In three patients with Crohn’s disease, interintestinal fistulae were detected both with MRC and conventional colonoscopy (fig 5 ▶). Acute diverticulitis was diagnosed both on conventional colonoscopy and MRC in the same five patients (fig 6 ▶). MRC identified diverticula without inflammation in 14 of 16 patients with conventional colonoscopy documented diverticulosis. There were no false positive readings based on the MRC data sets.
Figure 3.
Thirty nine year old female patient. Loss of haustral markings and increased contrast uptake of the colonic wall was determined (arrows). Conventional colonoscopy confirmed the diagnosis of ulcerative colitis.
Figure 4.
Dark lumen magnetic resonance colonography of a 34 year old female patient with known Crohn’s disease. The thickened bowel wall of the terminal ileum shows increased contrast uptake (arrow). Subsequent endoscopy and biopsy confirmed the presence of an acute inflammatory bowel disease.
Figure 5.
Twenty seven year old female patient with known Crohn’s disease. An interintestinal fistula between the ascending colon and ileum was detected (arrow).
Figure 6.
Seventy six year old patient with known diverticulosis of the sigmoid colon. This patient was transferred to the Department of Gastroenterology because of acute abdominal pain. Increased contrast uptake of the sigmoid bowel wall was seen (arrow) and the patient was rated as suffering from diverticulitis. This was subsequently confirmed by endoscopy.
Contrast to noise ratio (CNR)
Mean CNR between the bowel wall and bowel lumen was 31 (3.4) for the post contrast scans compared with 16 (2.6) for the pre contrast scans. Inflammatory wall alterations caused by Crohn’s disease as well as ulcerative colitis were associated with an increase in CNR (mean 54 (4.2)). On average, CNR values determined within colorectal masses also exceeded those seen in the normal colonic wall. Detailed analysis however revealed a rather heterogeneous distribution of CNR values. In 19 lesions (seven carcinomas and 12 polyps), CNR values exceeded those in the normal colonic wall by factors ranging between 1.35 and 2.4. The remaining eight polyps and two carcinomas revealed CNR values very similar to the normal colonic wall.
Additional findings by MRC
MRC permitted assessment of extraintestinal organs. A variety of relevant and non-relevant pathologies were identified. Hepatic metastases were observed in four patients (fig 7 ▶) and bone metastasis were seen in seven patients (fig 8 ▶). All extraluminal findings are listed in table 2 ▶.
Figure 7.
Sixty five year old female patient. A stenotic colonic carcinoma was detected in the sigmoid colon (arrow). In addition, multiple hepatic metastases were visualised simultaneously.
Figure 8.
Sixty six year old male patient with known colonic carcinoma. The magnetic resonance colonography examination showed a bright spot in the lumbar spine, which was rated as an osseous metastasis (arrow). Clinical follow up confirmed this diagnosis.
Table 2.
Extraintestinal findings of dark lumen magnetic resonance colonography (MRC) divided into two groups: non-therapy and therapy relevant pathologies
| Non-therapy relevant findings | n | Therapy relevant findings | n |
| Renal cysts | 29 | Hepatic metastases | 4 |
| Hepatic cysts | 16 | Osseous metastases | 7 |
| Uterus myomatosis | 14 | Uterus carcinoma | 2 |
| Osteochondrosis | 10 | Prostate carcinoma | 1 |
| Mesenterial lymph nodes | 5 | Peritoneal carcinosis | 1 |
| Sclerosis of the aorta | 2 | Infrarenal aortic aneurysma | 1 |
| Hepatomegaly | 2 | Focal cholangitis | 1 |
| Splenomegaly | 2 | Intrahepatic cholestasis | 1 |
| Cystolithiasis | 1 | Renal cell carcinoma | 1 |
| Cholecystolithiasis | 1 | CCC | 1 |
| Hiatus hernia | 1 | Hepatocellular carcinoma | 1 |
| Double VCI | 1 | ||
| Prostate hypertrophy | 1 | ||
| Thrombosis of confluens venosum | 1 | ||
| Haemangioma of LV | 1 | ||
| Ovarial cyst | 1 | ||
| Adenoma of suprarenal glands | 1 | ||
| Hepatic haemangioma | 1 | ||
| Paramilt | 1 |
VCI, vena cava inferior; LV, lumbar vertebra; CCC, cholangiocellular carcinoma.
DISCUSSION
The presented data indicate that “dark lumen MRC” represents an attractive alternative to conventional colonoscopy for the detection of colorectal masses and inflammatory bowel disease. MRC proved robust with 120 of 122 examinations characterised as diagnostic. Beyond positioning and localisation, the technique merely requires attainment of two three dimensional data acquisitions, each lasting 22 seconds. A break of more than one minute between the two acquisitions assures excellent patient compliance with breathholding instructions. All but two mass lesions exceeding 5 mm in size were depicted by MRC. Furthermore, alterations associated with diverticulitis and inflammatory bowel disease were also detected with great accuracy. In addition, the contrast enhanced three dimensional data sets permit a rather comprehensive assessment of extracolonic abdominal organ systems.
Diagnostic MRC is predicated on the fulfilment of two requirements: good bowel distension and sufficient contrast between the bowel lumen and the colonic wall as well as pathologies originating from it. Bowel distension requires rectal administration of either liquids13 or gases such as air or CO2.14 The contrast between the lumen and surrounding wall is largely determined by the contrast characteristics of the MR sequence employed, and depends on the signal characteristics inherent in the agent used for bowel distension.
To date, most MRC concepts were based on rectal administration of a gadolinium spiked water enema.9–11 On T1w three dimensional GRE data sets, the colonic lumen containing gadolinium spiked water is rendered bright whereas the colonic wall as well as pathologies arising from it remain dark. In a study evaluating this “bright lumen” MRC technique in 132 patients, Luboldt and colleagues15 reported a sensitivity rate of 93% for colorectal lesions >1 cm. Despite these promising results, implementation of the technique into routine patient care has been slow, largely reflecting the indirect nature of the technique. Thus colorectal masses are identified solely on a filling defect in the otherwise bright colonic lumen. Differentiation between polyps or carcinomas on the one hand from residual faecal material or air bubbles on the other can therefore prove difficult and in some cases even impossible. Without extensive expertise, this diagnostic dilemma may result in a considerable amount of both false positive and false negative findings. To compensate for the residual air within the colonic lumen, “bright lumen” MRC requires data collection both in the prone and supine patient positions. Turning the patient in the midst of the examination can be complicated, and always considerably prolongs the examination. In some cases the patient moves so much that a new landmark is required. In addition, a considerable part of the rectally applied contrast may escape through the ileocaecal valve during this procedure. Hence colonic distension may become insufficient and diagnostic quality may be reduced.
Dark lumen MRC overcomes the limitations inherent in bright lumen MRC. Intravenous application of the paramagnetic contrast technique allows for the direct depiction of the colorectal wall. Thus the bright colonic wall can be easily discriminated from the dark water filled colonic lumen. This form of direct visualisation of the bowel wall and of all colorectal pathologies originating from it reduces the incidence of false positive findings: residual stool or air bubbles which might mimic small polyps in the bright lumen technique remain dark and by virtue of their nature cannot take up paramagnetic contrast. Occasionally, the presence of residual stool bright on T1 weighted images may require direct comparison with the three dimensional data set collected prior to administration of paramagnetic contrast. As the patient remains stationary between the two three dimensional acquisitions, such an analysis is easy to accomplish. Lack of contrast enhancement between the pre and post contrast scans rules out the presence of a colorectal mass.
As part of its initial description, dark lumen MRC was compared with bright lumen MRC in 12 patients16 using conventional colonoscopy as the standard of reference. Based on “dark lumen” MRC image sets, five polyps exceeding 7 mm were correctly identified in four patients. On “bright lumen” MRC, two additional lesions were seen without any correlate in dark lumen MRC or conventional colonoscopy. Hence, these “bright lumen” findings were interpreted as false positive results. The present studies confirm these preliminary results. Dark lumen MRC proved to be a reliable diagnostic tool for the detection of colorectal masses exceeding 5 mm in size. Only two of 18 lesions ranging in size from 5 to 10 mm were missed by MRC whereas all 11 lesions exceeding 10 mm, including all nine histologically verified cancers, were correctly identified as such.
Beyond the identification of colorectal lesions, “dark lumen” MRC permits the detection and characterisation of colonic wall inflammation. Based on assessment of both bowel wall thickness and bowel wall contrast enhancement, diverticulitis as well as Crohn’s disease and ulcerative colitis were diagnosed with great accuracy. The underlying diagnostic criteria have been established by others.17 Common to all three entities, the colonic wall is thickened and characterised by increased contrast uptake. Whereas ulcerative colitis results in a classic loss of haustral folds extending orally from the rectum, skip lesions are the hallmark of Crohn’s disease. Diverticulitis is usually a focal process; differentiation from a large colorectal mass can be difficult based on assessment of colorectal morphology alone. In these cases the availability of clinical data is imperative.
This study confirms avid and rather homogeneous enhancement of the normal colorectal wall, increasing the signal intensities from a mean baseline of 16 (2.6) to 31 (4) Previous work has revealed that the most extensive enhancement occurs in the late venous phase, approximately 75 seconds following intravenous application of paramagnetic contrast.18,19 At this time, all colorectal lesions revealed enhancement equal to, or exceeding, the normal colorectal wall. Similar results have been reported with CT colonography.20 Based on 27 lesions identified on MRC, there was no correlation between the degree of contrast enhancement and histological differentiation into benign or malignant. Thus seven of nine carcinomas revealed contrast enhancement exceeding that of the normal colonic wall whereas two malignant tumours did not. Similarly, enhancement profiles among benign polyps were also heterogeneous.
Contrast enhancement played a rather dominant role in the detection and characterisation of colonic wall inflammation. All wall segments identified as diseased exhibited increased enhancement following administration of paramagnetic contrast. Most avid enhancement was seen in Crohn’s disease as well as in diverticulitis. Ulcerative colitis was however also associated with increased contrast uptake. Similar observations have been reported previously in the small bowel as well as in the colon.21,22
Contrast enhanced dark lumen MRC also proved reliable in the assessment of parenchymal abdominal organs. The combination of pre and post contrast three dimensional data sets permitted identification and characterisation of hepatic lesions, including metastases, hepatocellular carcinoma, and haemangiomas. Furthermore, bone metastases and an aortic aneurysm were readily identified.
Dark lumen MRC offers new possibilities regarding the optimisation of colonic distension. Recent work has shown that water can be replaced by CO2. The gas is signalless on T1w images and therefore easily permits delineation of the contrast enhanced bowel wall. Thus any potential advantage of gas over water regarding patient acceptance can be fully exploited with “dark lumen” MRC.
Clearly, there are limitations inherent to MRC as well as study design. Although apparently reliable as a diagnostic tool, MRC does not provide therapeutic options. All patients with colorectal pathologies detected by MRC must undergo subsequent conventional colonoscopy for polypectomy or biopsy. While featuring prominently in this type of disease enriched patient population, this issue is marginalised when considering a screening population with only a moderate risk of colorectal masses of below 5%.22,23 Hence the vast majority of patients would not require colonoscopy.
Another concern relates to the inability of the technique to identify colorectal lesions smaller than 5 mm in size. The significance of this limitation is reduced by the direct observational data on growth rates, indicating that small polyps (<10 mm) remain stable over a time range of 36–48 months. Furthermore, these small lesions are not prone to malignant degeneration.24,25 None the less, it is likely that lesions <5 mm will become detectable on dark lumen MRC as technical refinements, including parallel acquisition techniques, will be implemented.26 Flat adenomas are likely to remain elusive however.
This study confirms MRC as a relatively reliable technique for the detection of colorectal lesions. Although the enhancement characteristics do not permit lesion characterisation, the study confirms that colorectal lesions do take up contrast. Similarly, contrast enhancement was observed in all cases for diverticulosis and inflammatory bowel disease. In view of the diagnostic performance of the technique, it can be employed as an alternative to conventional colonoscopy for assessing diseases of the colon.
Abbreviations
MR, magnetic resonance
MRC, MR colonography
CT, computed tomography
ROI, regions of interest
CNR, contrast to noise ratios
REFERENCES
- 1.Andrew J, Aldridge, Jay NL, et al. Histological assessment of colorectal adenomas by size. are polyps less than 10 mm in size clinically important? Eur J Surg 2001;167:777–81. [DOI] [PubMed] [Google Scholar]
- 2.Angtuaco TL, Banaad-Omiotek GD, Howden CW. Differing attitudes toward virtual and conventional colonoscopy for colorectal cancer screening: surveys among primary care physicians and potential patients. Am J Gastroenterol 2001;96:887–93. [DOI] [PubMed] [Google Scholar]
- 3.Thomeer M, Bielen D, Vanbeckevoort D, et al. Patient acceptance for CT colonography: what is the real issue? Eur Radiol 2002;12:1410–15. [DOI] [PubMed] [Google Scholar]
- 4.Dachman AH, Kuniyoshi JK, Boyle CM, et al. CT colonography with three-dimensional problem solving for detection of colonic polyps. AJR Am J Roentgenol 1998;171:989–95. [DOI] [PubMed] [Google Scholar]
- 5.Luboldt W, Steiner P, Bauerfeind P, et al. Detection of mass lesions with MR colonography: preliminary report. Radiology 1998;207:59–65. [DOI] [PubMed] [Google Scholar]
- 6.Brant-Zawadzki MN. Screening CT: rationale. Radiographics 2002;22:1532–6. [DOI] [PubMed] [Google Scholar]
- 7.Yee J. CT screening for colorectal cancer. Radiographics 2002;22:1525–31. [DOI] [PubMed] [Google Scholar]
- 8.Luboldt W, Bauerfeind P, Steiner P, et al. Preliminary assessment of three-dimensional magnetic resonance imaging for various colonic disorders. Lancet 1997;349:1288–91. [DOI] [PubMed] [Google Scholar]
- 9.Luboldt W, Frohlich JM, Schneider N, et al. MR colonography: optimized enema composition. Radiology 1999;212:265–9. [DOI] [PubMed] [Google Scholar]
- 10.Saar B, Heverhagen JT, Obst T, et al. Magnetic resonance colonography and virtual magnetic resonance colonoscopy with the 1.0-T system: a feasibility study. Invest Radiol 2000;35:521–6. [DOI] [PubMed] [Google Scholar]
- 11.Pappalardo G, Polettini E, Frattaroli FM, et al. Magnetic resonance colonography versus conventional colonoscopy for the detection of colonic endoluminal lesions. Gastroenterology 2000;119:300–4. [DOI] [PubMed] [Google Scholar]
- 12.Lauenstein TC, Herborn CU, Vogt FM, et al. Dark lumen MR-colonography: initial experience. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2001;173:785–9. [DOI] [PubMed] [Google Scholar]
- 13.Wildermuth S, Debatin JF. Virtual endoscopy in abdominal MR imaging. Magn Reson Imaging Clin N Am 1999;7:349–64. [PubMed] [Google Scholar]
- 14.Lomas DJ, Rohit R, Sood A, et al. Colon carcinoma: MR imaging with CO2 enema. Radiology 2001;219:558–62. [DOI] [PubMed] [Google Scholar]
- 15.Luboldt W, Bauerfeind P, Wildermuth S, et al. Colonic masses: detection with MR colonography. Radiology 2000;216:383–8. [DOI] [PubMed] [Google Scholar]
- 16.Luboldt W, Bauerfeind P, Wildermuth S, et al. Contrast optimization for assessment of the colonic wall and lumen in MR colonography. J Magn Reson Imaging 1999;9:745–50. [DOI] [PubMed] [Google Scholar]
- 17.Hansmann HJ, Kosa R, Dux M, et al. The hydro-MRT of chronic inflammatory bowel diseases. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1997;167:132–8. [DOI] [PubMed] [Google Scholar]
- 18.Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, et al. MR enteroclysis protocol optimization: comparison between 3D FLASH with fat saturation after intravenous gadolinium injection and true FISP sequences. Eur Radiol 2001;11:908–13. [DOI] [PubMed] [Google Scholar]
- 19.Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, et al. MR imaging of the small bowel with a true-FISP sequence after enteroclysis with water solution. Invest Radiol 2000;35:707–11. [DOI] [PubMed] [Google Scholar]
- 20.Morrin MM, Hochmann MG, Farrell RJ, et al. MR colonography using colonic distension with air as the contrast material: work in progress. AJR Am J Roentgenol 2001;176:144–6. [DOI] [PubMed] [Google Scholar]
- 21.Aschoff AJ, Zeitler H, Merkle EM, et al. MR enteroclysis for nuclear spin tomographic diagnosis of inflammatory bowel diseases with contrast enhancement. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1997;167:387–91. [DOI] [PubMed] [Google Scholar]
- 22.Rieber A, Wruk D, Nussle K, et al. MRI of the abdomen combined with enteroclysis in Crohn disease using oral and intravenous Gd-DTPA. Radiologie 1998;38:23–8. [DOI] [PubMed] [Google Scholar]
- 23.Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA 2003;289:1297–302. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA 2003;289:1288–96. [DOI] [PubMed] [Google Scholar]
- 25.Villavicencio RT, Rex DK. Colonic adenomas: prevalence and incidence rates, growth rates, and miss rates at colonoscopy. Semin Gastrointest Dis 2000;11:185–93. [PubMed] [Google Scholar]
- 26.Beaulieu CF, Napel S, Daniel BL, et al. Detection of colonic polyps in a phantom model: implications for virtual colonoscopy data acquisition. J Comput Assist Tomogr 1998;22:656–63. [DOI] [PubMed] [Google Scholar]