Haemochromatosis is a common inherited disorder of iron metabolism, characterised by excessive iron absorption and deposition in tissues. The majority of cases are associated with mutations in the HFE gene and inherited in an autosomal recessive manner.1 Autosomal dominant forms of haemochromatosis have been reported, mainly associated with mutations in the ferroportin 1 gene.2 This syndrome, termed type 4 haemochromatosis or more recently ferroportin disease,3 is usually characterised by an early increase in serum ferritin with normal transferrin saturation. Iron accumulation is most prominent in Kupffer cells and other macrophages, in addition to hepatocytes. Some patients do not tolerate venesection therapy well and can develop anaemia. Hereditary iron overload disorders appear to be uncommon in Asia. Secondary iron overload due to beta thalassaemia is relatively common in the Indian subcontinent. However, primary iron overload disorders and HFE mutations appear to be rare and cases have not been well characterised in this region.4,5 We identified a patient from the Indian subcontinent with features typical of ferroportin disease.
A 36 year old female of Sri Lankan origin presented for a routine medical examination in December 2003. She was found to have an elevated serum ferritin of 3145 μg/l. Her serum iron (17.1 μmol/l) and transferrin saturation (29%) were normal. Liver functions tests, blood glucose, and thyroid studies were all normal. Physical examination was normal and she had no significant past medical history or risk factors for iron overload.
C282Y, H63D, and S65C HFE gene mutations were all negative and she had no family history of iron overload. Her mother and three siblings all had normal serum ferritin levels. Her father died of ischaemic heart disease aged 48 years.
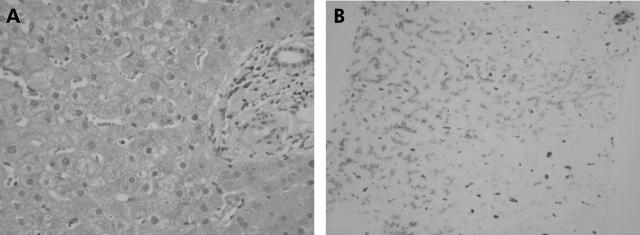
A magnetic resonance imaging scan showed hepatic iron overload. Liver biopsy showed grade 3–4 iron deposition within hepatocytes and Kupffer cells; no fibrosis or cirrhosis was evident (fig 1 ▶). The hepatic iron concentration was 17 700 μg/g dry weight and hepatic iron index was 9.1.
Figure 1.
Liver biopsy sections from our patient stained with (A) haematoxylin and eosin and (B) Perls’ Prussian blue (magnification 100×). Grade 3–4 iron is prominent in hepatocytes and Kupffer cells.
Venesection therapy was initially poorly tolerated with the development of anaemia following the first two 500 ml venesections. Her haemoglobin is now stable on a programme of 300–500 ml venesections every three weeks.
The features of ferroportin disease in this patient led us to sequence the ferroportin 1 gene, as previously described.6 Analysis of the DNA sequence revealed a heterozygous three base pair deletion (TTG) in exon 5. This is the same deletion, V162del, described by us and others in haemochromatosis patients from Australia, the UK, Italy, and Greece.6–9
This is the first report to identify V162del or indeed any ferroportin 1 mutation in an individual from the Indian subcontinent. Identification of V162del in an Asian patient confirms that this mutation is likely to be the most common mutation of ferroportin 1 and the most common cause of non-HFE associated haemochromatosis. The wide geographical distribution of this mutation suggests that it is a recurrent mutation that has repeatedly arisen in distinct populations, probably by slippage mispairing.
Iron overload in this patient was typical of ferroportin disease. At the time of diagnosis she was asymptomatic and had no fibrosis on liver biopsy. Whether fibrosis or clinical complications will develop with age if iron stores are not depleted is unclear.
In conclusion, we have identified the V162del mutation of ferroportin 1 in a fifth geographical location, emphasising that this mutation is the most common and widely distributed mutation which causes non-HFE haemochromatosis. We have identified V162del in a region where iron overload disorders have not been well characterised. Analysis of this and other ferroportin 1 mutations may be useful in the study of iron overload disorders in this region and may be the basis of hitherto unexplained cases of iron overload.
Acknowledgements
This work was supported in part by grants from the National Health and Medical Research Council of Australia (953219), the National Institutes of Health, USA (5R01DK057648-02), and the Haemochromatosis Society of Australia to VNS.
Conflict of interest: None declared.
References
- 1.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 1996;13:399–408. [DOI] [PubMed] [Google Scholar]
- 2.Pietrangelo A. Non-HFE hemochromatosis. Hepatology 2004;39:21–9. [DOI] [PubMed] [Google Scholar]
- 3.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis 2004;32:131–8. [DOI] [PubMed] [Google Scholar]
- 4.Kaur G, Rapthap CC, Xavier M, et al. Distribution of C282Y and H63D mutations in the HFE gene in healthy Asian Indians and patients with thalassaemia major. Natl Med J India 2003;16:309–10. [PubMed] [Google Scholar]
- 5.Thakur V, Guptan RC, Hashmi AZ, et al. Absence of hemochromatosis associated Cys282Tyr HFE gene mutation and low frequency of hemochromatosis phenotype in nonalcoholic chronic liver disease patients in India. J Gastroenterol Hepatol 2004;19:86–90. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DF, Pedersen P, Dixon JL, et al. Novel mutation in ferroportin1 is associated with autosomal dominant hemochromatosis. Blood 2002;100:692–4. [DOI] [PubMed] [Google Scholar]
- 7.Devalia V, Carter K, Walker AP, et al. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3). Blood 2002;100:695–7. [DOI] [PubMed] [Google Scholar]
- 8.Roetto A, Merryweather-Clarke AT, Daraio F, et al. A valine deletion of ferroportin 1: a common mutation in hemochromastosis type 4. Blood 2002;100:733–4. [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, Cremonesi L, Papaioannou M, et al. Genetic hyperferritinaemia and reticuloendothelial iron overload associated with a three base pair deletion in the coding region of the ferroportin gene (SLC11A3). Br J Haematol 2002;119:539–46. [DOI] [PubMed] [Google Scholar]