Abstract
Developmental exposure to oxytocin (OT) or oxytocin antagonists (OTAs) has been shown to cause long-lasting and often sexually dimorphic effects on social behaviors in prairie voles (Microtus ochrogaster). Because regulation of social behavior in monogamous mammals involves central receptors for OT, arginine vasopressin (AVP), and dopamine, we examined the hypothesis that the long-lasting, developmental effects of exposure to neonatal OT or OTA might reflect changes in the expression of receptors for these peptides. On postnatal day 1, prairie voles were injected intraperitoneally with either OT (1 mg/kg), an OTA (0.1 mg/kg), saline vehicle, or were handled only. At approximately 60 days of age, vasopressin V1a receptors, OT receptors (OTR) and dopamine D2 receptor binding were quantified using receptor autoradiography in brain tissue taken from males and females. Significant treatment effects on V1a binding were found in the bed nucleus of the stria terminalis (BNST), cingulate cortex (CgCtx), mediodorsal thalamus (MdThal), medial preoptic area of the hypothalamus (MPOA), and lateral septum (LS). The CgCtx, MPOA, ventral pallidum, and LS also showed significant sex by treatment interactions on V1a binding. No significant treatment or sex differences were observed for D2 receptor binding. No significant treatment difference was observed for OTR receptor binding, and only a marginal sex difference. Changes in the neuropeptide receptor expression, especially the V1a receptor, may help to explain sexually dimorphic changes in behavior that follow comparable neonatal manipulations.
Abbreviation List: AVP arginine vasopressin, BNST bed nucleus of the stria terminalis, CgCtx cingulate cortex, CTL control, D2 dopamine type 2 receptors, HAN handled only group, LS lateral septum, MdThal mediodorsal thalamus, MeA medial amygdala, MPOA medial preoptic area, OT oxytocin, OTA oxytocin antagonist, OTR oxytocin receptor, PND post-natal day, SAL saline, VP ventral pallidum, V1a vasopressin receptors type 1a
Introduction
The neurobiology of social behavior has been shown to be intimately linked to two central neuropeptide hormones, oxytocin and vasopressin. Oxytocin (OT) is a nine-amino-acid peptide associated with labor and milk let-down, as well as the formation of pair-bonds (particularly in females) and mother-infant bonds (Carter, 1998; Williams et al., 1994; Zingg, 2002). The related neuropeptide, arginine vasopressin (AVP), well-known for its peripheral effects on blood pressure and water balance, also plays a role in pair-bond formation and parental care, especially in males (Berecek, 1991; Wang et al., 1998; Winslow et al., 1993a). OT and AVP differ by two amino acids and may exhibit some receptor cross-reactivity (Barberis and Tribollet, 1996), allowing potential interactions between the two peptide systems. Changes in exposure to either OT or AVP during development may have significant potential to affect both social behavior and its regulation over a lifetime.
Variations in child-rearing practices have the capacity to affect OT exposure in offspring. Breast milk contains OT (Leake et al., 1981), and OT is also released by warmth and touch (Uvnas-Moberg, 1998). In dairy calves, suckling from the mother rather than drinking mother’s milk from a bucket raises the calf’s plasma level of OT (Lupoli et al., 2001). Developmental exposure to OT in rats is associated in later life with lower blood pressure (Holst et al., 2002), lower corticosterone levels (Sohlstrom et al., 2000), higher body weight (Sohlstrom et al., 2000), and can reverse the effects of maternal malnutrition (Olausson et al., 2003). In rodents, mothers that lick and groom their infants produced female offspring with significant increases in OT binding in the central amygdala and the bed nucleus of the stria terminalis (BNST). In contrast, male offspring of high licking and grooming mothers had higher AVP binding in the central nucleus of the amygdala (Francis et al., 2002). These neural differences were reflected in behavior, with offspring of high licking and grooming mothers also demonstrating high licking and grooming (Francis et al., 1999). Differences in perinatal exposure to OT, through breast-feeding or other forms of infant care, may thus have the ability to change neural systems in a long-term (perhaps life-long) manner.
In addition to the OT and AVP systems, the dopamine system appears to be crucial to the expression of social behavior. Dopamine has recently been identified for its crucial role both in pair-bonding (Aragona et al., 2003; Aragona et al., 2006; Liu and Wang, 2003; Wang et al., 1999) and parenting behavior (Lonstein, 2002). Access to D2 dopamine receptors is necessary for formation of a pair-bond, while activation of D1 dopamine receptors blocks pair-bond formation. Following formation of the bond, D1 receptors are up-regulated, preventing formation of a second bond (Aragona et al., 2006). Essential to the actions of dopamine in pair-bonding is co-localization of D2 receptors with OT receptors in the nucleus accumbens (in females) and with V1a receptors in the ventral pallidum (in males).
The prairie vole (Microtus ochrogaster), a socially monogamous rodent native to the Midwestern United States, is a well-studied model for sociality. Prairie voles exhibit selective pair-bonds (Williams et al., 1992) and high levels of both paternal and alloparental care (Roberts et al., 1998; Lonstein and De Vries, 2000). This species is sensitive to developmental manipulations in OT, showing long-lasting changes in behavior and physiology (Carter, 2003). In a series of experiments, prairie voles were injected on postnatal day 1 (PND1) with either 1 mg/kg OT, 0.1 mg/kg OTA, saline vehicle, or were handled only. Developmental exposure to OT facilitated pair-bond formation in adulthood in male voles (Bales and Carter, 2003b), whereas OTA exposure on PND1 produced a marked reduction in alloparental behavior in males (Bales et al., 2004b). Manipulation of either OT or OTA on PND1 altered the subsequent patterning of male sexual behavior and reduced male reproductive potential (Bales et al., 2004a). Finally, in males treatment with OTA resulted in fewer AVP-immunoreactive cells, and did not significantly alter the number of OT-immunoreactive cells (Yamamoto et al., 2004).
In females in general, the behavioral effects of neonatal exposure to OT or OTA were less pronounced than in males. However, neonatal OT did increase the mate-guarding component of pair-bonding shown by adult females (Bales and Carter, 2003a). Females were capable of responding to OTA, since female pups exposed to 0.1 mg/kg OTA on PND1 emitted significantly fewer ultrasonic vocalizations upon separation from their parents on PND8 (Kramer et al., 2003). PND1 OTA treated females showed, as adults, increased neural activation of the central amygdala when exposed to a member of the opposite sex (Kramer et al., 2006). Also in females, a single PND1 exposure to either OT or OTA resulted in higher numbers of OT-immunoreactive cells in the paraventricular nucleus of the hypothalamus on PND21 (Yamamoto et al., 2004).
The purpose of the present study was to examine the hypothesis that at least some of the functional changes that we observed following a single neonatal exposure to OT or OTA might be reflected in or due to the developmental capacity of these manipulations to influence receptor expression for neuropeptides or transmitters that have been previously implicated in social behavior. OT (OTR), AVP V1a and dopamine (D2) receptor binding were measured in adulthood using quantitative autoradiography. The brain areas selected for study were those in which these receptors were abundant and for which there was prior evidence of relevance to social behavior. Based on the behavioral changes that we had observed, we predicted that the effects of OT and OTA would (a) differ from each other, (b) be regionally specific and (c) differ in males and females.
Methods
Neonatal Treatments
Subjects were laboratory-bred male and female prairie voles, descendants of a wild stock originally captured near Champaign, Illinois. Stock was systematically outbred. Animals were maintained on a 14 h light:10 h dark cycle and given food (Purina rabbit chow) and water ad libitum. Breeding pairs were maintained in large polycarbonate cages (25 x 45 x 60 cm) and provided with cotton for nesting material. On PND21 offspring were removed and housed in same-sex sibling pairs in smaller (12 x 18 x 28 cm) cages. Sibling pairs were maintained in single-sex colony rooms. All studies were approved by the Animal Care and Use Committee of the University of Illinois at Chicago and complied with National Institutes of Health ethical guideline as set forth in the Guide for Lab Animal Care.
Within 24 hours of birth (postnatal day 1; PND1), test subjects randomly received either a single 1 mg/kg injection of oxytocin (Bachem, San Carlos, CA), a single 0.1 mg/kg injection of oxytocin antagonist (OTA), or were assigned to one of two control groups receiving either an injection of isotonic saline (SAL), or handling without injection (HAN). The OT receptor antagonist ([d(CH2)5, Tyr(Me)2, Orn8]-Vasotocin) (Bankowski et al., 1980) administered in this study also had been used in behavioral studies in this species, and is commercially available from Bachem. This antagonist is capable of affecting both OT and AVP V1a receptors, with a binding profile similar to Atosiban, the OTA most widely used to prevent premature labor (Bankowski et al., 1980; Manning et al., 1995). A lower dose of OTA than OT was used because in studies in rats, the OTA used here has been shown to be approximately 10 – 100 times more effective in receptor binding than the natural ligand (Barberis and Tribollet, 1996). In adult rodents, OT crossed the blood-brain-barrier (BBB) in small amounts (0.2 – 1.3%) when administered peripherally (Ermisch et al., 1985; Jones and Robinson, 1982; Banks and Kastin, 1985). The blood-brain-barrier of neonatal rodents should be more permeable than in adults (Vorbrodt, 1993). Finally, a study performed using these compounds and dosages in neonatal prairie voles showed specific and differential activations of c-Fos in various brain areas (Cushing et al., 2003); however, whether the consequences of neonatal OT or OTA were due to central or peripheral actions remains to be determined.
All injections were 50 ul in volume and administered intraperitoneally in 250 ul gastight Hamilton syringes. Infants were weighed and toe- clipped for identification on the day of birth. Although litters were not matched for pup number, infants were only used in the study if at least one control and one treatment animal of a given sex were available in the litter, and litters of more than six pups at birth were culled to six. Animals remained undisturbed in same-sex pairs until sacrifice at 60 days of age, and were not used in any behavioral testing.
Receptor Autoradiography
Following sacrifice, brains were quickly removed, flash-frozen on dry ice and stored at −80°C. Brains were sectioned at 20 μm thickness, mounted onto Super-frost slides and stored at −80°C until the time of assay. Sections were allowed to thaw to room temperature and then immersed in 0.1% paraformaldehyde for 2 min to optimize tissue integrity. Sections then were rinsed 3 times in 50mM Tris-HCl (pH 7.4) at room temperature for 5 min and incubated for 60 min at room temperature in a solution of 50 mM Tris-HCl (pH 7.4) with 10 mM MgCl2, 0.1% bovine serum albumin, and 50 pM of radiotracer. For OTR binding, [125I]-ornithine vasotocin analogue [(125I)OVTA] was employed [vasotocin, d(CH2)5[Tyr(Me)2,Thr4,Orn8, (125I)Tyr9-NH2]; 2200 Ci/mmol]; (NEN Nuclear, Boston, MA, USA). For V1a receptor binding, 125I-lin-vasopressin [125I-phenylacetyl-D-Tyr(ME)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2]; (NEN Nuclear) was used. For D2 binding, 125-I-iodospiperone (Perkin Elmer/NEN, MA), dissolved in 5.7 mM ascorbic acid and Tris-ions buffer (0.7% NaCl, 0.04% KCl, 0.02% CaCl2, 0.01% MgCl2 in Tris-base, pH 7.4), was used. Non-specific binding was determined by incubating adjacent sections with the radioactive specific ligand as well as with 50 μM of unlabelled Thr4, Gly7 oxytocin, a selective oxytocin ligand (Peninsula Laboratories, Belmont, CA, USA) or 50 μM of unlabelled [1-(-mercapto-,-cyclo-pentamethylene propionic acid),2-(O-methyl)-tyrosine]-arg8-vasopressin, selective for the V1a receptor. Following incubation, sections were washed 4 times at 5 min each in 50 mm Tris-HCl (pH 7.4) with 10 mM MgCl2 at 4°C, followed by a final rinse in this same buffer for 30 min while stirred with a magnetic bar. Slides then were quickly dipped in cold dH2O and rapidly dried with a stream of cold air. Sections were apposed to Kodak BioMaxMR film (Kodak, Rochester, NY, USA) with 125I microscale standards (Perkin-Elmer/NEN, Boston) for 72 h (for OTR and V1a) or 24 h (for D2). Autoradiographic 125I-receptor binding was quantified from film using the NIH Image program. 125I standards were used to convert uncalibrated optical density to disintegrations per minute (DPM). The number of slides scored for each area varied, but averaged approximately nine sections per area. Both sides of each area were quantified separately, compared for any differences according to side (which were not found), then a mean obtained for each slice. A mean for the area for each animal was then calculated, which was the value used in analyses. One set of slides was stained with acetylcholinesterase post-receptor binding to aid in identification of brain regions (Lim et al., 2004a).
Data Analysis
Initially the two control groups (SAL and HAN) were compared, and as they were in no cases statistically different, they were combined into one control group (CTL) for the rest of the analyses. Final group sizes were as follows: Male CTL = 17 animals, Male OT = 10, Male OTA = 9; Female CTL = 15 animals, Female OT = 8, Female OTA = 6 animals.
For each type of receptor binding (V1a, OTR, and D2), we first performed a multivariate analysis of variance (MANOVA)(O'Rourke et al., 2005) for all brain areas together. Brain regions selected for analysis were those with high levels of receptor binding and previously implicated in parenting and pair-bonding behavior. Independent factors included in the model were treatment, sex, and a sex*treatment interaction, as well as a random effect of litter. Following a significant MANOVA, we have performed mixed model ANOVAs using the same fixed and random factors tested above (Littell et al., 1996) to examine effects in individual brain regions.
Residuals were checked for normality and data transformed when necessary. All tests were two-tailed and significance was set to p = 0.05.
Results
Dopamine receptor binding (D2)
D2 binding was not affected by neonatal treatment, sex, or a sex by treatment interaction, although there was a significant effect of litter (MANOVAs for: treatment, Wilk’s lambda = 0.81, F6,72 = 1.31, p = 0.26; sex, Wilk’s lambda = 0.89, F3,36 = 1.48, p = 0.24; sex*treatment, Wilk’s lambda = 0.93, F6,72 = 0.45, p = 0.84; and litter, Wilk’s lambda = 0.13, F45, 107.73 = 2.35, p < 0.001). Raw means and standard errors (unadjusted for litter values) are presented in Tables 1a and b.
Table 1a.
Radiolabelled ligand binding for D2 receptors in males (DPM, means ± standard errors). N = 17 CTL, 10 OT, and 9 OTA. These are means unadjusted for litter CTLs; please see text for a full description of the data analysis.
| CTL | OT | OTA | |
|---|---|---|---|
| Nucleus accumbens shell | 332 ± 26 | 315 ± 33 | 334 ± 39 |
| Striatum | 805 ± 61 | 756 ± 37 | 952 ± 129 |
| Ventral pallidum | 236 ± 34 | 231 ± 36 | 280 ± 29 |
Table 1b.
Radiolabelled ligand binding for D2 receptors in females (DPM, means ± standard errors). N = 15 CTL, 8 OT, and 6 OTA. These are means unadjusted for litter CTLs; please see text for a full description of the data analysis.
| CTL | OT | OTA | |
|---|---|---|---|
| Nucleus accumbens shell | 330 ± 31 | 398 ± 41 | 405 ± 59 |
| Striatum | 796 ± 76 | 946 ± 114 | 944 ± 127 |
| Ventral pallidum | 193 ± 26 | 271 ± 46 | 222 ± 75 |
Oxytocin receptor binding (OTR)
OTR binding was not affected by neonatal treatment, or a sex by treatment interaction (MANOVAs for: treatment, Wilk’s lambda = 0.69, F12,72 = 1.24, p = 0.27; sex*treatment, Wilk’s lambda = 0.75, F12,72 = 0.92, p = 0.53). However, there was a significant litter effect (Wilk’s lambda = 0.05, F90, 209.06 = 1.65, p < 0.001). In addition, there was a marginally significant effect of sex (Wilk’s lambda = 0.72, F6,36 = 2.36, p = 0.05). Reanalysis of the model without the non-significant effects (treatment and treatment by litter) resulted in the sex effect becoming less, not more significant (Wilk’s lambda = 0.76, F6,40 = 2.16, p = 0.07). Raw means and standard errors (unadjusted for litter values) are presented in Tables 2a and 2b.
Table 2a.
Radiolabelled ligand binding for OT receptors in males (DPM, means ± standard errors). N = 17 CTL, 10 OT, and 9 OTA. These are means unadjusted for litter CTLs; please see text for a full description of the data analysis.
| CTL | OT | OTA | |
|---|---|---|---|
| Ventral pallidum | 173 ± 22 | 188 ± 31 | 195 ± 17 |
| Medial amygdala | 296 ± 48 | 284 ± 41 | 268 ± 40 |
| Central amygdala | 1096 ± 58 | 1029 ± 65 | 1122 ± 53 |
| BNST | 440 ± 43 | 458 ± 35 | 468 ± 59 |
| Lateral septum | 459 ± 38 | 497 ± 65 | 592 ± 80 |
| Cingulate cortex | 230 ± 27 | 219 ± 29 | 274 ± 61 |
Table 2b.
Radiolabelled ligand binding for OT receptors in females (DPM, means ± standard errors). N = 15 CTL, 8 OT, and 6 OTA. These are means unadjusted for litter CTLs; please see text for a full description of the data analysis.
| CTL | OT | OTA | |
|---|---|---|---|
| Ventral pallidum | 169 ± 18 | 162 ± 31 | 143 ± 44 |
| Medial amygdala | 226 ± 29 | 259 ± 49 | 167 ± 37 |
| Central amygdala | 1009 ± 69 | 964± 96 | 1113 ± 73 |
| BNST | 389 ± 24 | 352 ± 48 | 399 ± 45 |
| Lateral septum | 461 ± 42 | 465 ± 80 | 599 ± 100 |
| Cingulate cortex | 219 ± 30 | 189 ± 32 | 133 ± 41 |
Vasopressin receptor binding (V1a)
The AVP V1a receptor showed several changes as a function of neonatal OT or OTA. The overall MANOVA was significant for treatment (Wilk’s lambda = 0.38, F14,64 = 2.84, p < 0.01), as well as litter (Wilk’s lambda = 0.004, F2.74,105 = 2.74, p < 0.0001) and a treatment*sex interaction (Wilk’s lambda = 0.38, F14,64 = 2.85, p < 0.01); although not for the main effect of sex (Wilk’s lambda = 0.89, F7,32 = 0.54, p = 0.80). As the multivariate effect of treatment was significant, univariate analyses were carried out on individual brain areas. Raw means and standard errors are presented in Tables 3a and 3b. A representative photo of V1a receptor binding is shown in Figure 1. Change scores (means for each treatment subtracted from the control values for the litters) are presented in Figure 2 (for males), Figure 3 (for females), and Figure 4 (male and female data are regraphed to show sex by treatment interactions).
Table 3a.
Radiolabelled ligand binding for V1a receptors in males (DPM, means ± standard errors). N = 17 CTL, 10 OT, and 9 OTA. These are means unadjusted for litter CTLs.
| CTL | OT | OTA | |
|---|---|---|---|
| Ventral pallidum | 2199 ± 108 | 2469 ± 151 | 2285 ± 93 |
| Medial amygdala | 1263 ± 89 | 1178 ± 120 | 1064 ± 40 |
| MPOA* | 451 ± 48 | 484 ± 79 | 419 ± 42 |
| BNST* | 1256 ± 109 | 1102 ± 97 | 907 ± 27 |
| Lateral septum* | 421 ± 40 | 529 ± 68 | 376 ± 42 |
| Cingulate cortex* | 499 ± 65 | 513 ± 133 | 224 ± 106 |
| Mediodorsal thalamus* | 1403 ± 179 | 1179 ± 176 | 1254 ± 127 |
indicate a significant effect of treatment; please see text for a full description of the data analysis.
Table 3b.
Radiolabelled ligand binding for V1a receptors in females (DPM, means ± standard errors). N = 15 CTL, 8 OT, and 6 OTA. These are means unadjusted for litter CTLs.
| CTL | OT | OTA | |
|---|---|---|---|
| Ventral pallidum | 2355 ± 87 | 2201 ± 171 | 2321 ± 62 |
| Medial amygdala | 1252 ± 189 | 1242 ± 114 | 1174 ± 97 |
| MPOA* | 526 ± 53 | 409 ± 52 | 422 ± 84 |
| BNST* | 1133 ± 93 | 870 ± 68 | 931 ± 105 |
| Lateral septum* | 538 ± 46 | 316 ± 61 | 447 ± 76 |
| Cingulate cortex* | 642 ± 98 | 476 ± 74 | 550 ± 99 |
| Mediodorsal thalamus* | 1807 ± 125 | 1286 ± 104 | 1338 ± 217 |
indicate a significant effect of treatment; please see text for a full description of the data analysis.
Figure 1.
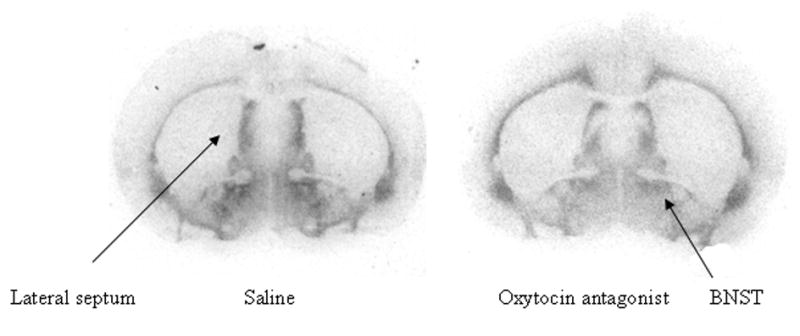
Representative photos of radiolabelled ligand binding for V1a receptors in males at the level of the lateral septum and bed nucleus of the stria terminalis (BNST).
Figure 2.

Radiolabelled ligand binding for V1a receptors (DPM) in males, represented as the change from the control (CTL) value for each litter. Asterisks indicate treatments that are significantly different (p < 0.05) from the CTL value when analyzed by post-hoc tests.
Figure 3.
Radiolabelled ligand binding for V1a receptors (DPM) in females, represented as the change from the control (CTL) value for each litter. Asterisks indicate treatments that are significantly different (p < 0.05) from the CTL value when analyzed by post-hoc tests.
Figure 4.

This figure is a regraphing of data from Figs 2 and 3, highlighting the differences between males and females in the brain areas that had significant sex by treatment interactions. Radiolabelled ligand binding for V1a receptors (DPM) in males and females treated with neonatal OT (left) and OTA (right), represented as the change from the control (CTL) value for each litter.
Ventral pallidum
The overall model for the VP was significant (F20 = 2.63, p < 0.001). The effect of treatment on V1a receptor binding, however, was not (F2 = 0.45, p = 0.64). Both the random litter effect (F15 = 2.94, p < 0.01) and the treatment by sex interaction (F2 = 4.27, p = 0.02) were significant. The effects of sex were not significant. Post-hoc tests were not performed because the treatment effect was not significant, but the significant sex by treatment interaction is shown in Figure 4.
Medial amygdala (MeA)
The overall model for the MeA was not significant (F20 = 1.68, p = 0.08).
Medial preoptic area (MPOA)
The overall model for the MPOA was significant (F20 = 5.09, p < 0.0001). The effect of treatment was significant on V1a receptor binding (F2 = 4.63, p = 0.02), as was the random litter effect (F15 = 5.85, p < 0.0001) and the treatment by sex interaction (F2 = 4.27, p = 0.02). The effects of sex were not significant. In males, OTA significantly reduced V1a binding in the MPOA (t = −2.80, p < 0.01; Figure 2), while in females OT (t = −3.10, p < 0.01; Figure 3) significantly reduced V1a binding. The significant sex by treatment interaction is presented in Figure 4.
Bed nucleus of the stria terminalis (BNST)
The overall model for the BNST was significant (F20 = 2.81, p < 0.01). The effect of treatment was significant on V1a receptor binding (F2 = 7.15, p < 0.01), as was the random litter effect (F15 = 2.49, p = 0.01). Sex and the treatment by sex interaction were not significant. In males, OTA significantly reduced V1a binding (t = −2.35, p = 0.02; Figure 2), while in females both OT (t = −3.33, p < 0.01) and OTA (t = −3.16, p < 0.01; Figure 3) significantly reduced V1a binding.
Lateral septum
The overall model for the LS was significant (F20 = 4.54, p < 0.0001). The effect of treatment was significant on V1a receptor binding (F2 = 8.18, p < 0.01), as was the random litter effect (F15 = 3.48, p < 0.001) and the treatment by sex interaction (F2 = 13.04, p < 0.0001). The effects of sex were not significant. In males, OTA significantly reduced V1a binding (t = −2.63, p = 0.01; Figure 2), while in females OT (t = −5.64, p < 0.001; Figure 3) significantly reduced V1a binding. The significant sex by treatment interaction is presented in Figure 4.
Cingulate cortex
The overall model for the CgCtx was significant (F20 = 4.93, p < 0.0001). The effect of treatment was significant on V1a receptor binding (F2 = 3.85, p = 0.03), as was the random litter effect (F15 = 5.97, p = 0.01) and the treatment by sex interaction (F2 = 5.17, p = 0.01). The effects of sex were not significant. In males, OT significantly increased V1a binding (t = 2.05, p < 0.05; Figure 2), while in females both OT (t = −2.62, p = 0.01) and OTA (t = −2.67, p = 0.01; Figure 3) significantly decreased V1a binding. The significant sex by treatment interaction is presented in Figure 4.
Mediodorsal thalamus
The overall model for the MdThal was significant (F20= 2.50, p < 0.01). The effect of treatment was significant on V1a receptor binding (F2 = 4.97, p = 0.01), as was the random litter effect (F15 = 2.51, p = 0.01). Sex and the treatment by sex interaction were not significant. In males, OTA showed a non-significant trend (t = 1.72, p = 0.09; Figure 2), while in females OT (t = −2.32, p = 0.03) significantly decreased V1a binding.
Discussion
The OT, V1a, and D2 receptor systems have been implicated in social behavior in the prairie vole, including the formation of pair-bonds as well as parental care (Cho et al., 1999; Carter, 1998; Winslow et al., 1993a; Aragona et al., 2003; Aragona and Wang, 2004; Lim et al., 2004b). In the present study we have observed that manipulations of OT on postnatal day 1 are associated with regional patterns of change in the V1a receptor system. Sex differences in the distribution of V1a receptors in prairie voles were not observed, consistent with earlier reports in this species (Phelps and Young, 2003). However, as shown here, neonatal manipulations of OT have sexually dimorphic effects on V1a receptor levels. Though in certain areas treatment effects were not statistically significant, the opposite direction of the changes in V1a binding in males and females resulted in a significant sex by treatment interaction. For example, in the VP, LS and CgCtx OT-treated males showed increases in V1a receptor binding while OT-treated females showed decreases in V1a receptor binding (Figure 4). The sexually dimorphic pattern of change in the V1a receptor in the LS is especially striking because this area is heavily innervated by androgen-dependent AVP-immunoreactive fibers (De Vries and Simerly, 2002). However, the effects of androgens on central AVP synthesis are usually detected following puberty. Thus the mechanisms through which exposure to OT would affect these regions in males, but not in females, remains to be determined.
In addition to the sexually dimorphic effects of OT, males exposed neonatally to OTA showed reductions in V1a receptor binding in several brain regions that have been implicated in both social behavior and emotionality, including the BNST, MPOA, and LS. AVP acting in these same brain regions has been associated later in life with both male parental care (Wang et al., 1994; Wang et al., 1998; Numan and Insel, 2003; Bester-Meredith and Marler, 2003) and with aggression (Bester-Meredith et al., 1999; Marler et al., 2003). In our own studies, we observed that males treated neonatally with OTA showed reduced alloparental behavior (Bales et al., 2004b), and tended to show lower levels of same-sex aggression as control males (Bales and Carter, 2003a), supporting a role for AVP and/or the V1a receptor in these behaviors. Other studies in prairie voles have revealed that neonatal exposure to AVP is associated with a later increase in same sex aggression, especially in males (Stribley and Carter, 1999), although the effects of neonatal AVP on V1a receptor binding have not yet been examined in voles. It is also notable that treatment with OTA in males in several of these areas (ex. the BNST, but NOT the LS) produced a pattern of changes that resemble the pattern of differences seen between polygynous and monogamous voles (Insel et al., 1994).
In contrast to males, it is more difficult to interpret the significance of the changes in V1a receptor binding in females, in which the functions of V1a receptors remain less well understood (Winslow et al., 1993b; Lim et al., 2004b; Bielsky et al., 2005). In the present study, OT-treated females displayed lower levels of V1a receptors in the LS, and we might, therefore, have expected changes in aggression. However, there is at present no strong evidence for a role for AVP or the V1a receptor in female aggression in voles or mice (Bielsky et al., 2005; Bowler et al., 2002). Among the other brain regions that were significantly altered by our developmental treatments (the MPOA and BNST in particular), are regions that are crucial to maternal behavior in many rodent species (Numan et al., 1998; Numan and Numan, 1994; Numan et al., 1988; Numan and Insel, 2003). While exogenous AVP is capable of facilitating maternal care in virgin rats (Pedersen et al., 1982), the behavioral role of endogenous AVP peptide or V1a receptors in these brain regions, or in female parental behavior, remains to be identified. The mechanism behind these changes in receptors also remains to be identified. As OT and AVP bind to each other’s receptors (Barberis and Tribollet, 1996), it is possible that early OT and OTA bound directly to the V1a receptor (especially given the relatively large dose of exogenous OT), activating or blocking it at a critical period of development.
There is increasing evidence that the long-lasting effects of social experiences can reflect sexually dimorphic actions of OT and AVP (Carter, 2003). In rats, experience-based changes in both the OT and V1a receptors are sexually dimorphic (Francis et al., 2002; Champagne et al., 2001). In mice, the consequences of genetically-induced deficiencies in the V1a receptor are also gender specific (Bielsky et al., 2005; Lim et al., 2004b). The very significant random litter effects found on all types of receptor binding studied here are also probably an additional reflection of the long-term effects of differing parental care, or perhaps of genetic background (Francis et al., 2002; Tyler et al., 2005).
The possible effects of early experience or hormonal manipulations have not been systematically studied in human development. The neural systems that are altered by neonatal peptide manipulations in prairie voles are evolutionarily ancient and have broad behavioral and physiological actions. Our studies suggest that these systems may be vulnerable during development to neural changes that could have long-lasting consequences. Results from the present study suggest the need for a deeper understanding of the mechanisms through which manipulations in endogenous or exogenous peptides might affect neuroanatomy, physiology and behavior.
Acknowledgments
This research was supported by NIH PO1 HD38490 to C. S. C., MH073022 to C.S.C. and K.L.B., MH 56539 to LJY, as well as NIH NRSA F32 HD08702 and NSF 0437523 to K.L. B. We thank Tim Hill, Drs. James Artwohl, and Dr. Terry Hewett for animal and veterinary care. We also thank Lorraine Smith, Sheryl Katta, Emily Harden, and Julie Hazelton for research assistance, as well as Caroline Hostetler and two anonymous reviewers for comments on previous versions of this paper.
References
- Aragona BJ, Liu Y, Curtis T, Stephan FK, Wang ZX. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang ZX. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang ZX. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. I L A R Journal. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Bales KL, Abdelnabi M, Cushing BS, Ottinger MA, Carter CS. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles. Physiology & Behavior. 2004a;81:519–526. doi: 10.1016/j.physbeh.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Hormones and Behavior. 2003a;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2003b;117:854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Developmental Psychobiology. 2004b;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Bankowski K, Manning M, Seto J, Haldar J, Sawyer WH. Design and synthesis of potent in vivo antagonists of oxytocin. Int J Pept Prot Res. 1980:382–391. doi: 10.1111/j.1399-3011.1980.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Permeability of the blood-brain barrier to neuropeptides: the case for penetration. Psychoneuroendocrinology. 1985;23:779–818. doi: 10.1016/0306-4530(85)90079-4. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Critical Reviews in Neurobiology. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Berecek KH. Role of vasopressin in central cardiovascular regulation. In: Kunos G, Ciriello J, editors. Central neural mechanisms in cardiovascular regulation Vol 2. Birkhauser; Boston: 1991. pp. 1–34. [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behavioral Neuroscience. 2003;117:455–463. doi: 10.1037/0735-7044.117.3.455. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Hormones and Behavior. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Young LJ. Sexual dimorphism in the vasopressin system; Lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behavioral Brain Research. 2005 doi: 10.1016/j.bbr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiology & Behavior. 2002;76:559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiology & Behavior. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Yamamoto Y, Hoffman GE, Carter CS. Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Developmental Brain Research. 2003;143:129–136. doi: 10.1016/s0165-3806(03)00105-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, editor. Hormones, brain, and behavior. Vol. 4. Academic Press; San Diego: 2002. pp. 137–192. [Google Scholar]
- Ermisch A, Ruhle H-J, Landgraf R, Hess J. Blood-brain barrier and peptides. Journal of Cerebral Blood Flow and Metabolism. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. Journal of Neuroendocrinology. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnas-Moberg K, Petersson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Autonomic Neuroscience: Basic and Clinical. 2002;99:85–90. doi: 10.1016/s1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Robinson ICAF. Differential clearance of neurophysin and neurohypophyseal peptides from the cerebrospinal fluid in conscious guinea pigs. Neuroendocrinology. 1982;34:297–302. doi: 10.1159/000123316. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Hormones and Behavior. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS. Developmental effects of oxytocin on stress response: single versus repeated exposure. Physiology & Behavior. 2003;79:775–782. doi: 10.1016/s0031-9384(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Leake RD, Wietzman RE, Fisher DA. Oxytocin concentrations during the neonatal period. Biology of the Neonate. 1981;39:127–131. doi: 10.1159/000241417. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. A method for acetylcholinesterase staining of brain sections previously processed for receptor autoradiography. Biotechnology and Histochemistry. 2004a;79:11–16. doi: 10.1080/10520290410001671344. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EAD, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. Journal of Neuroendocrinology. 2004b;16:325–332. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute Inc; Cary, NC: 1996. [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacology, Biochemistry and Behavior. 2002;74:11–19. doi: 10.1016/s0091-3057(02)00952-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neuroscience and Biobehavioral Reviews. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Lupoli B, Johansson B, Uvnas-Moberg K, Svennersten-Sjaunja K. Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves. Journal of Dairy Research. 2001;68:175–187. doi: 10.1017/s0022029901004721. [DOI] [PubMed] [Google Scholar]
- Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and Synthesis of Highly Selective In-Vitro and In-Vivo Uterine Receptor Antagonists of Oxytocin - Comparisons with Atosiban. International Journal of Peptide and Protein Research. 1995;46:244–252. doi: 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Marler CA, Bester-Meredith JK, Trainor BC. Paternal Behavior and aggression: Endocrine mechanisms and nongenomic transmission of behavior. Advances in the Study of Behavior. 2003;32:263–323. [Google Scholar]
- Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD. Axon-Sparing Lesions of the Preoptic Region and Substantia Innominata Disrupt Maternal-Behavior in Rats. Behavioral Neuroscience. 1988;102:381–396. doi: 10.1037//0735-7044.102.3.381. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- Numan M, Numan MJ. Expression of Fos-Like Immunoreactivity in the Preoptic Area of Maternally Behaving Virgin and Postpartum Rats. Behavioral Neuroscience. 1994;108:379–394. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Marzella SR, Palumbo A. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Research. 1998;792:348–352. doi: 10.1016/s0006-8993(98)00257-1. [DOI] [PubMed] [Google Scholar]
- O'Rourke N, Hatcher L, Stepanski EJ. Using SAS for Univariate and Multivariate Statistics. SAS Institute, Inc; Cary, NC: 2005. [Google Scholar]
- Olausson H, Uvnas-Moberg K, Sohlstrom A. Postnatal oxytocin alleviates adverse effects in adult rat offspring caused by maternal malnutrition. American Journal of Physiology: Endocrinology and Metabolism. 2003;284:E475–E480. doi: 10.1152/ajpendo.00297.2002. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): Patterns of variation and covariation. Journal of Comparative Neurology. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Williams JR, Wang AK, Carter CS. Cooperative breeding and monogamy in prairie voles: influence of the sire and geographical variation. Animal Behaviour. 1998;55:1131–1140. doi: 10.1006/anbe.1997.0659. [DOI] [PubMed] [Google Scholar]
- Sohlstrom A, Carlsson C, Uvnas-Moberg K. Effects of oxytocin treatment in early life on body weight and corticosterone in adult offspring from ad libitum-fed and food-restricted rats. Biology of the Neonate. 2000;78:33–40. doi: 10.1159/000014244. [DOI] [PubMed] [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prarie voles. Proceedings of the National Academy of Sciences. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler AN, Michel GF, Bales KL, Carter CS. Do brief early disturbances of parents affect parental care in the bi-parental prairie vole (Microtus ochrogaster)? Developmental Psychobiology. 2005;47:451. [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interactions and emotions. Psychoneuroendocrinology. 1998:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW. Morphological evidence of the functional polarization of brain microvascular epithelium. In: Pardridge WM, editor. The Blood-Brain Barrier. Raven Press; New York: 1993. pp. 137–164. [Google Scholar]
- Wang ZX, Ferris CF, Devries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Progress in Brain Research. 1998;119:483–499. doi: 10.1016/s0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Yu GZ, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): A mechanism for pair bonding? Behavioral Neuroscience. 1999;113:602–611. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Hormones and Behavior. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin centrally administered facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993a;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature. 1993b;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Zingg HH. Oxytocin. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain, and behavior. Vol. 3. Academic Press; New York: 2002. pp. 779–802. [Google Scholar]



