Since the identification of hepatitis C virus (HCV) in the late 1980s, chronic HCV infection has emerged as a complex multifaceted disease with manifestations extending beyond the liver. As such, hepatic steatosis, insulin resistance (IR), and type II diabetes have been observed to occur more frequently in association with HCV infection than other chronic inflammatory liver disease.1 A proportion of HCV infected patients with steatosis also exhibit several of the clinical features seen in non-alcoholic steatohepatitis (NASH), questioning the significance of these metabolic disorders in the pathogenesis of HCV related liver disease. Hence, considerable HCV research has recently been directed towards understanding the mechanisms underlying the development of these metabolic manifestations in HCV infected patients and their implications in the progression of liver disease. Several important questions have been examined: are these metabolic disorders in HCV infected patients a result of viral or host factors and, if viral, how do viral proteins interfere with lipid and insulin metabolism? What is the primary event in these patients (steatosis or IR) and what are the implications of steatosis and IR in the pathogenesis of liver disease? Finally, how can we exploit our current knowledge for developing effective therapeutic strategies for HCV infected patients?
In this issue of Gut, Fartoux and colleagues2 utilise the homeostasis model assessment for IR (HOMA IR) index to study the association between steatosis and IR in patients with chronic HCV infection (see page 1003). In order to examine this association, the authors excluded patients with alternate factors known to be associated with the development of steatosis (such as alcohol intake >20 g/day or diabetes). Their findings confirm earlier observations3–5 that differing and genotype specific mechanisms characterise the development of hepatic steatosis in HCV infected patients. Accordingly, steatosis in genotype 3 infected patients is primarily viral mediated (cytopathic) whereas host factors, principally those associated with IR and its clinical attributes, are responsible for the development of steatosis in the genotype 1 infected patient.
DIRECT STEATOGENIC EFFECT OF HCV
Evidence for a HCV genotype 3 specific steatogenic effect has come from several clinical observations (table 1 ▶).3,6–8 Furthermore, this direct steatogenic effect of HCV is highly reproducible in transgenic mice and cell culture models of hepatitis C, in which overexpression of viral protein (such as core and NS5A) has been shown to induce accumulation of intracytoplasmic triglyceride rich droplets.9
Table 1.
Clinical evidence for a steatogenic effect for genotype 3
| • Hepatic steatosis is present in the majority of patients with genotype 3 infection |
| • Hepatic steatosis correlates with the level of HCV RNA in serum and the liver |
| • In patients infected with genotype 3 (but not genotype 1), successful viral clearance with antiviral therapy results in the disappearance of steatosis, only to recur on viral relapse |
| • IR has been observed to be less common among genotype 3 infected patients, even in those with extensive hepatic steatosis. |
HCV, hepatitis C virus; IR, insulin resistance.
There are several identified mechanisms whereby HCV may alter lipid metabolism. Firstly, HCV core protein has been shown to directly inhibit the function of microsomal triglyceride transfer protein, a major regulator of hepatic assembly, and secretion of nascent triglyceride rich very low density lipoproteins (VLDL). The latter effect impairs the ability of hepatocytes to assemble and secret VLDL.10,11 Secondly, HCV core protein has been observed to induce mitochondrial injury resulting in oxidative stress. Oxidative stress perturbs lipid peroxidation, thereby contributing to the development of steatosis.12–14 Finally, microarray studies have illustrated the ability of HCV (in particular genotype 3) to induce transcription of several genes involved in lipid metabolism in the liver.15,16 Among these is stearoyl coenzyme A desaturase 4 (SCD4), a rate limiting enzyme in the synthesis of monounsaturated fats.15 Reduced expression of SCD4 in livers of ob/ob mice has been shown to significantly ameliorate hepatic steatosis.17
INSULIN RESISTANCE AND STEATOSIS IN HCV INFECTED PATIENTS
Steatosis in patients with genotype 1 infection is increasingly recognised as a component of the metabolic syndrome, a condition invariably associated with IR. In support, host factors such as age, body mass index (BMI), and central obesity, have been shown to correlate with the development of steatosis in genotype 1 infected patients (but not genotype 3).18 Fartoux and colleagues2 have further demonstrated that HOMA IR was the only risk factor independently associated with the development of steatosis in genotype 1 infected patients (p = 0.001). Moreover, the degree of steatosis was significantly predictive of HOMA IR (p = 0.004).
There are several uncertainties regarding the cascade of events leading to the development of IR and steatosis in HCV infected patients with features of the metabolic syndrome. As such, whether IR or steatosis is the primary or secondary event in these patients is unclear.
Overall, there are sufficient clinical and experimental data indicating that excess fat can perpetuate IR: large epidemiological studies reveal that the risk of IR rises as body fat content (determined by BMI) increases, from the very lean to the very obese.19 Experimental evidence also indicates that intracellular accumulation of fatty acid metabolites (either through increased delivery or decreased metabolism) in the liver or muscle directly promotes serine phosphorylation (in contrast with tyrosine phosphorylation) of insulin receptor substrate 1 (IRS-1). This effect leads to impaired glucose transport activity and other events downstream of insulin receptor signalling.20 In this respect, measures to reduce circulating free fatty acid and liver triglyceride content have been shown to restore insulin signalling and reverse both hepatic and muscle IR induced by high fat diets.17,21
Another common link in the development of IR and steatosis is the adipocyte secreted proteins leptin and adiponectin. Leptin is a protein product of the adipocyte obese (ob) gene. Serum leptin levels have been observed to be increased in patients with NASH and chronic HCV infection.22,23 Moreover, in each of these diseases, serum leptin level has been reported to independently correlate with hepatic steatosis.22,24 While it is recognised that the effect of leptin on insulin sensitivity is variable, increased expression of leptin in hepatocytes has been shown to attenuate several insulin induced activities, including tyrosine phosphorylation of IRS-1.25
Adiponectin is abundantly synthesised and secreted by adipose tissue. Serum levels of adiponectin correlate with systemic insulin sensitivity. More recently, low levels of serum adiponectin have been shown to correlate with the presence of steatosis in patients with chronic HCV infection. The latter observation suggests a role for adiponectin in the development of IR and steatosis in these patients.26
In contrast with the effect of fat on IR, insulin itself controls the regulation of a host of genes involved in lipid metabolism.27 The ability of insulin to activate lipogenesis is partly mediated by inducing the transcription of the sterol regulatory element binding protein 1c (SREBP-1c), a key regulator of fatty acid synthesis in the liver. The effect of insulin on SREBP-1c transcription is even more apparent in the presence of profound IR.28 In this setting, obese animals with IR have increased levels of SREBP-1c resulting in increased rates of fatty acid synthesis and the development of hepatic steatosis.29,30 Subsequent normalisation of SREBP-1 expression in these animals dramatically ameliorates steatosis.28
HCV AND INSULIN RESISTANCE
Several studies (including that of Fartoux and colleagues2) evaluating IR in patients with chronic HCV infection have found that the development of IR can occur early in the course of the disease. This effect appears to be independent of body weight, stage of liver disease, and presence or absence of overt diabetes.31–33 There are other clinical observations supporting a “fat independent” mechanism in the development of IR in HCV infected patients. Firstly, Fartoux and colleagues2 and others32 have observed that patients infected with HCV genotype 3 have more extensive hepatic steatosis but a lower incidence of IR.32 This observation supports a genotype specific mechanism underlying IR in HCV infected patients.32 Secondly, two studies demonstrated that clearance of HCV with antiviral therapy resulted in restoration of insulin sensitivity and euglycaemia.34,35 Finally, the extent of IR has been shown to correlate with the grade of portal inflammation in HCV infected patients.32 Collectively, these data suggest that either HCV per se or the inflammatory response incited by HCV in the liver is central to the development of IR. This first hypothesis has been recently verified in HCV core transgenic mice, observed to develop IR resistance as early as one month of age, in the absence of either overt liver injury or excessive body weight gain.
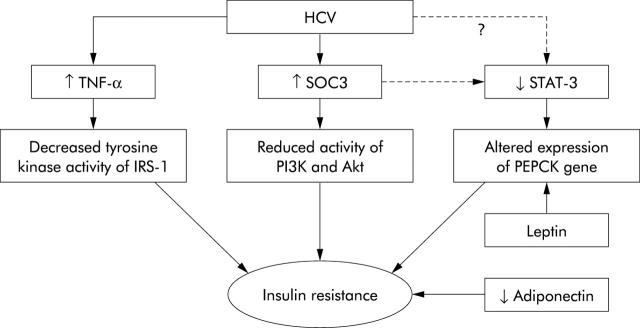
The precise mechanisms whereby HCV induces IR remain elusive but recent progress has shed light on several critical pathways. Impairment of IRS-1 and IRS-2 expression has been observed in the livers of both HCV infected patients as well as in HCV core transgenic mice.36,37 Specifically, HCV core protein has been shown to inhibit insulin induced phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and Akt, which are downstream components of IRS in the liver.37 Interestingly, HCV has been reported to mediate dysfunction of the insulin signalling pathways by upregulating the expression of suppressor of cytokine signalling 3 (SOC3).37 This observation is quite important in light of recent data demonstrating direct involvement of SOC1 and SOC3 in regulating expression of SREBP-1c in the livers of obese animals.38 In these animal models, overexpression of SOC1 and SOC3 proteins has been shown to enhance SREBP-1c promoter activity by attenuating signal transducer and activator of transcription 3 (STAT-3) mediated inhibition of this region.38 The net effect in these animals was development of systemic IR and hepatic steatosis. Conversely, inhibiting expression of SOCs protein in obese animals normalised levels of SREBP-1 and improved insulin sensitivity and hepatic steatosis. Consistent with these data, hepatic STAT-3 signalling has recently been shown to be essential for normal glucose homeostasis and insulin sensitivity.39 These observations are relevant as HCV is recognised as influencing the activity of STAT-3. It is therefore intriguing to speculate that STAT-3 may be one of the key molecules involved in HCV mediated IR (fig 1 ▶).
Figure 1.
Molecules that are likely to be involved in mediating insulin resistance (IR) in hepatitis C virus (HCV) infection. Tumour necrosis factor α (TNF-α) is increased in the serum and liver of HCV infected patients. TNF induces IR by decreasing the tyrosine kinase activity of insulin receptor substrate 1 (IRS-1).39 Treatment of HCV core transgenic mice with anti-TNF-α has been shown to restore insulin sensitivity. High levels of suppressor of cytokine signalling 3 (SOC3) have been detected in association with IR in HCV infection. This effect was associated with reduced insulin induced phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase (PI3K) and Akt.37 SOC3 (and possibly HCV) can attenuate the activity of signal transducer and activator of transcription 3 (STAT-3). Mice lacking STAT-3 specifically in the liver have been reported to exhibit IR and, when fed a high fat diet, glucose intolerance. The latter was associated with increased expression of the phosphoenolpyruvate carboxykinase (PEPCK) gene.38 The PEPCK gene itself is a target for leptin (also increased in HCV infection) mediated regulation of gluconeogenesis in the liver. Finally, reduced expression of adiponectin is associated with obesity and IR. Low serum levels of adiponectin have been shown to correlate with steatosis in HCV infected patients.26
ROLE OF CYTOKINES
Tumour necrosis factor α (TNF-α) levels are elevated in the liver and serum of patients with chronic HCV infection. TNF-α plays an important role in the development of IR through interfering with insulin signalling.40 In IR HCV core transgenic mice, treatment with anti-TNF-α restored insulin sensitivity, thus supporting the notion that TNF-α is a major factor for the development of IR in HCV infection.31
ROLE OF STEATOSIS AND INSULIN RESISTANCE IN PROGRESSION OF HCV LIVER DISEASE
In agreement with previous reports,3,4,7,41 Fartoux and colleagues2 observed an association between steatosis and severity of fibrosis, irrespective of HCV genotype. While insulin levels were predictive of fibrosis in their univariate analysis, subsequent multivariate analysis confirmed steatosis, but not insulin levels, to be independently associated with fibrosis (p = 0.02).
Thus two keys questions arise: is fat per se fibrogenic and what is the role of insulin in the fibrotic process?
Steatosis is associated with increased production of reactive oxygen species which initiate lipid peroxidation, resulting in hepatic stellate cell activation.42 However, in NASH, disease progression is recognised as being slower than that observed in patients with HCV infection and steatosis. Thus it is likely that the coexistence of HCV and steatosis aggravates and accelerates the injury induced by each alone. In this setting, hepatic inflammation induced by the host response, together with the increased production of several proinflammatory and profibrotic cytokines, provide the substrate for the “second hit” in the steatotic liver. Also, the ability of the virus itself to induce oxidative stress and promote lipid peroxidation may further aggravate the pathogenic process induced by steatosis. Consider also that fat may render HCV infected liver more vulnerable to injury. Livers with steatosis are more sensitive to TNF-α mediated inflammation and liver injury.43 Moreover, in HCV livers with steatosis, apoptosis activity has been noted to be increased compared with infected livers without steatosis.44
While Fartoux and colleagues2 could not find a direct association between IR and fibrosis, others have.32,33,45 The authors do concede that insulin plays a significant role in the development of fibrosis via a mechanism involving steatosis. In this regard, steatosis promotes cellular IR which, in turn, induces compensatory hyperinsulinaemia. Hyperinsulinaemia has been shown to directly stimulate hepatic stellate cell proliferation and increase expression of connective tissue growth factor, a key factor in the progression of fibrosis.46 Collectively, the data implicate both steatosis and IR in liver disease progression in HCV infected patients.
CONCLUSIONS
Our knowledge of the mechanisms and significance of steatosis and IR in patients with chronic HCV infection has advanced. The work by Fartoux and colleagues2 adds to the growing evidence that the relationship between HCV, steatosis, and IR is genotype specific and that steatosis and IR are closely linked to the progression of liver disease in HCV infected patients. As such, we need to clarify the molecular pathways involved in mediating these inter-relationships. In light of our current knowledge of the implications of steatosis and IR in liver disease, the importance of lifestyle changes (such as weight loss) need to be an emphasised in treating HCV infected patients. Similarly, new therapeutic approaches exploiting the interaction between HCV and lipid metabolism are eagerly awaited.
Acknowledgments
This work was supported by a grant from NIH, 2RO1 DK053792.
Abbreviations
HCV, hepatitis C virus
IR, insulin resistance
NASH,
non-alcoholic steatohepatitis,
MTP, microsomal transfer protein
VLDL, very-low density lipoproteins
SCD4, stearoyl coenzyme A desaturase 4
ob (obese),
BMI, body mass index
IRS, insulin receptor substrate
SREBP-1, sterol regulatory element binding protein-1c
SOC, suppressor of cytokine signaling
PI3K, phosphatidylinositol 3-kinase
STAT-3, signal transducer and activator of transcription-3
TNF, Tumor necrosis factor
PEPCK, phosphoenolpyruvate carboxykinase
Conflict of interest: None declared.
REFERENCES
- 1.Goodman ZD, Ishak KG. Histopathology of hepatitis C virus infection. Sem Liver Dis 1995;15:70–81. [DOI] [PubMed] [Google Scholar]
- 2.Fartoux L, Poujol-Robert A, Guéchot J, et al. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut 2005;54:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar D, Farrell GC, Fung C, et al. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology 2002;36:1266–72. [DOI] [PubMed] [Google Scholar]
- 4.Adinolfi LE, Gambardella M, Andreana A, et al. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001;33:1358–64. [DOI] [PubMed] [Google Scholar]
- 5.Rubbia-Brandt L, Quadri R, Abid K, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol 2000;33:106–15. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 2003;38:75–85. [DOI] [PubMed] [Google Scholar]
- 7.Patton HM, Patel K, Behling C, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 2004;40:484–90. [DOI] [PubMed] [Google Scholar]
- 8.Hui JM, Kench J, Farrell GC, et al. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J Gastroenterol Hepatol 2002;17:873–81. [DOI] [PubMed] [Google Scholar]
- 9.Moriya K, Yotsuyanagi H, Shintani Y, et al. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol 1997;78:1527–31. [DOI] [PubMed] [Google Scholar]
- 10.Perlemuter G, Sabile A, Letteron P, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J 2002;16:185–94. [DOI] [PubMed] [Google Scholar]
- 11.Serfaty L, Andreani T, Giral P, et al. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol 2001;34:428–34. [DOI] [PubMed] [Google Scholar]
- 12.Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002;122:366–75. [DOI] [PubMed] [Google Scholar]
- 13.Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 1998;4:1065–7. [DOI] [PubMed] [Google Scholar]
- 14.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology 2002;122:352–65. [DOI] [PubMed] [Google Scholar]
- 15.Bigger CB, Guerra B, Brasky KM, et al. Intrahepatic Gene Expression during Chronic Hepatitis C Virus Infection in Chimpanzees. J Virol 2004;78:13779–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shackel NA, McGuinness PH, Abbott CA, et al. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol 2002;160:641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiya M, Yahagi N, Matsuzaka T, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003;38:1529–39. [DOI] [PubMed] [Google Scholar]
- 18.Adinolfi LE, Utili R, Ruggiero G. Body composition and hepatic steatosis as precursors of fibrosis in chronic hepatitis C patients. Hepatology 1999;30:1530–1. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–6. [DOI] [PubMed] [Google Scholar]
- 20.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10:268–74. [DOI] [PubMed] [Google Scholar]
- 22.Chitturi S, Farrell G, Frost L, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology 2002;36:403–9. [DOI] [PubMed] [Google Scholar]
- 23.Patel K, Muir A, McHutchison JG, et al. A link between leptin and steatosis in chronic hepatitis C? Time to weigh up the fats. Am J Gastroenterol 2003;98:952–5. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Gomez M, Castellano-Megias VM, Grande L, et al. Serum leptin levels correlate with hepatic steatosis in chronic hepatitis C. Am J Gastroenterol 2003;98:1135–41. [DOI] [PubMed] [Google Scholar]
- 25.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science 1996;274:1185–8. [DOI] [PubMed] [Google Scholar]
- 26.Michel PJ, Anne M, Valerie J, et al. Decreased plasma adiponectin concentrations are closely related to steatosis in HCV-infected patients. J Clin Endocrinol Metab. 2005;not on p/m*. [DOI] [PubMed]
- 27.Shimomura I, Matsuda M, Hammer RE, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 2000;6:77–86. [PubMed] [Google Scholar]
- 28.Shimomura I, Shimano H, Korn BS, et al. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem 1998;273:35299–306. [DOI] [PubMed] [Google Scholar]
- 29.Yahagi N, Shimano H, Hasty AH, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem 2002;277:19353–7. [DOI] [PubMed] [Google Scholar]
- 30.Horton JD, Shimomura I, Ikemoto S, et al. Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J Biol Chem 2003;278:36652–60. [DOI] [PubMed] [Google Scholar]
- 31.Shintani Y, Fujie H, Miyoshi H, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology 2004;126:840–8. [DOI] [PubMed] [Google Scholar]
- 32.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression[corrected]. Gastroenterology 2003;125:1695–704. [DOI] [PubMed] [Google Scholar]
- 33.Petit JM, Bour JB, Galland-Jos C, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol 2001;35:279–83. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H, Shiota G, Kawasaki H. Changes in glucose tolerance after interferon-alpha therapy in patients with chronic hepatitis C. J Med 1997;28:335–46. [PubMed] [Google Scholar]
- 35.Konrad T, Zeuzem S, Vicini P, et al. Evaluation of factors controlling glucose tolerance in patients with HCV infection before and after 4 months therapy with interferon-alpha. Eur J Clin Invest 2000;30:111–21. [DOI] [PubMed] [Google Scholar]
- 36.Aytug S, Reich D, Sapiro LE, et al. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology 2003;38:1384–92. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol 2004;165:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue H, Ogawa W, Ozaki M, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 2004;10:168–74. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil GS, Murray DL, Choy LN, et al. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A 1994;91:4854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hourigan LF, Macdonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 1999;29:1215–9. [DOI] [PubMed] [Google Scholar]
- 42.Chavin KD, Yang S, Lin HZ, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 1999;274:5692–700. [DOI] [PubMed] [Google Scholar]
- 43.Yang SQ, Lin HZ, Lane MD, et al. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A 1997;94:2557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh MJ, Vanags DM, Clouston AD, et al. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology 2004;39:1230–8. [DOI] [PubMed] [Google Scholar]
- 45.McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut 2004;53:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001;34:738–44. [DOI] [PubMed] [Google Scholar]