Disease related prion protein (PrPSc) is readily detectable in lymphoreticular tissues in variant Creutzfeldt-Jakob disease (vCJD) but not in other forms of human prion disease.1–5 This distinctive pathogenesis together with the unknown population prevalence of asymptomatic vCJD infection1,5,6 has led to significant concerns that secondary transmission of vCJD prions will occur through a wide range of surgical procedures.1,3,7 Risk assessment for intestinal endoscopy, biopsy, and surgery is currently limited by a lack of knowledge about relative PrPSc levels and prion titres within intestinal tissues in vCJD patients. Because of its high content of lymphoid follicles, terminal ileum is regarded as the intestinal tissue having the highest potential for iatrogenic transmission of vCJD prions.8,9 Here we provide the first report of relative PrPSc concentrations in vCJD terminal ileum.
Tissues were obtained at autopsy with consent from relatives from four patients with neuropathologically confirmed vCJD and two patients with neuropathologically confirmed sporadic CJD (both PRNP codon 129MM with type 2 PrPSc in brain). Terminal ileum was analysed for PrPSc by high sensitivity immunoblotting3 and for abnormal PrP immunoreactivity by immunohistochemistry.6 Using these methods, terminal ileum from all four vCJD cases showed high levels of detectable PrPSc (fig 1A ▶). In three vCJD cases, 2/2 homogenates prepared from each ileum specimen were positive for PrPSc whereas 2/4 ileum homogenates were positive in the other vCJD case. The glycoform ratio of protease resistant fragments of di-, mono-, and non-glycosylated PrP in terminal ileum appeared to be closely similar to the type 4t PrPSc pattern seen in vCJD tonsil.2,3 Although there was variation in PrPSc concentration between different homogenates of vCJD terminal ileum, PrPSc levels in positive samples were typically in the range 0.1–1% of that present in vCJD brain (fig 1B ▶). With respect to both sampling variation and PrPSc concentration, terminal ileum appears to be closely similar to lymph nodes in vCJD.3 These findings, together with our previous studies, show that PrPSc deposition within the intestine is not uniform in vCJD. From the four cases of vCJD with PrPSc positive terminal ileum studied here, 0/2 cases with available tissue had detectable PrPSc in the appendix3,10 and only 1/3 cases had detectable PrPSc in the rectum.3 In contrast with findings with vCJD terminal ileum, no detectable PrPSc was found in homogenates of terminal ileum prepared from sporadic CJD patients (fig 1A ▶). The lack of detection of PrPSc in sporadic CJD terminal ileum extends our previous findings for one of these cases in which we have previously reported a lack of detectable PrPSc in tonsil, rectum, and appendix.3,10
Figure 1.
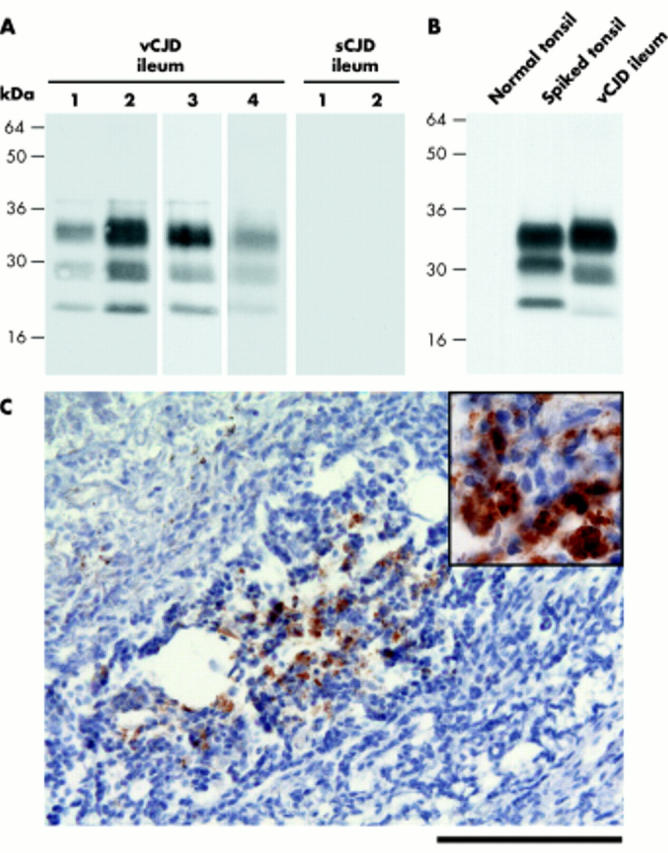
(A, B) High sensitivity immunoblots using anti-prion protein (PrP) monoclonal antibody 3F4. (A) Proteinase K digested sodium phosphotungstic acid pellets from 0.5 ml of 10% terminal ileum homogenates from variant Creutzfeldt-Jakob disease (vCJD) patients 1–4 or sporadic CJD (sCJD) patients 1 and 2. (B) Proteinase K digested sodium phosphotungstic acid pellets from 0.5 ml of 10% normal human tonsil homogenate (normal tonsil) or 0.5 ml of 10% normal human tonsil homogenate spiked with 2.5 μl of 10% brain homogenate from vCJD patient No 4 (spiked tonsil) were compared with a proteinase K digested sodium phosphotungstic acid pellet from 0.5 ml of 10% terminal ileum homogenate from the same vCJD patient. (C) Photomicrograph showing abnormal PrP immunoreactivity in a lymphoid follicle in vCJD terminal ileum (anti-PrP monoclonal antibody ICSM 35). Scale bar, 100 μm. Inset, high power magnification of PrP deposits.
In agreement with findings from immunoblotting, immunohistochemistry showed abnormal PrP deposition in the terminal ileum in vCJD (fig 1C ▶) but not in sporadic CJD (data not shown). The irregular distribution of abnormal PrP positive lymphoid follicles seen in vCJD terminal ileum is consistent with variation in PrPSc concentration detected in different terminal ileum samples by immunoblotting.
Albeit from necessarily limited numbers investigated, the uniform presence of PrPSc in vCJD terminal ileum, at concentrations of up to 1% of those found in vCJD brain, reinforces concerns that iatrogenic transmission of vCJD prions might occur through contaminated intestinal endoscopes, biopsy forceps, or surgical instruments.3,7,8,9,10 These findings should assist policy makers in the UK and elsewhere in risk assessments about the use of disposable forceps for intestinal biopsy. Alternative approaches to risk reduction may now be possible as practical means of prion decontamination for endoscopes and surgical instruments are now feasible using enzymatic methods.7,11
Acknowledgements
This study was funded by the UK Medical Research Council and was performed under the approval of the Institute of Neurology/National Hospital for Neurology and Neurosurgery Local Research Ethics Committee.
Supplementary Material
References
- 1.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet 1999;354:317–23. [DOI] [PubMed] [Google Scholar]
- 2.Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 1999;353:183–9. [DOI] [PubMed] [Google Scholar]
- 3.Wadsworth JDF, Joiner S, Hill AF, et al. Tissue distribution of protease resistant prion protein in variant CJD using a highly sensitive immuno-blotting assay. Lancet 2001;358:171–80. [DOI] [PubMed] [Google Scholar]
- 4.Head MW, Ritchie D, Smith N, et al. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: an immunohistochemical, quantitative, and biochemical study. Am J Pathol 2004;164:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton DA, Ghani AC, Conyers L, et al. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J Pathol 2004;203:733–9. [DOI] [PubMed] [Google Scholar]
- 6.Frosh A, Smith LC, Jackson CJ, et al. Analysis of 2000 consecutive UK tonsillectomy specimens for disease-related prion protein. Lancet 2004;364:1260–2. [DOI] [PubMed] [Google Scholar]
- 7.Jackson GS, McKintosh E, Flechsig E, et al. An enzyme-detergent method for effective prion decontamination of surgical steel. J Gen Virol 2005;86:869–78. [DOI] [PubMed] [Google Scholar]
- 8.Axon ATR, Beilenhoff U, Bramble MG, et al. Variant Creutzfeldt-Jakob disease (vCJD) and gastrointestinal endoscopy. Endoscopy 2001;33:1070–80. [DOI] [PubMed] [Google Scholar]
- 9.Bramble MG, Ironside JW. Creutzfeldt-Jakob disease: implications for gastroenterology. Gut 2002;50:888–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joiner S, Linehan J, Brandner S, et al. Irregular presence of abnormal prion protein in appendix in variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 2002;73:597–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichet G, Comoy E, Duval C, et al. Novel methods for disinfection of prion-contaminated medical devices. Lancet 2004;364:521–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


