Small intestinal transplantation should now be routinely considered for patients with irreversible intestinal failure and complications of parenteral nutrition (PN). Although technically possible for a century,1 and attempted in humans for more than 40 years,2 immunological graft intolerance presented an impenetrable barrier to successful engraftment until the development of the powerful calcineurin inhibitor immunosuppressive agents in the late 1970s. Their subsequent clinical use over the past 17 years has transformed small intestinal transplantation from a position of predictable failure to predominant success and routine clinical reality for a select group of patients
One year graft survivals for solid organs are now excellent and have been improving steadily since the introduction of ciclosporin to routine clinical practice in the early 1980s.3,4 In contrast, survival values for small intestinal transplantation have been slow to improve although are now comparable with those for lung, and the latest published values for one year survival approximate those for liver and kidney (table 1 ▶) Why then has progress with intestinal transplantation been so much more difficult?
Table 1.
Patient and graft survival of patients receiving small intestinal and solid organ grafts
| Graft type | 1 year survival (%) | 3 year survival (%) | 5 year survival (%) | |||
| Patient | Graft | Patient | Graft | Patient | Graft | |
| Kidney681990–1999 | 94 | 83 | 90 | 73 | 85 | 65 |
| Liver691994–2003* | 78 | 73 | 72 | 67 | 67 | 62 |
| Heart701990–2002 | 82 | 80 | 72 | 70 | 67 | 66 |
| Lung71 1990–2002 | 75 | 75 | 59 | 59 | 46 | 46 |
| Intestine321985–2004 | 72 | 64 | 57 | 42 | 47 | 35 |
| Intestine321999–2004 | 78 | 65 | 62 | 50 | 50** | 35** |
| Int and liver321985–2004 | 58 | 56 | 46 | 44 | 41 | 39 |
| Int and liver321999–2004 | 60 | 58 | 50 | 45 | 50** | 45** |
| MultiV32 1985–2004 | 58 | 58 | 50 | 45 | 50 | 40 |
| MultiV321999–2004 | 66 | 62 | 62 | 58 | 62** | 50** |
| Well performing individual centres | ||||||
| Sudan et al52Intestine (2000) | 93 | 71 | ||||
| Goulet et al54Intestine and liver (1999) | 80 | 80 | ||||
| Pinna et al53Multivisceral (2000) | 70 | 60 | ||||
| Fishbein et al23Intestinal (2002) | 92 | 92 | ||||
| Parenteral nutrition70(1990–1996) | 90 | 75 | ||||
Int, intestine; MultiV, multivisceral.
*Data given for first transplantation only. All other data include a small number of repeat transplantations (heart and lung 2%, intestine 7%) which are associated with a poorer outcome.
**Four year survival values are given.
Data in italics are most appropriate for comparison. Data, including during the early development of intestinal transplantation, are also given.
A BRIEF HISTORY OF SMALL INTESTINAL TRANSPLANTATION
The technical feasibility of the procedure has been established for a century1 but immunological feasibility was far more difficult to establish. The high density of lymphoid tissue and the large mucosal surface area of the small intestine expressing class 2 major histocompatability antigens fuels the mutual intolerance between graft and host. As a hollow organ whose lumen is colonised by a multitude of bacteria and other micro-organisms, it behaves as a potent vector of infection to the host, a problem that is made worse by the precarious barrier from the lumen provided by the thin and vulnerable monolayer of mucosal epithelium. Here then is the fine balance between immunosuppression and infection that has bedevilled its transplantation and led to failure in so many early attempts.2
Following early transplantation attempts, deaths were most commonly a consequence of acute graft rejection and subsequent sepsis associated multiorgan failure.5,6 This scenario was not improved even with the introduction of combination therapy with azathioprine, prednisolone, and antilymphocyte globulin.7,8 More powerful immunosuppression was needed and the target of most of the early research.
The introduction of ciclosporin in 1978 by Calne and colleagues3,4 both accelerated progress in solid organ transplantation and rekindled interest in intestinal grafts. Success in this new ciclosporin era led to the transition from success in animals to the first long term success in humans. In 1988 Grant and colleagues8 reported a patient with short gut syndrome following mesenteric infarction who had undergone combined liver and small intestine transplantation and remained alive one year after the procedure. Other groups soon reported similar experiences.8–11 Even though the barrier of chronic rejection was soon evident, the achievement of medium term survival was heralded as a defining moment.
The introduction of tacrolimus, a potent new calcineurin inhibitor, marked the next major step in allowing clinical intestinal transplantation to become a reality. Early rejection was then superseded by infection as the main cause of death,12 indicating the need to refine the target of immunological suppression to reduce infection while avoiding rejection. Another adverse effect of heavy immunosuppression appeared to be a substantial increase in the incidence of chronic rejection,13 and the near total loss of progressive tolerance that had allowed low dose maintenance immunosuppresion or immunosuppression free management in a proportion of the earlier procedures.14,15
A possible explanation for the divergent frequencies of acute and chronic rejection and the potential key to tolerance with minimal immunosuppression came from the report by Starzl et al that long term tolerance was associated with donor and host leucocyte chimerism.15,16 This apparent engagement of donor and host leucocytes seemed pivotal to long term tolerance but vulnerable to ablation by the powerful new immunosuppressants.17 Exhaustive deletion of helper and cytotoxic T cells18 and preferential differentiation to suppressor and regulatory T cells following chronic exposure of host T cells to donor antigens presented in non-inflamed conditions after graft “healing- in” have been postulated as the mechanisms of long term tolerance.19,20
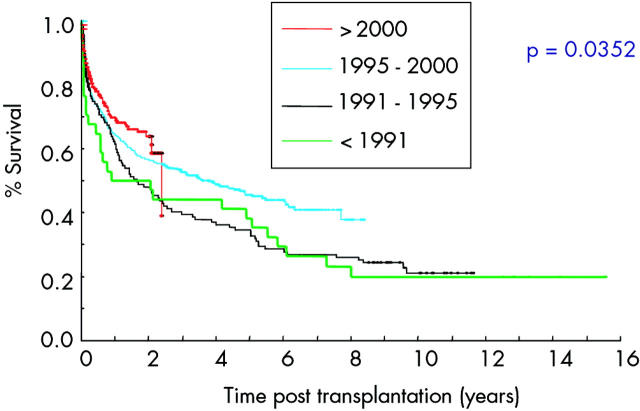
Monoclonal antibody therapy presented the potential to provide specific targeting of a wide range of immune mediators and, with the expected greater degree of selectivity, perhaps fewer problems with infection. It was postulated that preoperative induction with host lymphocyte depleting antibody might allow a more equitable engagement of graft with host and promote tolerance. Campath 1H, a monoclonal anti-CD 54 antibody which depletes both T and B cells19 was used for a multivisceral transplantation in Cambridge UK in 1995. In combination with tacrolimus the level of immunosuppression was profound, leading to episodes of severe infection but subsequently the immunosuppression could be steadily reduced without evidence of rejection. Initially composite grafting was thought to be entirely responsible for this greater degree of immunological tolerance21 but with the advancement of global experience and the benefits of a single international registry additional influence of the type and degree of immunosuppression on tolerance was appreciated.19 Judicious immunosuppression with the aim of engaging graft and host in a manner that encourages tolerance with minimal immunosuppression has become the goal of modern transplantation immunosuppression.14,19 Facilitated by deployment of the more selective monoclonal antibody technology, inroads into maximising host tolerance to minimise immunosuppression are being made19 and survival values are inexorably improving (fig 1 ▶).22
Figure 1.
Survival of patients transplanted in different eras (from the International Registry32).
Immunological engagement and mutual tolerance is fundamental to successful long term engraftment.19 Reliable achievement of this state of full or partial tolerance represents the next and perhaps last major barrier to the use of transplantation as first line treatment for intestinal failure.
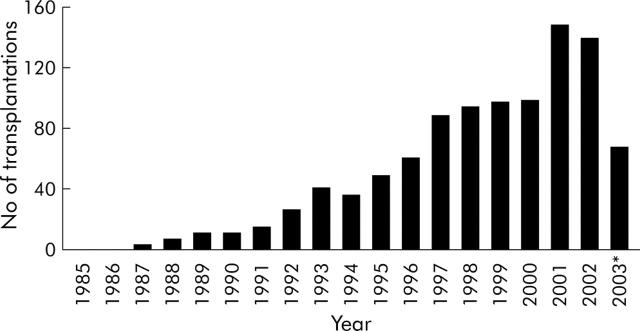
The success of the tacrolimus era led to an exponential increase in transplantation procedures (fig 2 ▶) and centres are now established in more than 30 countries. The development of monoclonal antibody technology has further improved survival. Recent reports of over 90% one year survival rates23 indicate the current incremental progression to a position that might eventually challenge PN as primary treatment for small intestinal failure. Such a broadening of the indications already practised sporadically in some centres will generate the next quantum increase in small intestinal transplantation.
Figure 2.
Number of intestinal transplantations reported to the International Registry per year (from the Transplant Registry32).*Data were acquired half way through 2003.
INDICATIONS FOR SMALL INTESTINAL TRANSPLANTATION
Patient selection
For the majority of patients, small intestinal transplantation is indicated only when irreversible small intestinal failure coexists with failure of PN. The main causes of PN failure are loss of venous access due to venous thrombosis, PN related liver disease, and recurrent line related sepsis.24–26 A global consensus for indications has yet to be published but is likely to be broadly in line with the healthcare finance administration of North American policy decision.27 Patients with significant complications of PN (table 2 ▶) should be discussed with an intestinal failure centre where they can be evaluated and PN optimised prior to referral to a transplantation centre if required.
Table 2.
Indications for referral of adults to an intestinal failure centre
| Complications of parenteral nutrition |
| (1) Impaired venous access |
| - Venous occlusion of one or more neck sites of central venous access (jugular or subclavian) |
| (2) Line related sepsis |
| - Two or more line changes in a year as a consequence of line related sepsis. |
| - One episode of life threatening line related sepsis (that is, cardiovascular shock). |
| - One episode of fungal line infection. |
| (3) Parenteral nutrition related liver disease. |
Patients with more advanced disease with one or less PN intravenous neck access sites but patent femoral veins, less than three PN intravenous neck vein access sites without bilateral femoral access, or severe liver dysfunction should be assessed by a transplant centre. Frequently recurrent line infections represent the indication in the majority of the other candidates. Evisceration during removal of large desmoid tumours, along with a few highly selected patients with malignant disease, may be considered suitable (table 3 ▶)
Table 3.
Indications for referral of adults to an intestinal transplantation centre
| (1) Complications of parenteral nutrition: |
| • Substantial venous occlusion with residual availability of: |
| - 2 or less major neck venous access sites with loss of one or more femoral sites or |
| - 1 or less major neck venous access site with both femoral sites available. |
| • Line related infection (events occurring despite review at an intestinal failure centre): |
| - recurrent episodes of severe line related sepsis requiring more than two line replacements in a year or |
| - recurrent fungal line related infections |
| • Parenteral nutrition related liver disease |
| - Impending or overt liver failure as indicated by portal hypertension, hepatic cirrhosis, or bridging fibrosis |
| (2) Requirement for extensive evisceration |
| • Desmoid tumours |
| - (malignant disease in very select circumstances, usually neuroendocrine or cholangiocarcinoma) (modified from Buchman and colleagues27) |
The incidence of severe PN related liver disease has been reported to affect as many as 15% of patients after one year28 and 50% of patients after five years. Although the latter report included patients with chronic hepatitis C and B infection it was based on data acquired from a central government coordinated data bank and has the advantage of better representing the entire population of patients on PN than single centre studies.29 This frequency of liver disease together with venous occlusion and infection would be expected to result in a considerable demand for small intestinal transplantation in the UK. The number of patients on PN in the UK has been steadily increasing, numbering approximately 500 in 2003.30 However, in the UK patients are rarely referred for consideration and only 14 adult transplantations have been undertaken over the last 15 years.
TIMING REFERRAL
Timely referral for assessment is essential to allow optimisation of physical and psychological factors and should be at a stage when central venous access is adequate for surgery and management of postoperative complications which may include renal replacement therapy. Patients referred to an intestinal failure centre can then be followed closely and familiarised with the options for treatment in the event of further deterioration. Patients who have lost all venous access in the neck and are receiving PN via the femoral vein are at a considerable disadvantage. Not only are they more prone to line infections26 but if composite grafting of two or more organs is to be considered central venous access above the hepatic veins will be necessary. Time should be available to minimise comorbidity, particularly that which might increase the likelihood of postoperative complications, such as chronic infections which may be the focus of serious postoperative infections during immunosuppression. Mental health problems can present a threat to patients of similar severity to physical disease and should not be underestimated or overlooked.31
Patients who are well enough to be admitted for transplantation from home have a significantly better prognosis than those who are medically unstable and require hospitalisation during the pretransplant period.32 This underscores the importance of preoperative preparation and timing intervention which requires fine judgement to find the window of opportunity that avoids inappropriate intervention without allowing the patient’s condition to deteriorate to a point that excludes transplantation or makes it unduly hazardous.
Time on the waiting list is often protracted, with approximately 40% waiting longer than a year and relatively longer if composite grafts are required.33 Early referral before the patient develops severe irreversible liver disease may allow timely isolated small intestinal transplantation, avoiding the need for more complicated grafts.
As a hollow non-sterile organ the small intestine is very prone to damage, and cold ischaemia times must be minimised. Donor suitability is further restricted by accommodation of the graft within an often damaged peritoneal cavity. Late referral of patients and slow donor acquisition cause considerable waiting list mortality which has been reported to be as high as 50%.32,34–36 Most of these deaths (two thirds) occur in infants who in particular should be referred or at least discussed with the appropriate transplant centre at an early stage. Discussion of full referral guidelines for children37,38 are beyond the scope of this article. However, in general, intestinal disorders with a poor prognosis, PN related liver dysfunction, or protracted PN dependence in infants and children require prompt referral.
Occasionally PN fails because of frequent line infections. In most cases re-education and modification of line care can resolve the problem. However, for some these infections are frequent and recurrent39 and in the event of serious life threatening infection, transplantation should be considered.
In the UK, Addenbrooke’s Hospital in Cambridge and St James’s hospital in Leeds are national centres for adult small intestinal transplantation, and St Mark’s Hospital Northwick Park, London and The Hope hospital in Manchester are the national centres for intestinal failure. Referral of children should be made to the Birmingham Children’s Hospital.
IMMUNOSUPPRESSION
Current regimens are usually based on the powerful calcineurin inhibitor tacrolimus. The combination of tacrolimus and sirolimus does not have additional benefit over tacrolimus alone and current regimens are evolving away from combination maintenance therapy with adjunctive agents such as the antiproliferative agent mycophenolate mofetil,40 the non-calcineurin inhibitor immunosuppressant sirolimus,23 or steroids, towards monotherapy with tacrolimus17 or sirolimus41 following induction with lymphocyte depleting antibodies such as antithymocyte globulin,17Campath 1H (alemtuzumab),24,42 OKT3,13 or with monoclonal anti-interleukin 2 (IL-2) receptor antibodies (daclizumab or basiliximab).43–47 Antibody preconditioning seems to allow less potent subsequent maintenance immunosuppression and improved tolerance, allowing lower levels of tacrolimus.17 Despite the use of lower levels of tacrolimus, monitoring of haematological and renal parameters remains important. Cardiomyopathy and neurological complications also continue to be considerations. Antibody therapies are occasionally associated with severe infusion related adverse reactions caused by hypersensitivity or profound cytotoxin release but in most cases they are well tolerated. There is an increasing trend to minimise the use of steroids although they remain part of most regimens. In addition, some have used donor bone marrow infusions48,49 in an attempt to achieve better immunological engagement and improve subsequent long term tolerance, so reducing chronic rejection.14 Lymphocyte depletion of the graft by ex vivo intestine irradiation49 has shown promise but is limited by concerns related to graft radiation damage.
SURGERY
The surgical procedure is usually technically demanding. Many of the patients who are candidates for small bowel transplantation will have a heavily scarred abdominal wall from multiple abdominal procedures and previous bowel resections, resulting in difficult access to a reduced volume abdominal cavity. At times this already difficult situation can be further compounded by portal hypertension as a result of concomitant liver disease. Selecting smaller size donors and thus smaller grafts to be transplanted will help with this issue but further graft size reduction may be necessary, and securing a satisfactory abdominal wall closure represents a substantial challenge. In many cases an end ileostomy will be appropriate and in the short term allows access for monitoring of the graft by repeated biopsies to look for evidence of rejection.
Isolated small bowel transplantation is in principle the simplest surgical procedure and offers a solution to the patient who has problems with venous access and no evidence of irreversible liver disease. It has the theoretical advantage that the graft can be removed if necessary and the patient returned to PN, although this frequently proves not to be the case due to sepsis and other postoperative complications. A graft is obtained from a cadaveric donor based on the superior mesenteric artery (SMA) with a narrow patch of aortic wall (a Carrel patch) and the superior mesenteric vein (SMV)/proximal portal vein (PV). The graft is placed in the abdominal cavity and revascularised by anastomosing the narrow patch of aortic wall around the orifice of the SMA with the front wall of the aorta in the recipient. The SMV/PV are anastomosed to either the right side of the recipient portal vein which can be approached just behind the bile duct above the upper border of the duodenum after mobilising the duodenum and head of pancreas, or more simply to the front surface of the inferior vena cava (IVC) at the same level as the anastomosis to the aorta. The former technique is more technically demanding but does offer restoration of physiological drainage of the gut via the portal system. In practice, the latter technique is technically easier and is seldom associated with major problems in terms of outcome.50 Intestinal continuity is restored by performing a proximal anastomosis to the remaining recipient gut and a distal ileostomy fashioned. Grafts have also been obtained from living donors based on the distal SMA and SMV, removing approximately one third of the donor’s small bowel and anastomosing the graft to the aorta and IVC in the same way as for a cadaveric graft.51
Combined liver and small bowel transplantation offers a treatment option in cases where there is irreversible liver damage and has been more commonly applied in paediatric cases, where PN related liver disease has been more of a problem than in the adult population. There are a number of technical issues with such procedures. Excision of the liver will leave any residual upper abdominal organs (usually stomach, duodenum, pancreas, and spleen) without any venous drainage unless the remaining native portal vein (which will have been divided in removing the diseased liver) is anastomosed to the cava to form a porta-caval shunt or “piggy backed” onto the portal vein of the liver/intestinal graft. The former usually offers the simplest option. Biliary drainage of the liver is restored either by a direct anatomosis to the remaining native bile duct or to a roux loop created from the transplanted bowel (identical to the biliary drainage options in conventional liver transplantation). More recently a composite graft including the liver, intestine, duodenum, and head of pancreas (the pancreas being divided in the line of the portal vein/SMV) has been described which includes all of the donor bile duct and avoids the need for an additional biliary anastomosis and eliminates the risk of biliary leakage or anastomotic stricture.48
Vascular reconstruction for the graft in these cases will involve anastomosis of the caval venous outflow from the liver, either in the classical orthotopic liver transplant fashion or using a piggy back technique to the hepatic veins (or the more recently described technique of cavo-cavostomy) and an arterial anastomosis to the recipient aorta, usually involving a conduit of donor iliac artery or donor aorta anastomosed to a vascular patch from the donor, including the origins of the coeliac axis and SMA (which are closely related).
Multivisceral grafting offers an option in cases with large intra-abdominal desmoid tumours or other extensive disease processes which may necessitate excision of most or even all of the intra-abdominal organs.
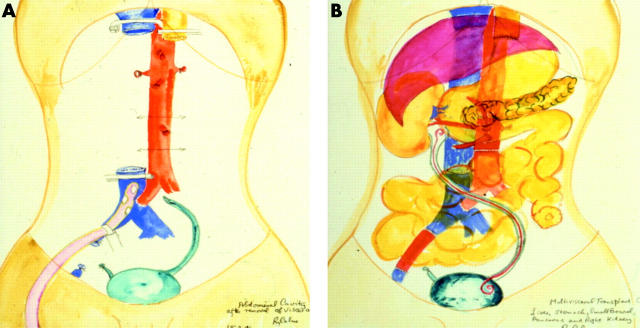
Such grafts may involve a variety of organs, usually including the liver, stomach, pancreas, duodenum, and small bowel. The spleen is removed to avoid immunological sequelae and if necessary a kidney can also be included. The vascular reconstruction required will depend on the nature of the graft but can commonly be achieved by substitution of a segment of recipient IVC (if a kidney is also being included in the graft the cava draining the right renal vein can also be included) and a segment of donor aorta, including the coeliac axis and SMA (and if needed the right renal artery for an orthotopic kidney graft), or simply an aortic patch anastomosed to the front of the aorta for arterial inflow. Figure 3 ▶ illustrates such a case of our own where a patient with Gardner’s syndrome complicated by extensive intra-abdominal desmoid disease required total exenteration (fig 3A ▶). He received a graft consisting of stomach, liver, pancreas, small intestine, and a single kidney (graft shown in position in fig 3B ▶). This patient died following head trauma five years after the procedure with a fully functioning graft.
Figure 3.
Illustration of multivisceral transplantation. (A) Following preparation of the recipient for grafting. Clamps are shown on the inferior vena cava and oesophagus. All abnormal organs apart from the bladder have been excised. (B) Following grafting of the stomach, liver, pancreas, kidney, and small intestine. A stent was placed in the re-anastomosed ureter. A segment of iliac vein on the right has been replaced. Original water colours by Sir Roy Calne, with kind permission. This illustration originally appeared in Chan and colleagues.73
OUTCOME
Patient and graft survival
Recent reports from certain centres are encouraging, with one year patient and graft survival rates for small intestine alone of approximately 90% and 80%,52,53 which are similar to those for children of 80% and 80%, respectively,54 and comparable with those of solid organ transplantation (table 1 ▶) Combined international data indicate overall one year patient and graft survival of procedures undertaken since 2000 of approximately 70%, improving from about 50% in the early 1990s (fig 1 ▶).32
Longer term survival values, which include patients receiving earlier immunosuppression strategies, are poorer but in most cases preferable to continuation on failing PN. Patients receiving intestinal or composite grafts since 1999 are reported by the International Registry32 to have one and five year graft and patient survival rates of approximately 60% and 45%, and 39% and 48%, respectively This is similar to the UK experience since 1995 of 57% and 43% respectively for both grafts and patients.31
The main reasons for graft loss in adults are rejection (48%), thrombosis/ischaemia (28%), and sepsis (12%). Infection is the main cause of death followed by rejection.32
Quality of life
Several groups have reported an improvement in the quality of life following intestinal transplantation for patients who had complications of home parenteral nutrition (HPN) preoperatively.55,56 It also compares favourably with patients on stable HPN57 and is better in transplanted patients than in patients who continue with frequent complications of PN.56
Cost effectiveness
Although not extensively studied, the available evidence suggests that intestinal transplantation is less costly than HPN.58,59 The precise costs of HPN are case sensitive but are approximately £40 000 per patient per year. Patients with frequent complications will require greater health care provision with associated additional costs. On the other hand, the first and by far the most expensive year of intestinal transplantation costs is approximately £80 000 and falls considerably in subsequent years. Cost effectiveness has not been formally compared with PN. However, when assessed by quality of life years, it might currently be expected to be better than patients with frequent complications of PN but inferior to those on stable HPN as the latter have a lower long term mortality.
Factors influencing outcome
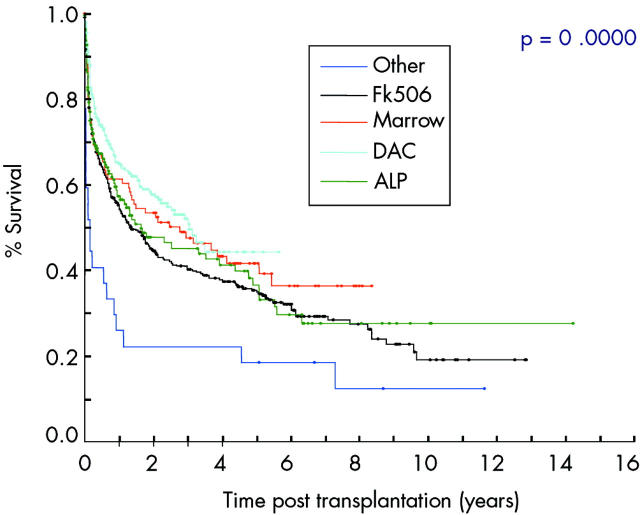
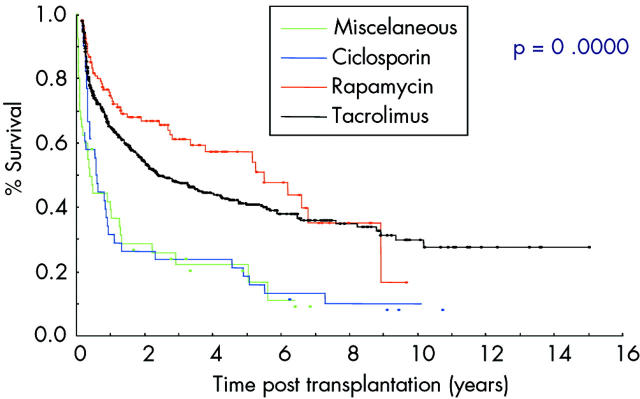
Patients admitted for transplantation from their home have significantly better graft survival which underscores the need for good preoperative preparation to optimise patient health. Graft survival is also better in those who have received antibody induction therapy with lymphocyte depleting or anti-IL-2 receptor antibody (fig 4 ▶) and when the centre has had experience of more than nine procedures.32 The highest success rates have been reported using antilymphocytic antibodies and post-transplantation tacrolimus monotherapy which is hoped to reduce postoperative immunosuppression related complications.17 The type of maintenance immunosuppression also seems to influence patient and graft survival (fig 5 ▶). (Age)2 is a significant risk associated continuous variable. Interestingly, the primary diagnosis, sex of the recipient, type of graft, retransplantation, portal venous drainage, and donor graft irradiation were not significant prognostic factors.32
Figure 4.
Graft survival related to induction therapy (from the International Registry32). DAC, daclizumab; ALP, lymphocyte depleting antibodies; FK506, tacrolimus.
Figure 5.
Graft survival related to maintenance therapy (from the International Registry32).
Acute graft rejection
Until recently, acute host versus graft rejection was reported to affect between 50%32,54 and 100%,52,54 with most having averaged values for all types of graft between 70% and 80%.27 However, novel regimens using antibody induction have seen the incidence fall, and Campath IH (alemtuzimab) is associated with a particularly low rate of 20% in some series.22 Composite grafts that include the liver tend to have a protective effect on acute intestinal rejection48 and this may depend upon establishment of physiological perfusion preserving both arterial and portal venous blood flow. Prompt histological diagnosis60,61 and treatment is usually effective to prevent severe rejection and graft loss but can be problematic when rejection is patchy.62 Episodes are usually steroid responsive and can also be effectively treated with antilymphocyte antibodies such as OKT3.48 A positive lymphocyte crossmatch is associated with a higher frequency of rejection whereas the degree of HLA mismatch is not. Most episodes of rejection occur within the first three months but can occur more than a year after transplantation.63
Graft versus host disease
The large quantity of lymphoid tissue present in the small intestine gave rise to early concerns that graft versus host disease would be a common problem. However, in practice, the incidence is far lower than expected, reportedly affecting between 5% and 16% of patients. The condition often manifests as a skin rash and is treated by increasing immunosuppression. However, occasionally it can be severe and may progress to multiorgan failure.32,48
Chronic graft rejection
With the gradual reduction of acute rejection and better short term survival, chronic rejection has become a more significant problem and the main limitation to long term survival after the first year. It affects approximately 8% of grafts62 and occurs insidiously, which often results in late diagnosis. The pathological changes of chronic rejection are seen in the deep layers of the gut wall and the gut vasculature, with relative mucosal sparing. Superficial biopsies obtained during endoscopic routine surveillance can miss these changes and full thickness biopsies are often required which further hampers the diagnosis. The clinical picture of chronic watery diarrhoea, weight loss, and chronic abdominal pain is the result of the underlying obliterative endarterectomy with neointimal hyperplasia that is the pathological hall mark of the condition.61
Postoperative infection
Clinical signs of impending sepsis in the postoperative period are often subtle but can proceed extremely rapidly, commonly making this the most challenging part of the procedure. Acute rejection can first manifest itself as infection caused by disruption of the mucosal barrier and bacterial translocation, leading to a complex clinical picture. Infection is now the most frequent cause of death32 in contrast with the pre-tacrolimus era when rejection was predominant. Almost all patients experience at least one infection following transplantation.64 Serious infections are most usually bacterial and commonly affect central venous lines, the lower respiratory tract, and the urinary system. Intra-abdominal collections can also occur often as a consequence of intestinal bacterial translocation65 or peritoneal contamination during surgery.64
Cytomegalovirus (CMV) infection affects up to one third of patients and is higher when grafts are CMV positive. Strategies to encourage tolerance and allow lower levels of immunosuppression will be important to reduce the incidence of CMV disease which is quite resistant to ganciclovir prophylaxis.66
POTENTIAL DEMAND
The British artificial nutrition survey reported that the point prevalence of adults on HPN in 2002 was 465, which is part of an increasing trend from 206 in 1996, 306 in1998, and 400 in 2000.30 With greater awareness of the life saving potential of PN and a more interventional approach to conditions such as small intestinal infarction, this value is expected to continue to increase. The annual mortality statistics for England and Wales indicate that the death rate for small intestine catastrophe is 15, 95, and 800 and volvulus 12, 33, and 350 per 100 000 for ages up to 45, 60, and over 60 years, respectively.67 Should more of these patients become established on PN the requirement for small intestinal transplantation would consequently increase. Additionally, further improvements in long term survival may soon see transplantation challenge PN as the treatment of choice for those patients who find that PN adversely affects their quality of life.
THE FUTURE
As patient survival approaches that of PN the improved quality of life will make small intestinal transplantation an increasingly attractive prospect and eventually an alternative to PN. Certain centres are now offering carefully selected patients transplantation as an alternative to PN on this basis.58 The ability to reliably achieve immunological tolerance is likely to be the key to improved long term survival.
Summary.
Small intestinal transplantation should be considered routinely as an option for patients with irreversible small intestinal failure who have complications of parenteral nutrition.
Patient and graft survival rates are now comparable with those of lung, and approaching those of liver and heart transplantation.
Quality of life of patients following small intestinal transplantation is improved and similar to that of patients on stable uncomplicated home parenteral nutrition.
The most recent results using more targeted immunosuppression with lymphocyte depleting antibodies and less potent maintenance immunosuppression indicate a further improvement in one year survival, reaching 90% in some centres.
Small intestinal transplantation may become an alternative to parenteral nutrition for patients with irreversible small intestinal failure if the current trend of improvement in survival values continues.
Patients with complications of parenteral nutrition should be referred to an intestinal failure or transplantation centre at an appropriate stage, as indicated in this article, before their deterioration substantially increases their surgical risk.
Strategies to increase residual intestinal function with the use of growth factors such as glucagon-like peptide-274,75 or engineer the growth of additional “intestinal” tissue with the capacity to digest and absorb nutrients76,77 are being developed and may provide alternatives to transplantation in the future.
Progress with the development of small intestine transplantation in the UK has been impaired by the apparently low demand for the procedure. It is however probable that the actual requirement is much greater.
Small intestinal transplantation is now established as the favoured treatment option for patients with significant complications of PN. The timing of transplantation is critical and patients should be referred for assessment before debilitation and inadequate venous access substantially increases the associated risks.
Conflict of interest: None declared.
REFERENCES
- 1.Carrel A. The transplantation of organs. A preliminary communication. JAMA 1905;45:1645. [PMC free article] [PubMed] [Google Scholar]
- 2.Okumura M, Mester M. The coming of age of small bowel transplantation: a historical perspective. Transplant Proc 1992;24:1241–2. [PubMed] [Google Scholar]
- 3.Calne RY, Rolles K, White DJG, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978;2:1323–7. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, White DJG, Thiru S, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaver organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979;ii:1033–6. [DOI] [PubMed] [Google Scholar]
- 5.Deterling R. Discussion. In: Alican F, Hardy JD, Cayrili M, et al, eds. Intestinal transplantation: laboratory experience and report of a clinical case, Am Surg 1971;121:150. [DOI] [PubMed] [Google Scholar]
- 6.Lilehei RC, Idezuki Y, Feemster JA, et al. Transplantation of stomach, intestine and pancreas: experimental and clinical observations. Surgery 1967;62:721. [PubMed] [Google Scholar]
- 7.Okumura M, Fujimura I, Ferrari A, et al. Transplante de intestine Delgado. Apresentacao de um caso. Rev Hosp Clin Fac Med S Paulo 1969;24:39. [PubMed] [Google Scholar]
- 8.Grant D, Wall W, Mineault R, et al. Successful small bowel/liver transplantation. Lancet 1990;335:181. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA 1989;261:1449. [PMC free article] [PubMed] [Google Scholar]
- 10.Williams J, Sankary H, Foster P, et al. Splanchnic transplantation: an approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA 1989;261:1458. [DOI] [PubMed] [Google Scholar]
- 11.Goulet O, Sarnacki S. Isolated and combined liver-small bowel transplantation in Paris 1987–1995. Transplant Proc 1996;28:2750. [PubMed] [Google Scholar]
- 12.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg 1995;222:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Murase N, Demetris A, et al. Lessons of organ-induced tolerance learned from historical clinical experience. Transplantation 2004;77:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecka JM, Terasaki PI. World transplant records. Clinical Transplants 2001. Los Angeles: Los Angeles UCLA Immunogenetics Centre, 2002:279.
- 15.McGeown M, Douglas JF, Donaldson RA, et al. Ten year results of renal transplantation with azathioprine and prednisolone as only immunosuppression. Lancet 1998;1:983–5. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Demetris AJ, Mirase N, et al. Cell migration, chimerism and graft acceptance. Lancet 1992;339:1579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet 2003;361:1502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation 1995;60:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis for graft acceptance. Hepatology 1993;17:1127–52. [PMC free article] [PubMed] [Google Scholar]
- 20.Cortesini R, Suciu-Foca N. The concept of “partial” clinical tolerance. Transplant Immunol 2004;13:101–4. [DOI] [PubMed] [Google Scholar]
- 21.Calne RY, Sells Ra, Pena JR. et al Induction of immunological tolerance by porcine liver allografts. Nature 1969;223:472–4. [DOI] [PubMed] [Google Scholar]
- 22.Garcia D, Weppler D, Mittal S, et al. Campath-1H immunosuppressive therapy reduces incidence and intensity of acute rejection in intestinal and multivisceral transplantation. Transplant Proc 2004;36:323–4. [DOI] [PubMed] [Google Scholar]
- 23.Fishbein TM, Florman S, Gondolesi G, et al. Intestinal transplantation before and after the introduction of sirolimus. Transplantation 2002;73:1538–42. [DOI] [PubMed] [Google Scholar]
- 24.Messing B, Crenn P, Beau P. et al Long term survival and parenteral nutrition dependence in adult patients with short bowel syndrome. Gastroenterology 1999;117:1043–50. [DOI] [PubMed] [Google Scholar]
- 25.Jeppsen PB, Staun M, Mortensen PB. Adult patients receiving home parenteral nutrition in Denmark from 1991–1996: who will benefit form intestinal transplantation? Scand J Gastroenterl 1998;338:839–46. [DOI] [PubMed] [Google Scholar]
- 26.Bozzetti F, Mariani L, Boggio Bertinet D. Central venous catheter complications in 447 patients on home parenteral nutrition: an analysis of over 100 000 catheter days. Clin Nutr 2002;21:475–85. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AL, Scolapio J, Fryer J. AGA Technical review on Short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111–34. [DOI] [PubMed] [Google Scholar]
- 28.Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end stage liver disease in patients receiving home total parenteral nutrition. Surgery 1999;126:28–34. [DOI] [PubMed] [Google Scholar]
- 29.Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 2000;132:525–32. [DOI] [PubMed] [Google Scholar]
- 30.Glencorse C, Meadows N, Holden C, et al. Trends in artificial nutritional support in the UK Between 1996 and 2002. Report by the British Artificial Nutrition Survey (BANS). Secure Hold Business Centre, Redditch, Worcestershire: British Association for Parenteral and Enteral Nutrition (BAPEN), http://www.bapen.org.uk (accessed 22 August 2005).
- 31.Middleton SJ. Pollard S, Friend PJ, et al. Adult small intestinal transplantation in England and Wales. B J Surg 2003;90:723–727. [DOI] [PubMed] [Google Scholar]
- 32.Intestinal Transplant Registry, 1985–2003. http://www.intestinaltransplant.org/ (accessed 22 August 2005).
- 33.Harper AM, McBride MA, Ellison MD. The UNOS OPTN waiting list, 1988–1998. Clin Transpl 1999;9:71–82. [PubMed] [Google Scholar]
- 34.Fryer J, Pellar S, Ormond D, et al. Mortality in candidates waiting for combined liver intestine transplants exceeds that for other candidates waiting for liver transplants. Liver Transpl 2003;9:748–53. [DOI] [PubMed] [Google Scholar]
- 35.The US Scientific Registry of Transplant Recipients and The Organ Procurement and Transplantation Network. Transplant data 1990–1999. 2000 Annual Report. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation and Richmond, VA, United Network of Organ Sharing.
- 36.Bueno J, Ohwada S, Kocoshis S, et al. Factors impacting the survival of children with intestinal failure referred for intestinal transplantation. J Pediatric Surg 1999;34:27–32. [DOI] [PubMed] [Google Scholar]
- 37.American Gastroenterological Association. American Gastroenterological Association medical position statement: short bowel syndrome and intestinal transplantation. Gastroenterology 2004;124:1105–10. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant 2001;5:80–7. [DOI] [PubMed] [Google Scholar]
- 39.Scolapio SJ, Fleming CR, Kelly DG, et al. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo Clinic. Mayo Clinic Proc 1999;74:217–22. [DOI] [PubMed] [Google Scholar]
- 40.Kartzas T, Khan F, Tzakis AG. Clinical intestinal transplantation: Experience in Miami. Transplant Proc 1997;29:1787–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappas PA, Weppler D, Pinna AD, et al. Sirolimus in pediatric gastrointestinal transplantation: the use of sirolimus for pediatric transplant patients with tacrolimus related cardiomyopathy. Pediatr Transplant 2000;4:45–9. [DOI] [PubMed] [Google Scholar]
- 42.Tzakis AG, Kato T, Nishida S, et al. Alematuzumab (Campath-1H) combined with tacrolimus in intestinal and multivisceral transplantation. Transplantation 2003;75:1512–17. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Elmagd K, Fung J, McGhee W, et al. The efficacy of daclizumab for intestinal transplantation: preliminary report. Transplant Proc 2000;32:1195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farmer DG, McDiarmid SV, Yersiz H, et al. Outcomes after intestinal transplantation: a single-center experience over a decade. Transplant Proc 2002;34:896–7. [DOI] [PubMed] [Google Scholar]
- 45.Goulet O, Lacaille F, Colomb V, et al. Intestinal transplantation in children: Paris experience. Transplant Proc 2002;34:1887–8. [DOI] [PubMed] [Google Scholar]
- 46.Carreno MR, Kato T, Weppler D, et al. Induction therapy with daclizumab as part of the immunosuppressive regimen in human small bowel and multi organ transplants. Transplant Proc 2001;33:1015–17. [DOI] [PubMed] [Google Scholar]
- 47.Tzakis AG, Tryphonopoulos P, Kato T, et al. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation 2004;78:489. [DOI] [PubMed] [Google Scholar]
- 48.Abu-Elmagd K, Reyes J, Todo S, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg 1998;186:512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murase N, Ye Q, Lee RG. et al. Immunomodulation of intestinal transplant with allograft irradiation and simultaneous donor bone marrow infusion. Transplant Proc 1999;31:565–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SM, Roberts PJ, Watson CJ, et al. Relapsing encephalopathy following small bowel transplantation. Transplant Proc 2003;35:1565–6. [DOI] [PubMed] [Google Scholar]
- 51.Calne RY, Friend PJ, Middleton S, et al. Intestinal transplant between two identical triplets. Lancet 1997;350:1077–8. [DOI] [PubMed] [Google Scholar]
- 52.Sudan DL, Kaufman SS, Shaw BW Jr, et al. Isolated intestinal transplantation for intestinal failure. Am J Gastroenterol 2000;95:1506–15. [DOI] [PubMed] [Google Scholar]
- 53.Pinna AD, Webbler D, Nevy J, et al. Intestinal transplanation at the University of Miami: five years of experience. Transplant Proc 2000;32:1226–7. [DOI] [PubMed] [Google Scholar]
- 54.Goulet O, Jan D, Lacaille F, et al. Intestinal transplantation in children: preliminary experience in Paris. JPEN J Parenter Enteral Nutr 1999;23:S121–5. [DOI] [PubMed] [Google Scholar]
- 55.DiMartini A, Giuseppe RM, Toby G, et al. Quality of life after small intestinal transplantation and amoung home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 1998;22:357–62. [DOI] [PubMed] [Google Scholar]
- 56.Cameron EAB, Binnie JAH, Jamieson NV, et al. Quality of life an adults following small bowel transplantation. Tranaplant Proc 2002;34:965–6. [DOI] [PubMed] [Google Scholar]
- 57.Guiseppe RM, DiMartini A, Schoen RE, et al. Quality of life of patients after intestinal transplantation. Transplantation 1998;66:1141–5. [DOI] [PubMed] [Google Scholar]
- 58.Abu-Elmagd K, Reyes J, Fung JJ, et al. Evolution of clinical intestinal transplantation: improved outcome and cost effectiveness. Transplant Proc 1999;31:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schalamon J, Mayr JM, Hollwarth ME, et al. Mortality and economics in short bowel syndrome. 2003;17:931–42. [DOI] [PubMed]
- 60.Bryant J. Observations upon the growth and length of the human intestine. Am J Med Sci 1924;167:499–520. [Google Scholar]
- 61.Lee RG, Nakamura K, Tsamanda AC, et al. Pathology of human intestinal transplantation. Gastroenterology 1996;110:1820–34. [DOI] [PubMed] [Google Scholar]
- 62.Sigurdsson L, Reyes J, Todos. et al Anatomic variability of rejection in intestinal allografts after pediatric intestinal transplantation. J Pediatr Gastroenterol Nutr 1998;27:403–6. [DOI] [PubMed] [Google Scholar]
- 63.Sudan DL, Kaufman SS, Shaw BW Jr, et al. : Isolated intestinal transplantation for intestinal failure.. Am J Gastroenterol 2000;95:1606–15. [DOI] [PubMed] [Google Scholar]
- 64.Kusne S, Furukawa H, Abu EK. et al : Infectious complications after small bowel transplantation in adults: an update. Transplant Proc 1996;28:2761–2. [PMC free article] [PubMed] [Google Scholar]
- 65.Cicalese L, Sileri P, Green M, et al. Bacterial translocation in clinical intestinal transplantation. Transplant Proc 2000;32:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajendra Prassad K, Pollard SG. Small bowel transplantation. Current Opin Gastroenterol 2000;16:126–33. [DOI] [PubMed] [Google Scholar]
- 67.Office for National Statistics England and Wales. London: Office for National Statistics England and Wales, 2000, series DH2 No. 26: 134–9, ISBN 011 621383 3. ISSN 0265–9670. http://www.statistics.gov.uk (accessed 22 August 2005).
- 68.Morris PJ, Johnson RJ, Fuggle SV, et al. Analysis of factors that affect outcome of primary cadaveric renal transplatation in the UK. HLA Task Force of the Kidney Advisory Group of the United Kingdom Transplant Support Service Authority (UKTSSA). Lancet 1999;354:1147–52. [DOI] [PubMed] [Google Scholar]
- 69.Jacob M, Copley LP, Lewsey JD, et al. UK and Ireland liver transplant audit. London: Annual Report to the National Specialist Commissioning Advisory Group (NSCAG), 2004.
- 70.Taylor DO, Edwards LB, Boucek MM, et al. The registry for the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report—2004. J Heart Lung Transplant 2004;23:796–803. [DOI] [PubMed] [Google Scholar]
- 71.Trulock EP, Edwards LB Taylor DO, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report. J Heart Lung Transplant 2004;23:804–15. [DOI] [PubMed] [Google Scholar]
- 72.Howard L, Malone M. Current status of home parenteral nutrition in the United States. Transplant Proc 1996;28:2691. [PubMed] [Google Scholar]
- 73.Chan WK, Friend PJ, Jamieson NV, et al. Multivisceral grafting for Gardener’s syndrome. J Wound Care 1995;4:214–16. [PubMed] [Google Scholar]
- 74.Jeppesen PB, Mortensen PB. Enhancing bowel adaptation in short bowel syndrome. Curr Gastroenterol Rep 2002;4:338–47. [DOI] [PubMed] [Google Scholar]
- 75.Jeppesen PB. Clinical significance of GLP-2 in short-bowel syndrome. J Nutr 2003;133:3721–4. [DOI] [PubMed] [Google Scholar]
- 76.Grikscheit TC, Siddique A, Ochoa ER, et al. Tissue engineered small intestine improves recovery after massive small bowel resection. Ann Surg 2004;240:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Day RM, Boccaccini AR, Shurey S, et al. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004;25:5857–66. [DOI] [PubMed] [Google Scholar]