Abstract
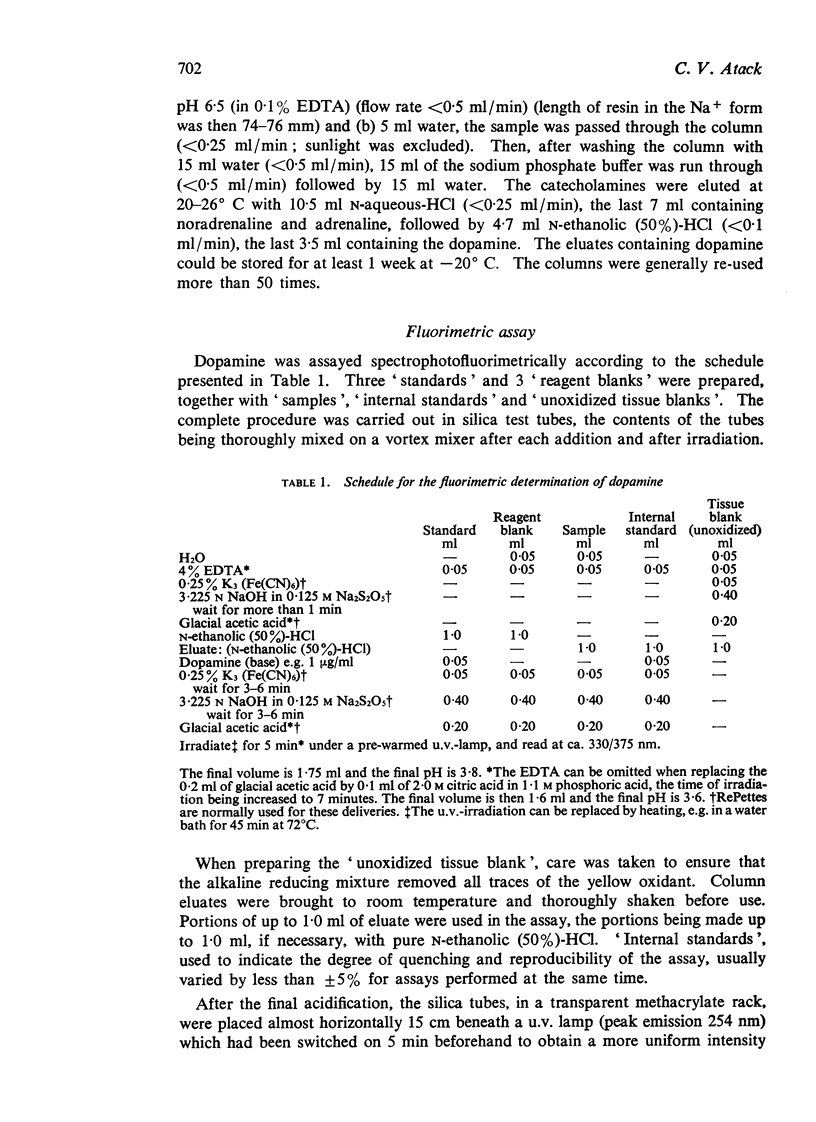
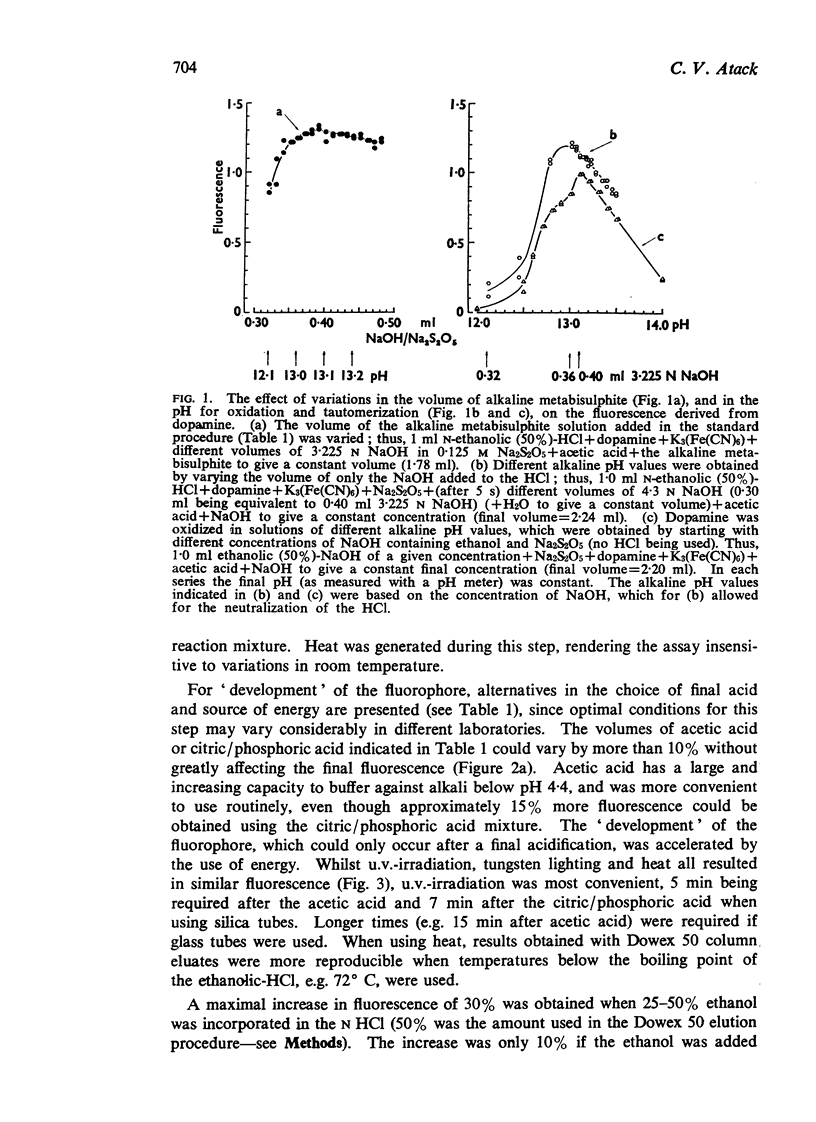
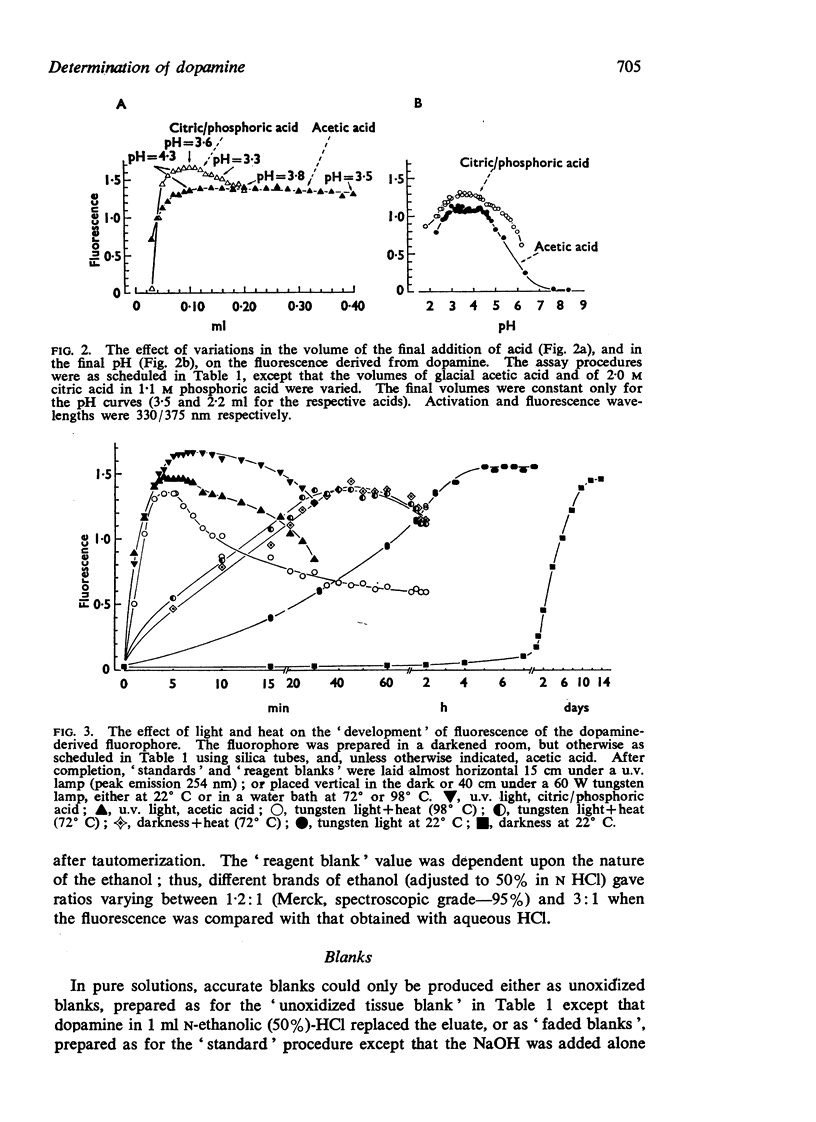
1. A simplified sensitive fluorimetric assay for dopamine based on the hydroxyindole principle is described. Oxidation of dopamine by ferricyanide and subsequent tautomerization both occur in the same, strongly alkaline, ethanolic solution in the presence of metabisulphite. The reaction is self-regulating, and since times of additions of reagents are relatively unimportant and the final fluorescence is very stable, a large number of samples can be assayed together.
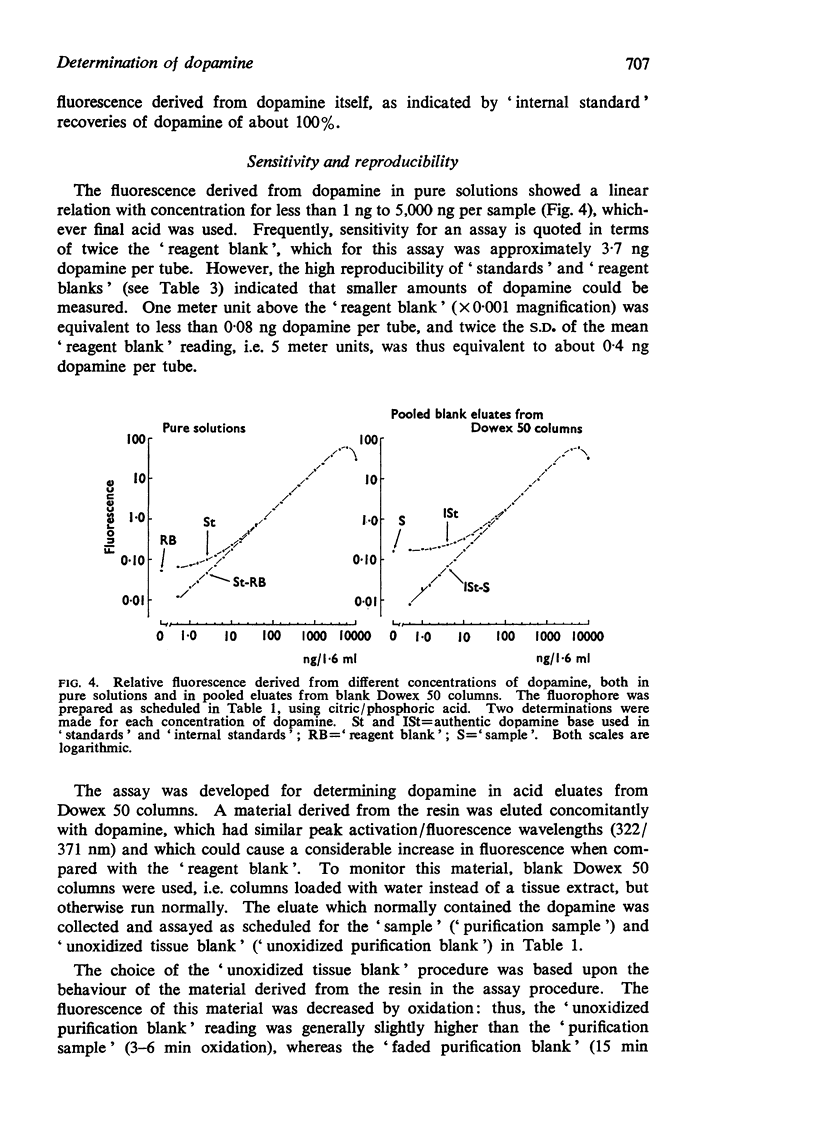
2. The method was developed for assaying dopamine in N-ethanolic (50%)-HCl eluates, after purification on a strongly acidic cation exchange column as previously described (Atack & Magnusson, 1970). Portions, up to 1 ml volume, of the 3·5 ml eluate can be taken. No preliminary, time-consuming, neutralization step is required before oxidation. Fluorescence intensity shows a linear relation with concentration for 1-5,000 ng of dopamine per 1·6 ml of final solution, both in pure solutions and in column eluates.
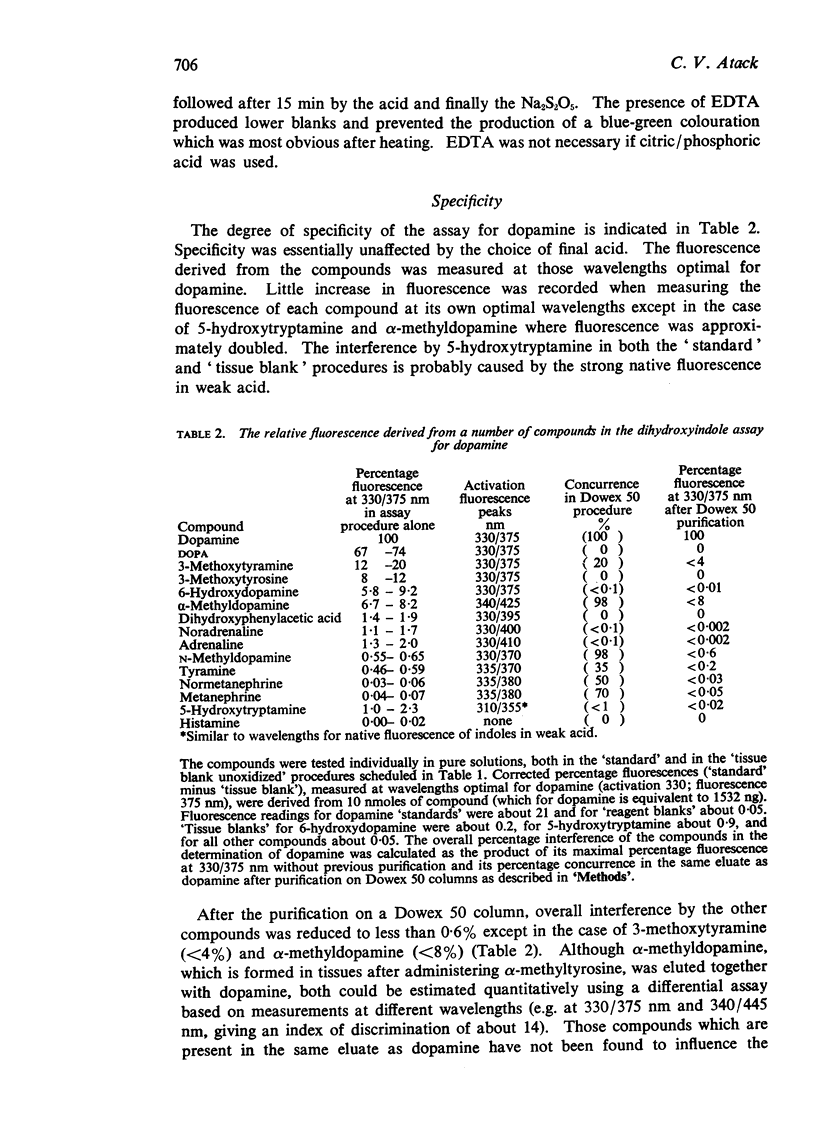
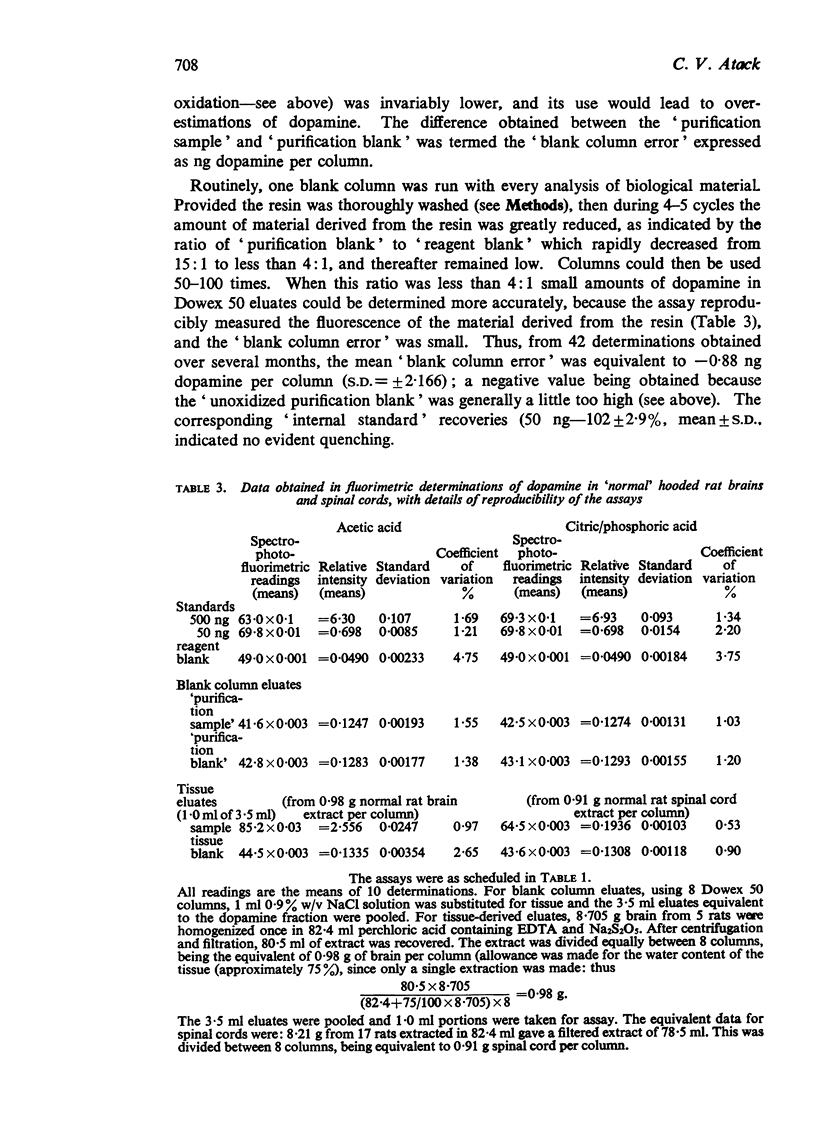
3. Fluorescence readings from biological material are given, with evidence for their reproducibility. These, together with other data, demonstrate the relative accuracy of the `tissue blank' and indicate that amounts of dopamine greater than 3 ng per column can confidently be detected, and amounts greater than 10 ng can be measured quantitatively.
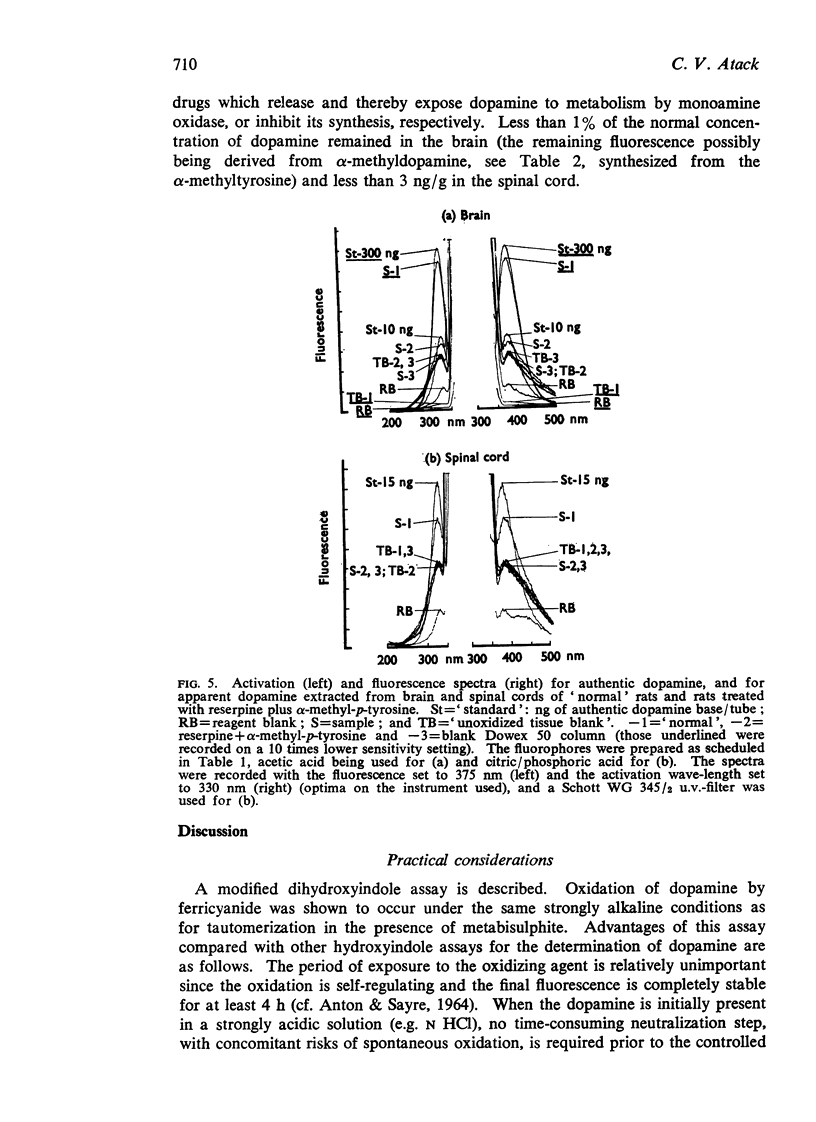
4. Fluorescence spectra are presented for the fluorophore derived from authentic dopamine, and from suspected dopamine extracted from spinal cord of normal rats and rats treated with reserpine plus an inhibitor of synthesis. Values for the concentration of dopamine of 20 and <3 ng/g, respectively, were obtained.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. THE DISTRIBUTION OF DOPAMINE AND DOPA IN VARIOUS ANIMALS AND A METHOD FOR THEIR DETERMINATION IN DIVERSE BIOLOGICAL MATERIAL. J Pharmacol Exp Ther. 1964 Sep;145:326–336. [PubMed] [Google Scholar]
- Atack C. V., Ericson L. E., Melander A. Intracellular distribution of amines and calcitonin in the sheep thyroid gland. J Ultrastruct Res. 1972 Dec;41(5):484–498. doi: 10.1016/s0022-5320(72)90051-2. [DOI] [PubMed] [Google Scholar]
- Atack C. V., Magnusson T. Individual elution of noradrenaline (together with adrenaline), dopamine, 5-hydroxytryptamine and histamine from a single, strong cation exchange column, by means of mineral acid-organic solvent mixtures. J Pharm Pharmacol. 1970 Aug;22(8):625–627. doi: 10.1111/j.2042-7158.1970.tb10584.x. [DOI] [PubMed] [Google Scholar]
- BERTLER A., CARLSSON A., ROSENGREN E. A method for the fluorimetric determination of adrenaline and noradrenaline in tissues. Acta Physiol Scand. 1958 Dec 15;44(3-4):273–292. doi: 10.1111/j.1748-1716.1958.tb01627.x. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. In-vivo decarboxylation of alpha-methyl DOPA and alpha-methyl metatyrosine. Acta Physiol Scand. 1962 Jan;54:87–94. doi: 10.1111/j.1748-1716.1962.tb02331.x. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M., MAGNUSSON T., WALDECK B. On the presence of 3-hydroxytyramine in brain. Science. 1958 Feb 28;127(3296):471–471. doi: 10.1126/science.127.3296.471. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., WALDECK B. A METHOD FOR THE FLUORIMETRIC DETERMINATION OF 3-METHOXYTYRAMINE IN TISSUES AND THE OCCURRENCE OF THIS AMINE IN BRAIN. Scand J Clin Lab Invest. 1964;16:133–138. doi: 10.1080/00365516409060495. [DOI] [PubMed] [Google Scholar]
- CARLSSON A., WALDECK B. A fluorimetric method for the determination of dopamine (3-hydroxytyramine). Acta Physiol Scand. 1958 Dec 15;44(3-4):293–298. doi: 10.1111/j.1748-1716.1958.tb01628.x. [DOI] [PubMed] [Google Scholar]
- CHANG C. C. A SENSITIVE METHOD FOR SPECTROPHOTOFLUOROMETRIC ASSAY OF CATECHOLAMINES. Int J Neuropharmacol. 1964 Dec;3:643–649. doi: 10.1016/0028-3908(64)90089-9. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Davis J. N., Kehr W., Lindqvist M., Atack C. V. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(2):153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- DRUJAN B. D., SOURKES T. L., LAYNE D. S., MURPHY G. F. The differential determination of catecholamines in urine. Can J Biochem Physiol. 1959 Oct;37:1153–1159. [PubMed] [Google Scholar]
- Dulière W. L., Raper H. S. The tyrosinase-tyrosine reaction: The action of tyrosinase on certain substances related to tyrosine. Biochem J. 1930;24(2):239–249. doi: 10.1042/bj0240239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg H. C., Sharman D. F., Tegerdine P. R. Some observations on the estimation of 3-methoxytyramine in brain tissue. Br J Pharmacol. 1971 Aug;42(4):505–511. doi: 10.1111/j.1476-5381.1971.tb07136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAEGGENDAL J. AN IMPROVED METHOD FOR FLUORIMETRIC DETERMINATION OF SMALL AMOUNTS OF ADRENALINE AND NORADRENALINE IN PLASMA AND TISSUES. Acta Physiol Scand. 1963 Nov;59:242–254. doi: 10.1111/j.1748-1716.1963.tb02739.x. [DOI] [PubMed] [Google Scholar]
- HAGGENDAL J. On the use of strong exchange resins for determinations of small amounts of catechol amines. Scand J Clin Lab Invest. 1962;14:537–544. doi: 10.3109/00365516209051276. [DOI] [PubMed] [Google Scholar]
- Harrison W. H., Whisler W. W., Hill B. J. Catecholamine oxidation and ionization properties indicated from the H+ release, tritium exchange, and spectral changes which occur during ferricyanide oxidation. Biochemistry. 1968 Sep;7(9):3089–3094. doi: 10.1021/bi00849a010. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Karoum F., Cattabeni F., Costa E., Ruthven C. R., Sandler M. Gas chromatographic assay of picomole concentrations of biogenic amines. Anal Biochem. 1972 Jun;47(2):550–561. doi: 10.1016/0003-2697(72)90149-2. [DOI] [PubMed] [Google Scholar]
- Kehr W., Carlsson A., Lindqvist M. A method for the determination of 3,4-dihydroxyphenylalanine (DOPA) in brain. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(3):273–280. doi: 10.1007/BF00501936. [DOI] [PubMed] [Google Scholar]
- Koslow S. H., Cattabeni F., Costa E. Norepinephrine and dopamine: assay by mass fragmentography in the picomole range. Science. 1972 Apr 14;176(4031):177–180. doi: 10.1126/science.176.4031.177. [DOI] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. THE ESTIMATION OF SMALL QUANTITIES OF 3,4-DIHYDROXYPHENYLETHYLAMINE IN TISSUES. Br J Pharmacol Chemother. 1965 Apr;24:538–548. doi: 10.1111/j.1476-5381.1965.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty R., Taylor K. M. The fluorometric assay of catecholamines and related compounds: improvements and extensions to the hydroxyindole technique. Anal Biochem. 1968 Feb;22(2):269–279. doi: 10.1016/0003-2697(68)90316-3. [DOI] [PubMed] [Google Scholar]
- Lindqvist M. Quantitative estimation of 5-hydroxy-3-indole acetic acid and 5-hydroxytryptophan in the brain following isolation by means of a strong cation exchange column. Acta Pharmacol Toxicol (Copenh) 1971;29(4):303–313. doi: 10.1111/j.1600-0773.1971.tb00592.x. [DOI] [PubMed] [Google Scholar]
- MONTAGU K. A. Catechol compounds in rat tissues and in brains of different animals. Nature. 1957 Aug 3;180(4579):244–245. doi: 10.1038/180244a0. [DOI] [PubMed] [Google Scholar]
- Snider S. R., Carlsson A. The adrenal dopamine as an indicator of adrenomedullary hormone biosynthesis. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(4):347–357. doi: 10.1007/BF00501124. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- VON EULER U. S. The development and applications of the trihydroxyindole method for catecholamines. Pharmacol Rev. 1959 Jun;11(2 Pt 2):262–268. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. The chemical estimation of adrenaline-like substances in blood. Biochem J. 1952 Jun;51(3):311–318. doi: 10.1042/bj0510311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H. The estimation of total (free + conjugated) catecholamines and some catecholamine metabolites in human urine. Methods Biochem Anal. 1968;16:293–326. doi: 10.1002/9780470110348.ch6. [DOI] [PubMed] [Google Scholar]
- Welch A. S., Welch B. L. Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin, and 5-hydroxyindoleacetic acid in a single mouse brain. Anal Biochem. 1969 Aug;30(2):161–179. doi: 10.1016/0003-2697(69)90387-x. [DOI] [PubMed] [Google Scholar]


