Abstract
1. The effects of depletion of endogenous noradrenaline by reserpine-pretreatment on [3H]-noradrenaline overflow elicited by nerve stimulation were determined in the isolated nerve-muscle preparation of the cat's nictitating membrane.
2. Reserpine pretreatment (0·3 mg/kg, s.c., 4 days prior to the experiment) reduced the noradrenaline levels in the smooth muscle of the nictitating membrane to about 10% of the control values while granular retention of [3H]-noradrenaline had recovered to nearly 40% of the controls.
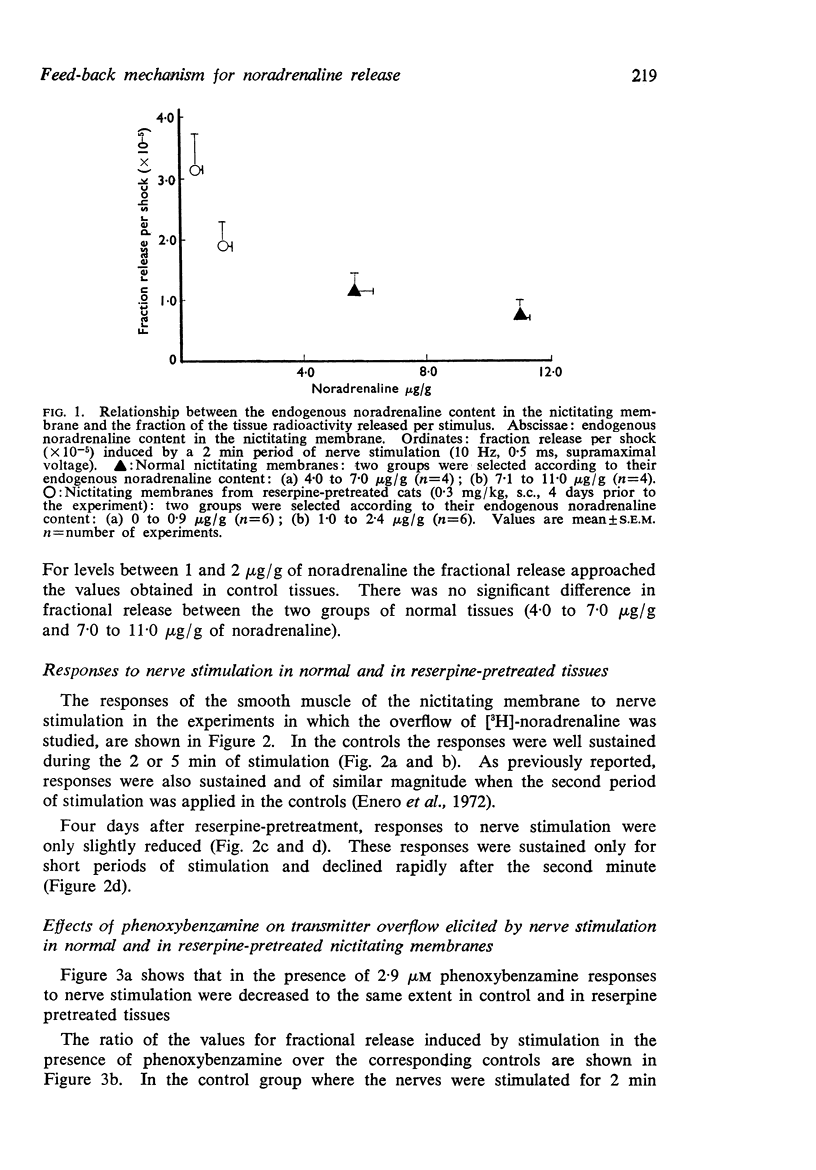
3. In the reserpine-pretreated tissue the fraction release per shock induced by nerve stimulation was 2·2-fold higher than the value obtained in the untreated tissues. This effect was correlated with the degree of depletion of the noradrenaline stores rather than with the decrease in the response of the effector organ.
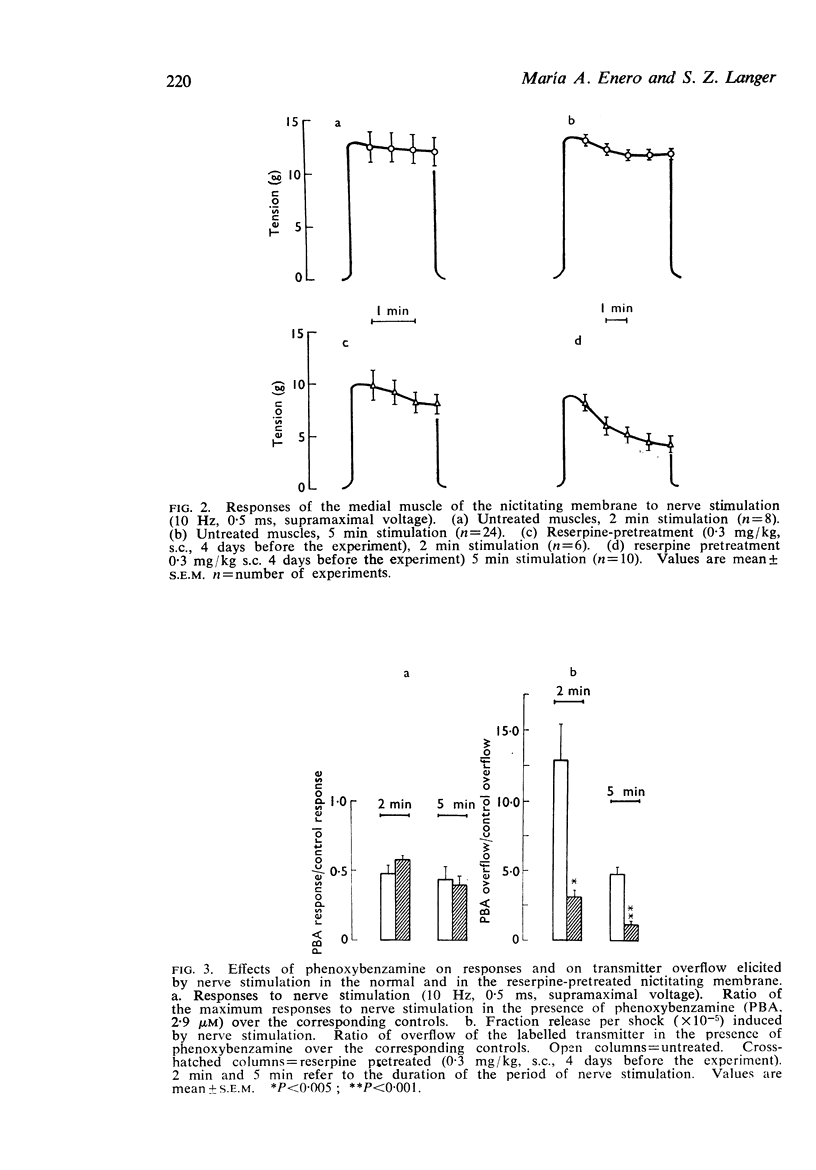
4. Phenoxybenzamine, 2·9 μM reduced the responses to nerve stimulation to the same extent in control and in reserpine-pretreated tissues. Yet, this concentration of phenoxybenzamine increased by 13-fold the overflow of the labelled transmitter in the controls and only by 3-fold in reserpine-pretreated tissues.
5. The decrease in effectiveness of phenoxybenzamine in enhancing transmitter overflow after reserpine-pretreatment appears to be due to the decrease in the total release of the transmitter.
6. The results obtained support the view that in reserpine-pretreated tissues decreased transmitter output reduces the activation of the presynaptic α-adrenoceptors which mediate the negative feed-back mechanism that regulates transmitter release by nerve stimulation.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Graschinsky E., Langer S. Z., Rubio M. C. Metabolism of norepinephrine released by phenoxybenzamine in isolated guinea-pig atria. J Pharmacol Exp Ther. 1972 Feb;180(2):286–301. [PubMed] [Google Scholar]
- Andén N. E., Henning M. Adrenergic nerve function, noradrenaline level and noradrenaline uptake in cat nictitating membrane after reserpine treatment. Acta Physiol Scand. 1966 Jul-Aug;67(3):498–504. doi: 10.1111/j.1748-1716.1966.tb03335.x. [DOI] [PubMed] [Google Scholar]
- BLAKELEY A. G., BROWN G. L., FERRY C. B. Pharmacological experiments on the release of the sympathetic transmitter. J Physiol. 1963 Jul;167:505–514. doi: 10.1113/jphysiol.1963.sp007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z., Rothlin R. P., Stefano F. J. Role of the -adrenoceptor in regulating noradrenaline overflow by nerve stimulation. Br J Pharmacol. 1972 Apr;44(4):672–688. doi: 10.1111/j.1476-5381.1972.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O. Effect of reserpine on release of ( 3 H)noradrenaline, ( 3 H)dopamine and ( 3 H)metaraminol from field stimulated rat iris. Biochem Pharmacol. 1971 Oct;20(10):2715–2726. doi: 10.1016/0006-2952(71)90181-x. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of 3 H-monoamines from field stimulated rat brain slices. Acta Physiol Scand Suppl. 1971;371:35–44. doi: 10.1111/j.1748-1716.1971.tb05213.x. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B., Jonsson G. Release of ( 3 H)noradrenaline and ( 3 H)dopamine from field stimulated cerebral cortex slices. Effect of tyrosine hydroxylase and dopamine- -hydroxylase inhibition. J Neurochem. 1971 Dec;18(12):2491–2500. doi: 10.1111/j.1471-4159.1971.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Malamfors T. 3 H-noradrenaline release and mechanical response in the field stimulated mouse vas deferens. Acta Physiol Scand Suppl. 1971;371:1–18. doi: 10.1111/j.1748-1716.1971.tb05210.x. [DOI] [PubMed] [Google Scholar]
- Ferry C. B. The autonomic nervous system. Annu Rev Pharmacol. 1967;7:185–202. doi: 10.1146/annurev.pa.07.040167.001153. [DOI] [PubMed] [Google Scholar]
- Graffe K. H., Stefano F. J., Langer S. Z. Preferential metabolism of (-) 3 H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol. 1973 May 15;22(10):1147–1160. doi: 10.1016/0006-2952(73)90231-1. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Langer S. Z. Effects of phenoxybenzamine on the uptake and metabolism of noradrenaline in the rat heart and vas deferens. Br J Pharmacol. 1969 Nov;37(3):627–637. doi: 10.1111/j.1476-5381.1969.tb08501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Puig M. Effect of flow-stop on noradrenaline release from normal spleens and spleens treated with cocaine, phentolamine or phenoxybenzamine. Br J Pharmacol. 1971 Oct;43(2):359–369. [PMC free article] [PubMed] [Google Scholar]
- LINDMAR R., MUSCHOLL E. DIE WIRKUNG VON PHARMAKA AUF DIE ELIMINATION VON NORADRENALIN AUS DER PERFUSIONSFLUESSIGKEIT UND DIE NORADRENALINEUFNAHME IN DAS ISOLIERTE HERZ. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 Jul 28;247:469–492. doi: 10.1007/BF00329896. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Stefano F. J., Enero M. A. Pre- and postsynaptic origin of the norepinephrine metabolites formed during transmitter release elicited by nerve stimulation. J Pharmacol Exp Ther. 1972 Oct;183(1):90–102. [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Vogt M. Noradrenaline release from isolated muscles of the nictitating membrane of the cat. J Physiol. 1971 Apr;214(1):159–171. doi: 10.1113/jphysiol.1971.sp009425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty R., Taylor K. M. The fluorometric assay of catecholamines and related compounds: improvements and extensions to the hydroxyindole technique. Anal Biochem. 1968 Feb;22(2):269–279. doi: 10.1016/0003-2697(68)90316-3. [DOI] [PubMed] [Google Scholar]
- Starke K. Influence of extracellular noradrenaline on the stimulation-evoked secretion of noradrenaline from sympathetic nerves: evidence for an -receptor-mediated feed-back inhibition of noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):11–23. doi: 10.1007/BF00505064. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H., Schümann H. J. Influence of cocaine and phenoxybenzamine on noradrenaline uptake and release. Naunyn Schmiedebergs Arch Pharmakol. 1971;270(2):210–214. doi: 10.1007/BF00997091. [DOI] [PubMed] [Google Scholar]
- THOMPSON J. W. Studies on the responses of the isolated nictitating membrane of the cat. J Physiol. 1958 Apr 3;141(1):46–72. doi: 10.1113/jphysiol.1958.sp005954. [DOI] [PMC free article] [PubMed] [Google Scholar]


