Abstract
Germ-line mutations of LKB1 (STK11) lead to Peutz–Jeghers syndrome characterized by gastrointestinal polyps and cancer of different organ systems. The mutations lead to loss or severe impairment of Lkb1 serine/threonine kinase activity. Therefore LKB1 has been implicated as a tumor suppressor gene, but only a few mutations in the coding exons of LKB1 have been detected in sporadic tumors. Here, we have identified tumor cell lines with severely reduced mRNA levels and impaired Lkb1 kinase activity. Reintroducing Lkb1 into these cells suppressed cell growth. The Lkb1-mediated growth inhibition was caused by a G1 cell cycle block and was not detected with several naturally occurring Lkb1 mutants. These results indicate that LKB1 has functional and specific growth-suppressing activity.
Peutz–Jeghers syndrome (PJS) is a dominantly inherited disease characterized by predisposition to gastrointestinal polyposis, mucocutaneous melanin pigmentation, and various neoplasms (reviewed in ref. 1). PJS-associated hamartomatous polyps are benign and have a specific branched structure consisting of a smooth muscle and stromal tissue core surrounded by glandular epithelium. Patients with PJS have an elevated risk of malignancy, most commonly affecting the gastrointestinal tract, pancreas, breast, testis, and ovary.
A susceptibility locus for PJS was found on 19p13.3 by linkage analysis, and subsequently, mutations in the LKB1 (STK11) gene in most PJS cases were identified (2–4). Moreover, tumors associated with PJS have acquired somatic mutations in the remaining wild-type allele of LKB1, strongly implicating LKB1 as a tumor susceptibility gene (2, 5).
LKB1 encodes a 60-kDa serine/threonine kinase of unknown function (6, 7). Apparent homologs have been identified from Xenopus (Xeek1; 82% identity; (8) and from mouse (88% identity; GenBank accession no. AF129870). Kinases most related to Lkb1 include the Saccharomyces cerevisiae SNF1 and its apparent mammalian homologue AMPK involved in cellular stress responses (9). LKB1 mRNA is ubiquitously expressed at the highest levels in testis and liver (4, 10). The cellular function and substrates of Lkb1 are yet to be characterized, but Lkb1 has autocatalytic kinase activity that is lost or severely impaired by mutant LKB1 alleles identified in PJS (6, 7).
Although LKB1 is implicated as a tumor suppressor gene in PJS, only a limited number of mutations in the coding region of LKB1 have been identified in sporadic tumors (11–15). Here, we have identified tumor cell lines with severely reduced Lkb1 mRNA expression. Moreover, we provide functional evidence indicating that Lkb1 has growth-suppressing activity when reintroduced into these cells.
MATERIALS AND METHODS
Cell Culture and Transfections.
G361 (melanoma), HeLa S3 (cervical carcinoma), SW480 (colorectal adenocarcinoma), U2OS (osteosarcoma), and NIH 3T3 (fibroblast) cells were grown in DMEM supplemented with 10% (vol/vol) FCS. The cells were transfected by using the calcium phosphate transfection method as described (16). For determining the colony forming ability, the transfected cells were subjected to 2–3 mg/ml G418 (Boehringer Mannheim) selection for 16–20 days. For counting, the colonies were fixed with 5% (vol/vol) trichloroacetic acid and stained with 3% (vol/vol) Giemsa stain.
Lkb1 Expression Vectors.
The following expression vectors were used: pCMVβ-Gal (17), pCMVCD20 (a gift from Ed Harlow, Harvard Medical School, Boston), pCI-Neo (Promega), pCI-Neo containing N-terminal myc or hemagglutinin epitope tags (pAMC and pAHC, respectively), Lkb1/pCI-Neo, Lkb1/pAHC, Lkb1/pAMC, Lkb1G163D/pAMC, Lkb1-SL8/pAHC, and Lkb1-SL26/pAHC as described by Ylikorkala et al. (7).
Immunoprecipitation and Kinase Assays.
The cells were lysed as described by Ylikorkala et al. (7). A similar amount of total proteins of each cell line was incubated with a specific polyclonal antiserum raised against a 15-aa C-terminal peptide of human Lkb1. Control immunoprecipitations were performed with anti-Lkb1 preincubated with the antigenic peptide. The immunoprecipitates were subjected to a kinase assay (7), followed by separation in 10% SDS/PAGE and analysis by PhosphoImager or autoradiography.
Western Blotting.
Total proteins (40 μg) were analyzed by 10% SDS/PAGE and blotted according to standard protocols (16). Monoclonal antihemagglutinin (12CA5, Babco, Richmond, CA) or polyclonal anti-Lkb1 (see above) was used to detect transfected proteins by enhanced chemiluminescence.
Northern Blotting.
CLONTECH Multiple Tissue Northern Blot (7757-1) was hybridized according to standard protocols (16). The Raji (Burkitt’s lymphoma) lane that was present in the Northern filter was excluded from the analysis because of low levels of mRNA.
Immunofluorescence.
Cells were seeded on coverslips and fixed with 3.5% (vol/vol) paraformaldehyde 45 h after transfection. Double immunofluorescence was performed with polyclonal anti-Lkb1 (see above) and monoclonal anti-β-galactosidase (Promega), which were detected with rhodamine-conjugated anti-rabbit and fluorescein-conjugated anti-mouse secondary antibodies (both from Boehringer Mannheim), respectively. The nuclei were visualized with Hoechst 33342 (Sigma; 0.5 μg/ml), and the immunostainings were viewed and documented with a Zeiss Axiophot microscope.
Flow Cytometry Analysis.
G361 cells were cotransfected with pCMVCD20 and indicated plasmids as described above for 42 h. The cells were treated with nocodazole (70 ng/ml, Sigma) for an additional 24 h to induce a G2/M phase block. Subsequently, cells were detached, incubated with anti-CD20-FITC (Becton Dickinson), and fixed with 80% (vol/vol) ethanol. Propidium iodide (30 μg/ml, Sigma) was used to stain the nuclei. The cell cycle distribution of cells testing positive and negative for CD20 was analyzed with a Coulter EPICS flow cytometer. Percentages of cells in G1, S, and G2/M phases were determined with the cellfit cell cycle analysis program (Becton Dickinson).
RESULTS
Down-Regulation of LKB1 mRNA Expression in Human Tumor Cell Lines.
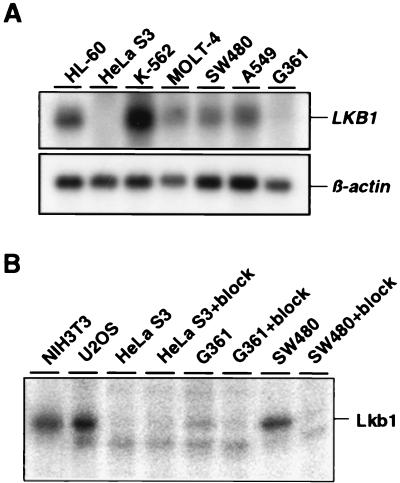
To study LKB1 mRNA expression in human tumors, we performed Northern blotting analyses on a panel of human tumor cell lines originating from various tissues. As described (4), a major band of ≈3 kb was detected in the majority of cell lines (Fig. 1A, LKB1). However, HeLa S3 cells (cervical adenocarcinoma) had undetectable levels, and G361 cells (melanoma) had severely reduced levels of LKB1 mRNA (Fig. 1A). This result is striking, as all analyzed normal adult and embryonic tissues have ubiquitous expression (3, 4, 10), suggesting that mRNA down-regulation could be a mechanism to impair Lkb1 function in human tumors. One possibility for silencing of LKB1 mRNA expression is epigenetic inactivation caused by promoter methylation, and indeed the HeLa S3 cell line has complete methylation of the LKB1 promoter region (L. A. Aaltonen and J. G. Herman, personal communication).
Figure 1.
Lkb1 expression and activity in human tumor cell lines. (A) LKB1 mRNA expression in indicated cell lines (Upper). β-Actin mRNA is shown as a control (Lower). (B) Autocatalytic kinase activity of Lkb1 in indicated cell lines. Immunoprecipitates from cell lysates containing 400 μg of total protein with anti-Lkb1 antiserum or antiserum blocked with the antigenic peptide (+ block) were subjected to an in vitro kinase reaction. Subsequently, samples were analyzed by SDS/PAGE, and radioactivity was detected with a PhosphorImager.
Impaired Lkb1 Kinase Activity in HeLa S3 and G361 Cells.
To assess whether the reduced or undetectable LKB1 mRNA was reflected in Lkb1 function, we analyzed Lkb1 activity in HeLa S3 and G361 cell lines as described (7). For this purpose, Lkb1 was immunoprecipitated with a specific polyclonal antiserum (see Materials and Methods), followed by an in vitro kinase reaction to assess the autocatalytic activity of Lkb1 (Fig. 1B). Lkb1 activity was evident in immunoprecipitates from SW480 colon carcinoma cells, U2OS osteosarcoma cells, and NIH 3T3 fibroblasts used as controls. In contrast, no activity was detected in HeLa S3 cells, and a markedly reduced activity was detected in G361 cells, in accordance to their LKB1 mRNA levels.
Reintroducing LKB1 into G361 Cells Restores Lkb1 Activity.
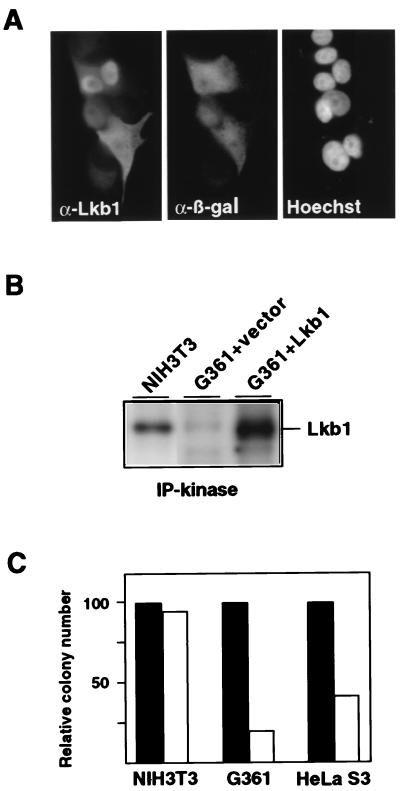
Down-regulation of LKB1 mRNA and Lkb1 activity in HeLa S3 and G361 cells may have provided a growth advantage to these cells. To investigate this possibility, we first examined whether Lkb1 could be expressed in cells with low endogenous LKB1. After a transient transfection of an LKB1 expression vector (Lkb1/pCI-Neo) into G361 cells, Lkb1 protein was readily detected by immunofluorescence by using α-Lkb1 (Fig. 2A), as well as by Western blotting (not shown). Interestingly, the subcellular localization of Lkb1 was predominantly nuclear in most cells, but in a significant fraction (≈30%), Lkb1 was found mainly in the cytoplasm. The apparent Xenopus homolog Xeek1 was reported to be exclusively cytoplasmic in frog oocytes by subcellular fractionation (8), suggesting that Lkb1 and Xeek1 may have distinct functions.
Figure 2.
Ectopic expression of Lkb1 in cells with impaired endogenous Lkb1 activity. (A) Subcellular localization of ectopic Lkb1 in G361 cells. Double immunofluorescence with anti-Lkb1 (Left) and anti-β-galactosidase (Center), indicating transfected cells. Nuclei were visualized by Hoechst staining (Right). (B) Lkb1 kinase activity in untransfected NIH 3T3 cells and G361 cells transfected with a Lkb1 encoding plasmid (G361 + Lkb1) or an empty vector (G361 + vector). Immunoprecipitation from cell lysates containing 400 μg of total protein and kinase activity assays were as performed as described in Fig. 1, except that samples were visualized by autoradiography. (C) G418-resistant colony formation by NIH 3T3, G361, and HeLa S3 cells after transfection with a vector control (solid bars) or with an Lkb1-expressing plasmid (open bars). For each cell line, the colony numbers obtained in the Lkb1 transfection were normalized to the vector transfection. A representative experiment is shown.
To verify that the ectopically expressed Lkb1 was functional in G361 cells, the protein was immunoprecipitated from the transfected cells and analyzed for autocatalytic activity as described above. As shown in Fig. 2B, the Lkb1 kinase activity detected in the Lkb1-transfected G361 cells was higher than that observed in NIH 3T3 cells used as a control. This result indicates that any cellular factors that Lkb1 may require for kinase activity are present in G361 cells.
Restoring Lkb1 Activity in Tumor Cells Induces Growth Suppression.
To study whether Lkb1 expression affects growth of G361, HeLa S3, and NIH 3T3 cells, they were transfected with an expression vector encoding both Lkb1 and a neomycin resistance gene (Lkb1/pCI-Neo) or a vector encoding the selection marker only (pCI-Neo). Subsequently, the transfections were subjected to G418 selection for 16 days. After the selection, a strongly reduced number of colonies were detected in Lkb1 transfections compared with the vector transfections of both G361 and HeLa S3 cells (Fig. 2C). The growth suppression by ectopic Lkb1 was limited to cells with impaired Lkb1 activity, as determined by the fact that no suppression occurred when using NIH 3T3 cells (Fig. 2C) or other cell lines (HCT116, DLD-1, and 293; data not shown) with clearly detectable Lkb1 activity.
Growth Suppression by Lkb1 Requires a Functional Kinase Domain.
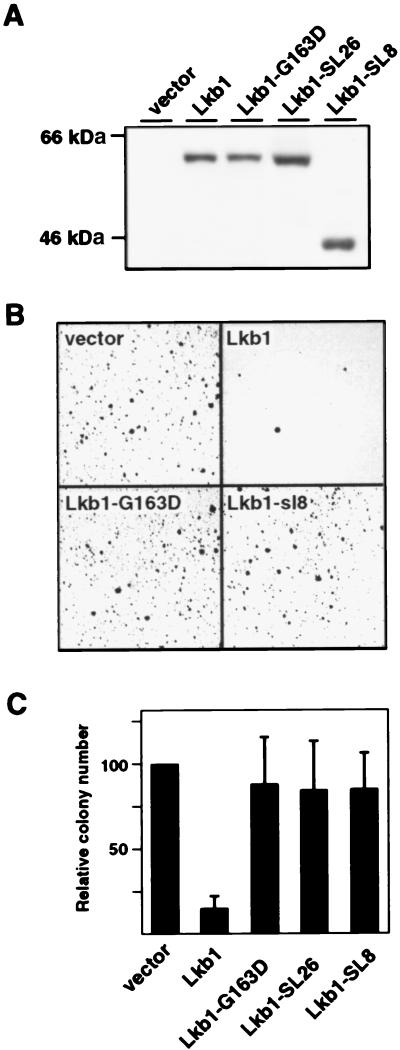
To investigate whether the kinase activity of Lkb1 was necessary for growth inhibition, we took advantage of three naturally occurring LKB1 mutant alleles that impair the kinase activity (3, 7, 11). Of these mutants, two encode PJS-associated kinase-deficient proteins: Lkb1-SL8 (C-terminal truncation caused by a frameshift in codon 307) and Lkb1-SL26 (a deletion of amino acids 303–306). The third mutant, Lkb1-G163D, identified from a sporadic testicular cancer, has retained a low level of activity (≈1% of that of wild type; ref. 7). G361 cells were used in these experiments, as transfection efficiencies were significantly higher than in HeLa S3 (data not shown). Cells transfected with plasmids encoding these mutants or a wild-type control expressed comparable levels of the corresponding proteins 2 days after transfection (Fig. 3A). Subsequently, these same transfections were subjected to G418 selection for 16–20 days. As above, ectopic expression of wild-type Lkb1 strongly inhibited growth of G361 cells, which was detected as a reduced number of colonies compared with those in the control transfection (Fig. 3 B and C). By contrast, all three naturally occurring Lkb1 mutants, including Lkb1-G163D with severely reduced kinase activity, were unable to suppress growth of G361 cells (Fig. 3 B and C). Thus, restoring the Lkb1 expression in G361 cells led to growth suppression that depended on a functional kinase domain.
Figure 3.
Growth effects of wild-type and naturally occurring mutant alleles of LKB1 in G361 melanoma cells. (A) Western blotting analysis of Lkb1 in cells transfected with indicated expression vectors. Detection was performed with anti-Lkb1, except for Lkb1-SL8, which was detected by antihemagglutinin because of a C-terminal truncation. (B) Photographs of Giemsa-stained G418-resistant colonies of G361 cells from plates transfected with indicated expression vectors. (C) Relative numbers of G418-resistant colonies after transfections with the indicated expression vectors shown in B. Standard deviations are from nine (vector, Lkb1) or six (G163D, SL26, SL8) independent experiments.
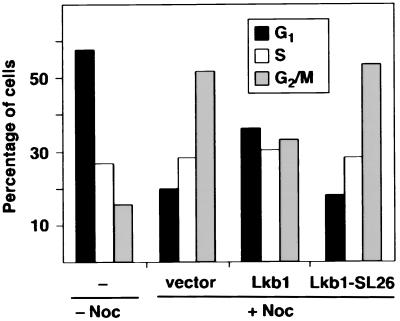
Expression of Lkb1 in G361 Cells Leads to a G1 Cell Cycle Arrest.
The observed growth suppression by Lkb1 could be caused by either increased apoptosis or inhibition of cell proliferation. However, no increase in the rate of apoptosis in Lkb1-expressing cells was detected in immunofluorescence experiments similar to those described in Fig. 2 (data not shown). Therefore, possible cell cycle changes in Lkb1-transfected cells were studied by using flow cytometry. Transfected G361 cells were identified by coexpression of the CD20 cell surface antigen, and 48 h after transfection, cells were processed for analysis with a fluorescence-activated cell sorter. A comparison of G1, S, and G2/M cell cycle phases between vector- and Lkb1-transfected cells did not reveal significant differences from an untransfected population (data not shown and Fig. 4, − Noc). As the relatively large fraction (58%) of cells was in G1, a possible growth inhibition may have been masked. To explore this possibility, proliferating cells were arrested in mitosis with the microtubule-stabilizing agent nocodazole to identify cells blocked in G1 as described (18). Both vector-transfected as well as Lkb1-SL26-transfected cells accumulated in G2/M (Fig. 4), and both had a significant decrease in the G1 population. By contrast, the Lkb1-transfected cells were blocked also in G1. The G1 fraction of Lkb1-transfected cells was 36% compared with 20% of vector-transfected cells and 18% of SL26-transfected cells. These results indicate that Lkb1 growth suppression results from G1 cell cycle arrest.
Figure 4.
Cell cycle distribution of G361 cells after Lkb1 transfection. Cells cotransfected with the cell surface marker CD20 (CMV-CD20) together with vector control (vector), wild-type Lkb1 (Lkb1), or a naturally occurring kinase-deficient mutant (Lkb1-SL26) were treated with nocodazole (+ Noc) to induce a G2/M arrest. Subsequently, transfected (CD20 positive) cells were analyzed by using flow cytometry as detailed in Materials and Methods. The cell cycle distribution of nontransfected, unsynchronized cells is shown as a control (− Noc).
DISCUSSION
This study provides functional evidence indicating that the LKB1 gene inactivated in PJS and in some sporadic tumors has growth-suppressing activity. The demonstration of a growth suppressive function establishes LKB1 as a tumor suppressor gene. Although the cellular function(s) of Lkb1 remain to be elucidated, the growth suppression was mediated through a G1 cell cycle arrest and did not involve increased apoptosis. These findings suggest that Lkb1 is involved in a G0/G1 checkpoint rather than regulation of cell death.
The growth suppression by ectopic Lkb1 was limited to cells with undetectable or low endogenous levels of Lkb1 (G361 and HeLa S3 cells). Cells resistant to growth inhibition by ectopic Lkb1 may have acquired mutations that prohibit suppression by Lkb1. However, LKB1 mRNA is expressed also in normal proliferating cells (10), and thus expression of LKB1 per se is not sufficient to suppress growth. In this respect, it is interesting to note that growth inhibition by the PTEN tumor suppressor is also limited to cells with mutated PTEN (19). Germ-line mutations in LKB1 and PTEN lead to closely related diseases, namely PJS (3, 4) and Cowden disease (20, 21), suggesting that these genes may be involved in the same or related growth inhibitory pathways.
Inhibition of colony formation by Lkb1 provides a model system to study the mechanism by which Lkb1 exerts its growth suppressive effect. This effect was shown by using several naturally occurring mutants of Lkb1, which had been shown to be deficient for the autocatalytic kinase activity (7) and, herein, was shown to be incapable of growth suppression. This model system provides the means to define further the domains of Lkb1 that mediate growth suppression and may be useful in identifying regulators of Lkb1 function.
The ability of LKB1 to suppress growth and the increased tumor incidence in patients with PJS indicate that abrogation of Lkb1 function is advantageous for tumor cell proliferation. However, only a limited number of LKB1 mutations have been detected in sporadic tumors. Here, we identified down-regulation of LKB1 mRNA expression as a potential mechanism of inactivating Lkb1 function in tumors. The silencing of LKB1 expression in HeLa S3 is caused by epigenetic inactivation by promoter methylation (L. A. Aaltonen and J. G. Herman, personal communication), and thus LKB1 down-regulation in G361 could be caused by partial methylation.
It would be of interest to study whether primary tumors may have loss of functional Lkb1 because of the mechanisms not detected in prior analyses (3–7, 11–15). Using the functional Lkb1 assay described here to assess Lkb1 status in tumors would provide a convenient assay for detecting all LKB1 defects, regardless of the mechanism.
Acknowledgments
We thank Arno Pihlak and Monica Schoultz for help in fluorescence-activated cell sorter analysis, Birgitta Tjäder for technical assistance, J. G. Herman and Lauri Aaltonen for communication of unpublished results, and Ed Harlow for providing reagents. This study was supported by grants from the Academy of Finland, Biocentrum Helsinki, University of Helsinki, Finnish Cancer Society, Finnish Cancer Institute, and the Sigrid Juselius Foundation. M.T. is a Biocentrum Helsinki postdoctoral fellow, and A.Y. is a M.D./Ph.D. student of the Helsinki Biomedical Graduate School.
ABBREVIATION
- PJS
Peutz–Jeghers syndrome
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Tomlinson I P, Houlston R S. J Med Genet. 1997;34:1007–1011. doi: 10.1136/jmg.34.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemminki A, Tomlinson I, Markie D, Jarvinen H, Sistonen P, Bjorkqvist A M, Knuutila S, Salovaara R, Bodmer W, Shibata D, et al. Nat Genet. 1997;15:87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, et al. Nature (London) 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 4.Jenne D E, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 5.Gruber S B, Entius M M, Petersen G M, Laken S J, Longo P A, Boyer R, Levin A M, Mujumdar U J, Trent J M, Kinzler K W, et al. Cancer Res. 1998;58:5267–5270. [PubMed] [Google Scholar]
- 6.Mehenni H, Gehrig C, Nezu J, Oku A, Shimane M, Rossier C, Guex N, Blouin J L, Scott H S, Antonarakis S E. Am J Hum Genet. 1998;63:1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylikorkala A, Avizienyte E, Tomlinson I P M, Tiainen M, Roth S, Loukola A, Hemminki A, Johansson M, Sistonen P, Markie D, et al. Hum Mol Genet. 1999;8:45–51. doi: 10.1093/hmg/8.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Su J Y, Erikson E, Maller J L. J Biol Chem. 1996;271:14430–14437. doi: 10.1074/jbc.271.24.14430. [DOI] [PubMed] [Google Scholar]
- 9.Salt I, Celler J W, Hawley S A, Prescott A, Woods A, Carling D, Hardie D G. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luukko K, Ylikorkala A, Tiainen M, Mäkelä T P. Mech Dev. 1999;83:187–190. doi: 10.1016/s0925-4773(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 11.Avizienyte E, Roth S, Loukola A, Hemminki A, Lothe R A, Stenwig A E, Fossa S D, Salovaara R, Aaltonen L A. Cancer Res. 1998;58:2087–2090. [PubMed] [Google Scholar]
- 12.Bignell G R, Barfoot R, Seal S, Collins N, Warren W, Stratton M R. Cancer Res. 1998;58:1384–1386. [PubMed] [Google Scholar]
- 13.Resta N, Simone C, Mareni C, Montera M, Gentile M, Susca F, Gristina R, Pozzi S, Bertario L, Bufo P, et al. Cancer Res. 1998;58:4799–4801. [PubMed] [Google Scholar]
- 14.Wang Z J, Taylor F, Churchman M, Norbury G, Tomlinson I. Am J Pathol. 1998;153:363–366. doi: 10.1016/S0002-9440(10)65579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avizienyte E, Loukola A, Roth S, Hemminki A, Tarkkanen M, Salovaara R, Arola J, Butzow R, Husgafvel-Pursiainen K, Kokkola A, et al. Am J Pathol. 1999;154:677–681. doi: 10.1016/S0002-9440(10)65314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.MacGregor G R, Caskey C T. Nucleic Acids Res. 1989;17:2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L, Enders G, Lees J A, Beijersbergen R L, Bernards R, Harlow E. EMBO J. 1995;14:1904–1913. doi: 10.1002/j.1460-2075.1995.tb07182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furnari F B, Lin H, Huang H S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 21.Nelen M R, van Staveren W C, Peeters E A, Hassel M B, Gorlin R J, Hamm H, Lindboe C F, Fryns J P, Sijmons R H, Woods D G, et al. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]