Abstract
1. Trains of end-plate potentials (e.p.p.s) have been recorded from the isolated tenuissimus of the cat. The muscle was paralyzed either by transversely cutting the muscle fibres or by non-depolarizing blocking drugs.
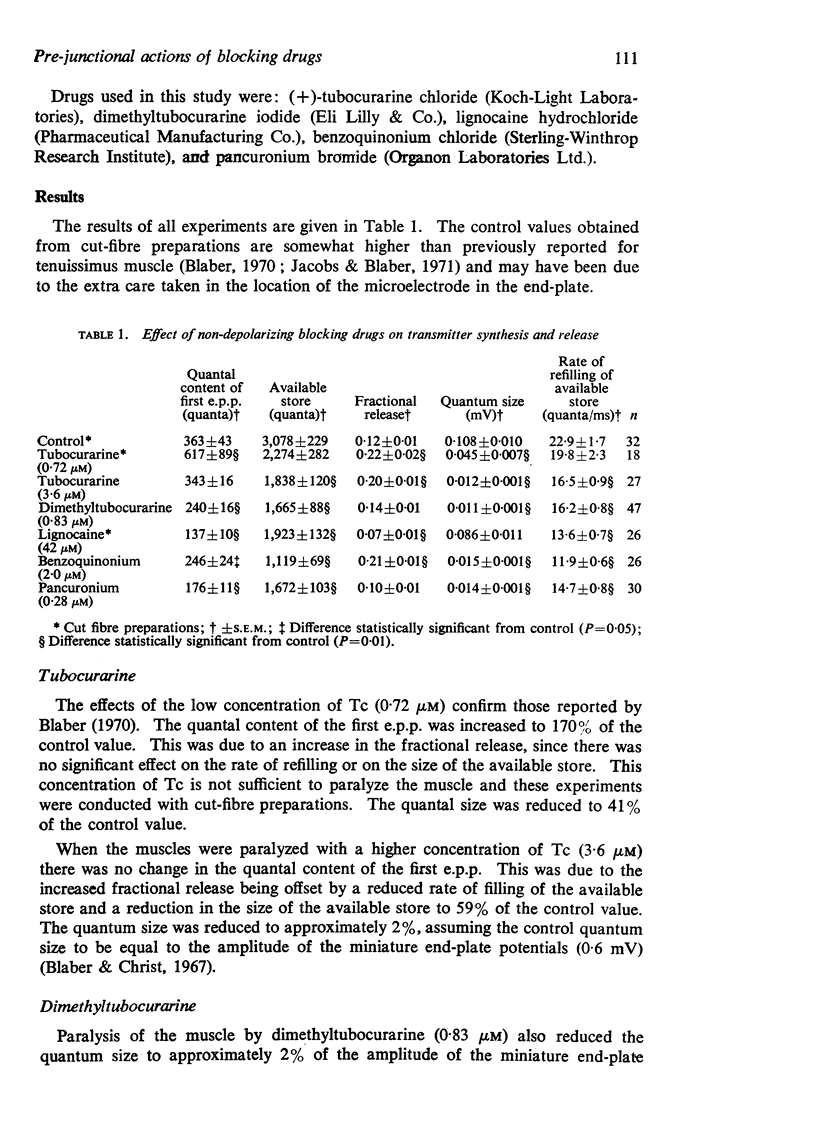
2. The following parameters of transmitter synthesis, storage and release have been calculated: the quantal content of the first e.p.p. in the train, the size of the available store, fractional release, quantum size, and the rate of refilling of the available store.
3. Tubocurarine and benzoquinonium depressed the rate of refilling of the available store causing its depletion at high rates of stimulation. This was offset by an increase in fractional release, which in the case of tubocurarine was sufficient for the quantal content of the first e.p.p. to be unchanged.
4. Dimethyltubocurarine and pancuronium had a similar effect to tubocurarine on the rate of refilling of the store and depletion of the store at high rates of stimulation but did not increase fractional release. There was, therefore, a decrease in the quantal content of the first e.p.p.
5. Lignocaine depressed the rate of refilling of the store and depleted the store at high rates of stimulation. Fractional release was also depressed.
6. It is suggested that the non-depolarizing drugs have a weak local anaesthetic action retarding the influx of sodium into the nerve terminal which slows the rate of refilling of the store. This effect is due to the quaternary ammonium head. The presence of a phenolic group increases fractional release due either to an increased influx of calcium into the nerve terminal or to a potentiation of the actions of calcium.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEANI L., BIANCHI C., LEDDA F. THE EFFECT OF TUBOCURARINE ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. J Physiol. 1964 Nov;174:172–183. doi: 10.1113/jphysiol.1964.sp007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G. Stimulus frequency and neuromuscular block. Br J Pharmacol Chemother. 1963 Feb;20:5–16. doi: 10.1111/j.1476-5381.1963.tb01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H. Die Freisetzung von Acetylcholin an der motorischen Nervenendigung unter dem Einfluss von d-Tubocurarin. Pflugers Arch. 1971;326(2):162–183. doi: 10.1007/BF00586908. [DOI] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Burstyn P. G., Firth D. R. The form of sodium-calcium competition at the frog myoneural junction. J Gen Physiol. 1968 Dec;52(6):887–907. doi: 10.1085/jgp.52.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber L. C., Christ D. D. The action of facilitatory drugs on the isolated tenuissimus muscle of the cat. Int J Neuropharmacol. 1967 Nov;6(6):473–484. doi: 10.1016/0028-3908(67)90047-0. [DOI] [PubMed] [Google Scholar]
- Blaber L. C. The mechanism of the facilitatory action of edrophonium in cat skeletal muscle. Br J Pharmacol. 1972 Nov;46(3):498–507. doi: 10.1111/j.1476-5381.1972.tb08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaer L. C. The effect of facilitatory concentrations of decamethonium on the storage and release of transmitter at the neuromuscular junction of the cat. J Pharmacol Exp Ther. 1970 Dec;175(3):664–672. [PubMed] [Google Scholar]
- Chang C. C., Cheng H. C., Chen T. F. Does d-tubocurarine inhibit the release of acetylcholine from motor nerve endings? Jpn J Physiol. 1967 Oct 15;17(5):505–515. doi: 10.2170/jjphysiol.17.505. [DOI] [PubMed] [Google Scholar]
- Christ D. D., Blaber L. C. The actions of benzoquinonium in the isolated cat tenuissimus muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):159–165. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Feldberg W., Vogt M. Release of acetylcholine at voluntary motor nerve endings. J Physiol. 1936 May 4;86(4):353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Prejunctional effect of curare: its relative importance. J Neurophysiol. 1971 Mar;34(2):289–301. doi: 10.1152/jn.1971.34.2.289. [DOI] [PubMed] [Google Scholar]
- Gergis S. D., Dretchen K. L., Sokoll M. D., Long J. P. Effect of pancuronium bromide on acetylcholine release. Proc Soc Exp Biol Med. 1972 Jan;139(1):74–76. doi: 10.3181/00379727-139-36080. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F., Miyamoto M. Reduction of transmitter release by D-tubocurarine. Nature. 1969 Aug 2;223(5205):531–533. doi: 10.1038/223531a0. [DOI] [PubMed] [Google Scholar]
- Jacobs R. S., Blaber L. C. The anti-curare action of sodium fluoride at the neuromuscular junction of cat tenuissimus muscle. Neuropharmacology. 1971 Sep;10(5):607–612. doi: 10.1016/0028-3908(71)90026-8. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KELLY J. S. ANTAGONISM BETWEEN NA+ AND CA2+ AT THE NEUROMUSCULAR JUNCTION. Nature. 1965 Jan 16;205:296–297. doi: 10.1038/205296a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Tomita T. Noradrenaline action on nerve terminal in the rat diaphragm. J Physiol. 1971 Aug;217(1):19–31. doi: 10.1113/jphysiol.1971.sp009557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEHEIL G., NAESS K. A presynaptic effect of d-tubocurarine in the neuromuscular junction. Acta Physiol Scand. 1961 Jun;52:120–136. doi: 10.1111/j.1748-1716.1961.tb02208.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E. Effect of procaine on electrical properties of squid axon membrane. Am J Physiol. 1959 May;196(5):1071–1078. doi: 10.1152/ajplegacy.1959.196.5.1071. [DOI] [PubMed] [Google Scholar]
- WERNER G. Antidromic activity in motor nerves and its relation to a generator event in nerve terminals. J Neurophysiol. 1961 Jul;24:401–413. doi: 10.1152/jn.1961.24.4.401. [DOI] [PubMed] [Google Scholar]


