Abstract
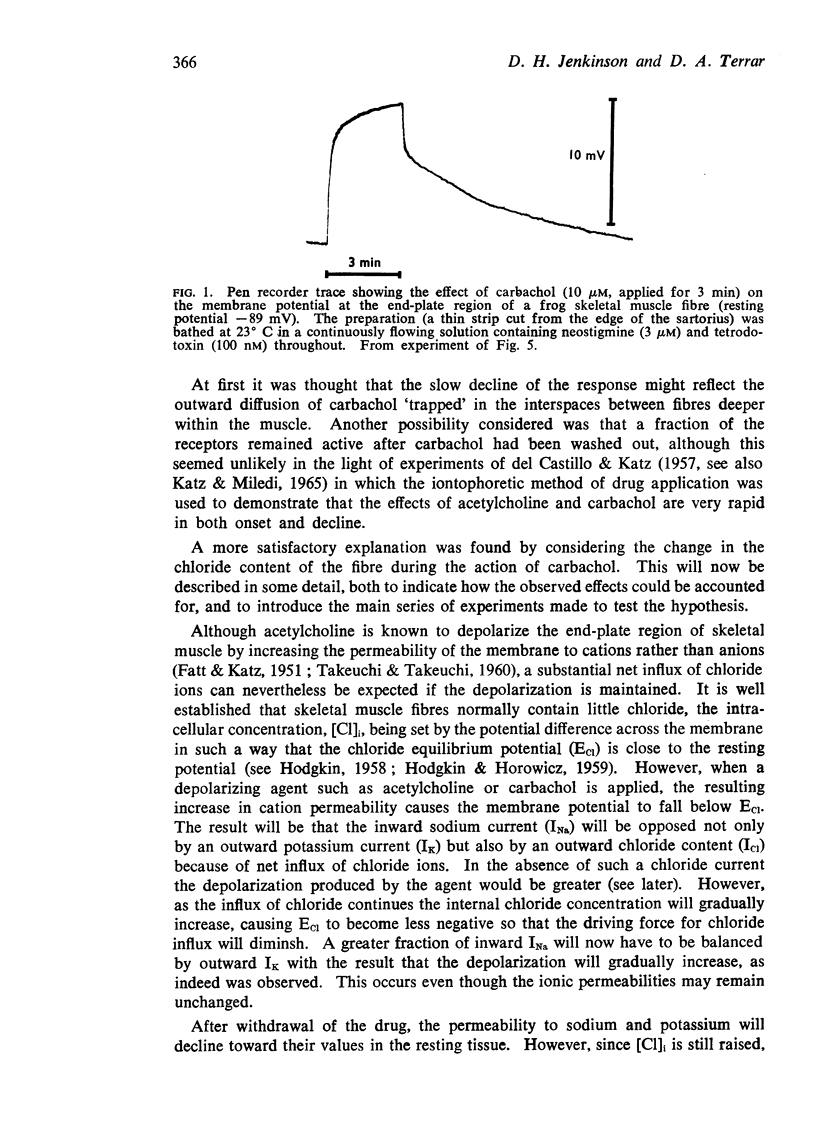
1. Micro-electrodes were used to follow changes in the membrane potential at the end-plate region of single fibres in narrow strips of frog skeletal muscle exposed to carbachol applied in continuously flowing Ringer solution containing tetrodotoxin (200 nM) and neostigmine (3 μM).
2. The depolarizations elicited by carbachol (5-20 μM) usually developed in two phases, the first of which was generally complete within 30 s whereas several min were required for the second.
3. Repolarization after carbachol also occurred in two phases, the second of which outlasted the time needed to clear the bath, and varied with the magnitude and duration of the depolarization which carbachol had caused.
4. These findings could best be explained in terms of the consequences of net entry of chloride ions into the fibre during the depolarization caused by carbachol. This hypothesis is supported by three lines of evidence:
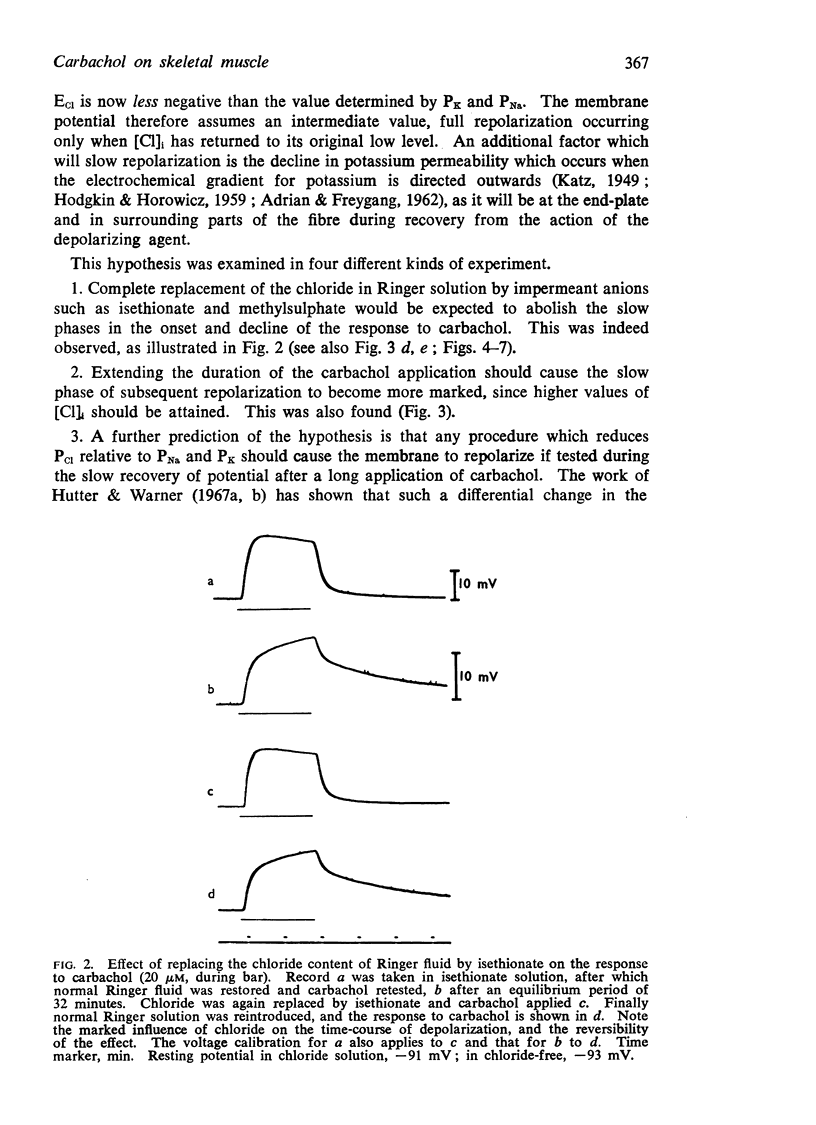
(a) Replacement of the chloride content of the Ringer solution by the less permeant anion isethionate abolished the slow phases of the carbachol response.
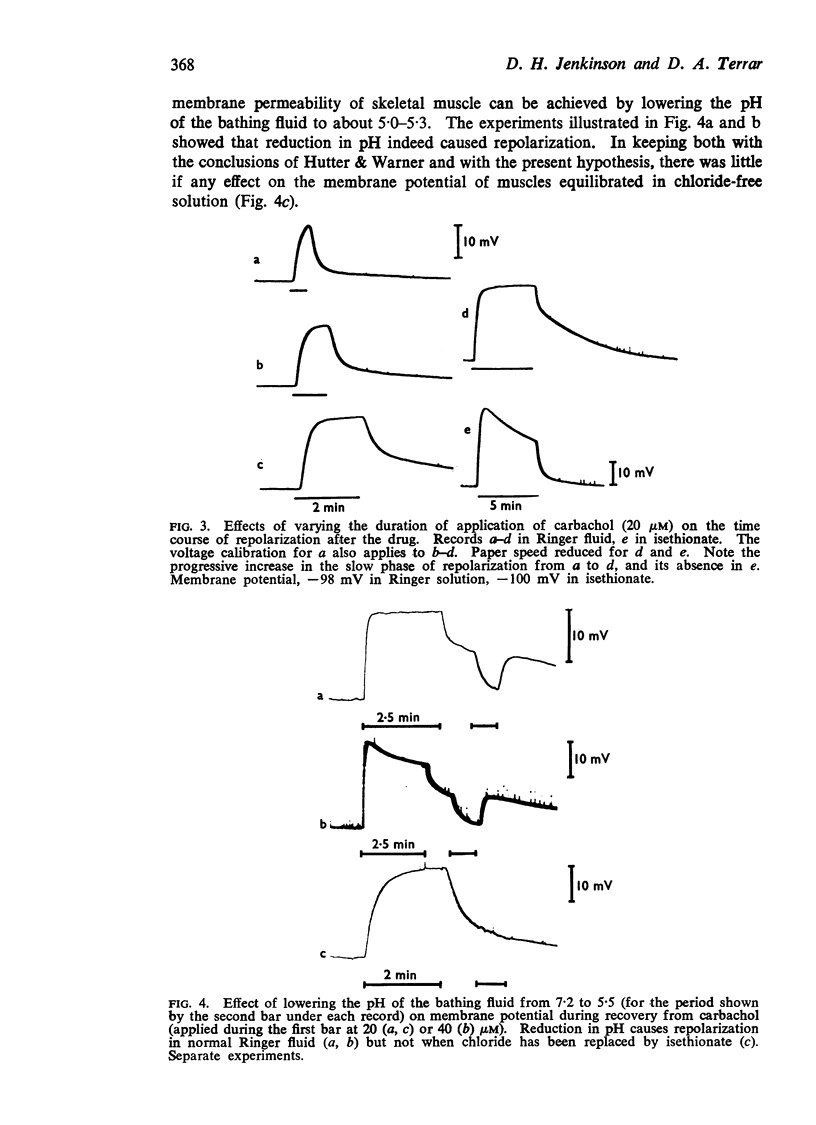
(b) Reduction of chloride permeability (by lowering pH) caused rapid repolarization during the recovery period after carbachol.
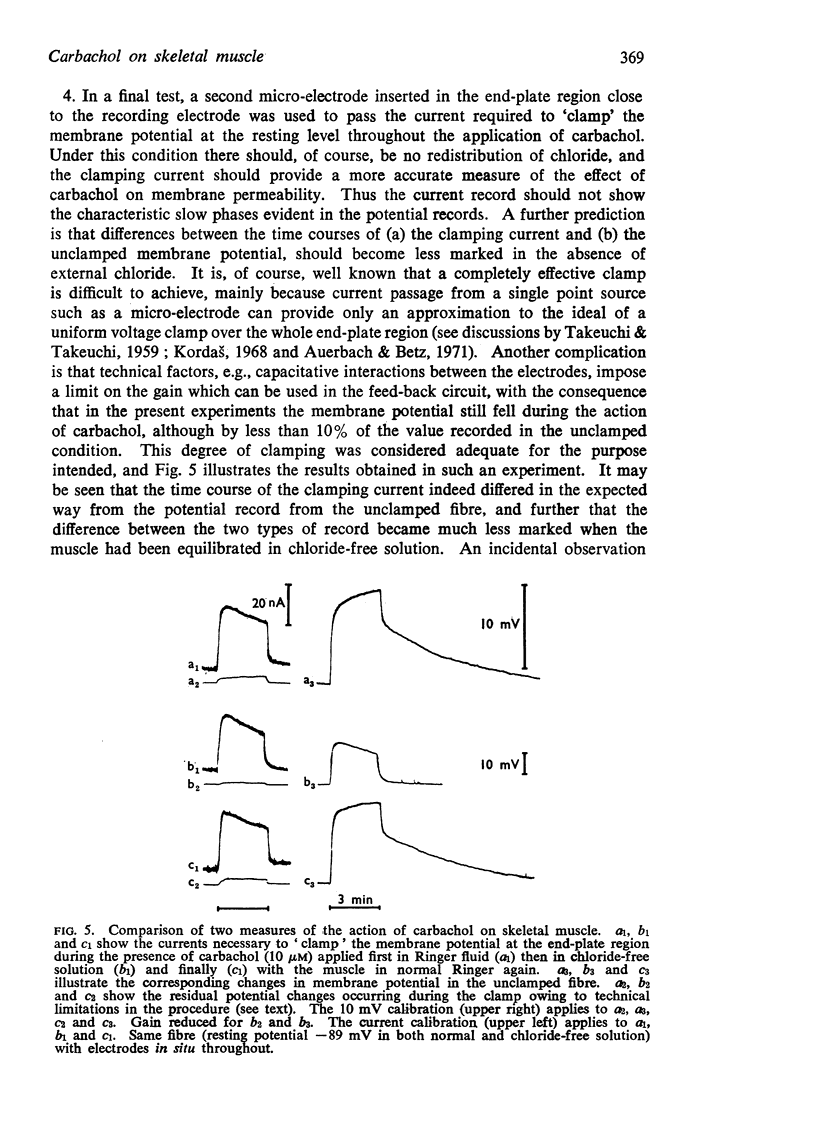
(c) When the membrane potential was clamped at the resting level throughout the action of carbachol, so avoiding chloride redistribution, the clamping current records did not show the slow phases attributed to chloride movement.
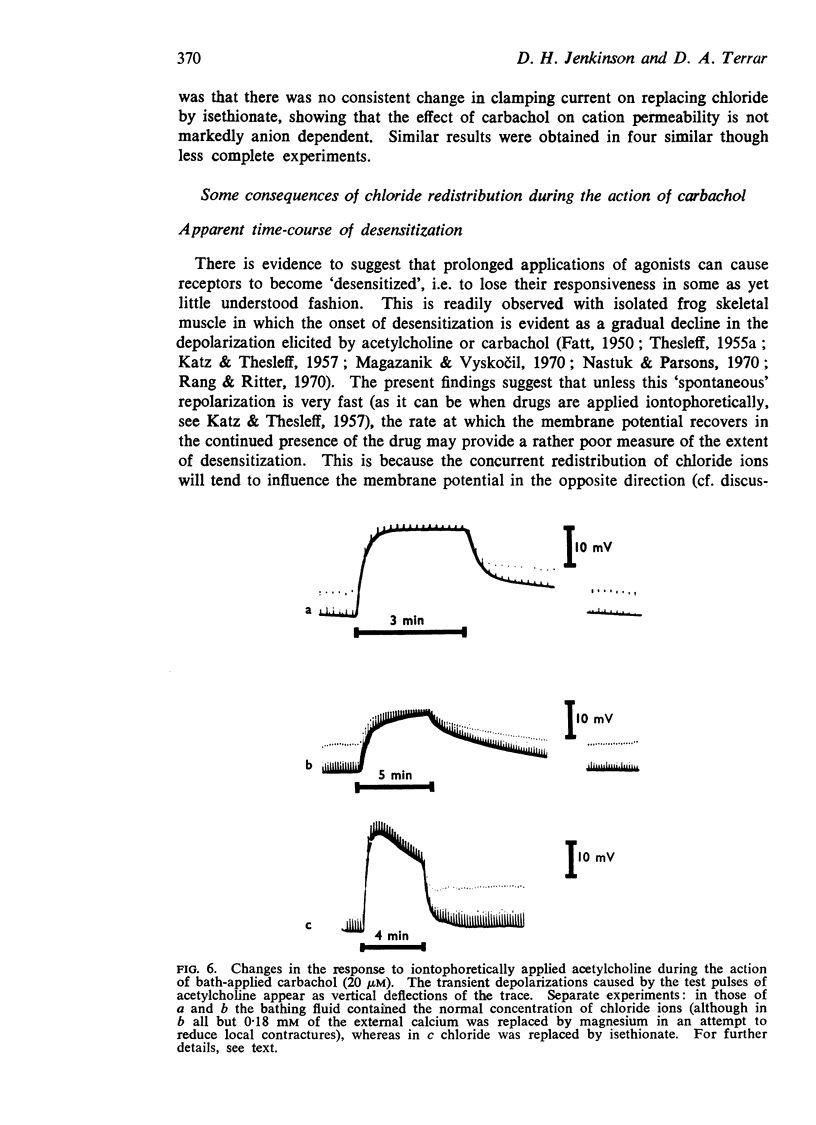
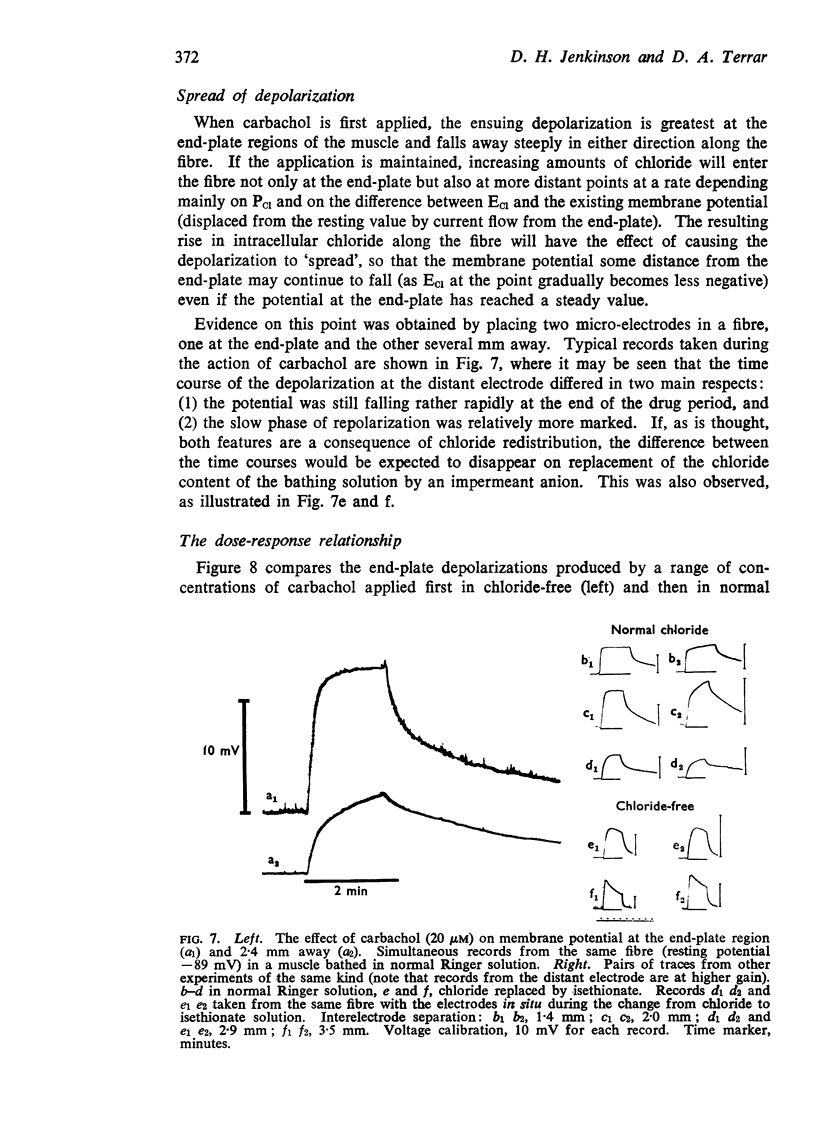
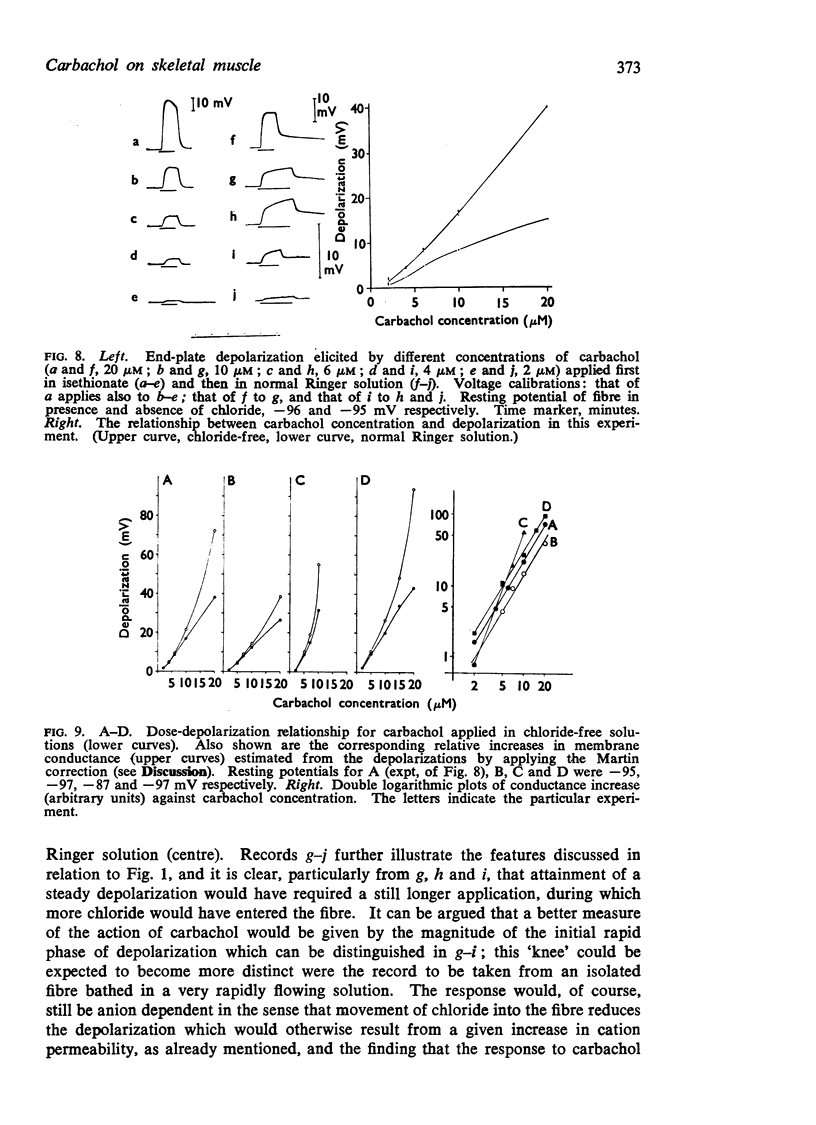
5. Chloride redistribution contributes to the gradual spread of depolarization during prolonged applications of depolarizing agents to skeletal muscle. It also complicates the interpretation of the dose-response relationship, and may make it more difficult to assess the extent to which the receptors become desensitized during the action of agonists applied in the bath.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Betz W. Does curare affect transmitter release? J Physiol. 1971 Mar;213(3):691–705. doi: 10.1113/jphysiol.1971.sp009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R., Changeux J. P. About the changes of internal ionic concentration in the isolated electroplax during chemical excitation. Biochim Biophys Acta. 1970 Dec 1;219(2):398–404. doi: 10.1016/0005-2736(70)90217-8. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R. On the excitability and cooperativity of the electroplax membrane. Proc Natl Acad Sci U S A. 1968 Mar;59(3):944–950. doi: 10.1073/pnas.59.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Dunant Y. Diffusion of drugs through stationary water layers as the rate limiting process in their action at membrane receptors. Br J Pharmacol. 1970 Nov;40(3):508–521. doi: 10.1111/j.1476-5381.1970.tb10632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. A comparison of acetylcholine and stable depolarizing agents. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):362–368. doi: 10.1098/rspb.1957.0017. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. Ionic permeability changes induced by some cholinergic agonists on normal and denervated frog muscles. J Physiol. 1971 Oct;218(1):101–116. doi: 10.1113/jphysiol.1971.sp009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGMAN H. B., PODLESKI T. R., BARTELS E. APPARENT DISSOCIATION CONSTANTS BETWEEN CARBAMYLCHOLINE, DELTA-TUBOCURARINE AND THE RECEPTOR. Biochim Biophys Acta. 1963 Sep 24;75:187–193. doi: 10.1016/0006-3002(63)90597-3. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The effect of pH on the 36-Cl efflux from frog skeletal muscle. J Physiol. 1967 Apr;189(3):427–443. doi: 10.1113/jphysiol.1967.sp008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Karlin A. Permeability and internal concentration of ions during depolarization of the electroplax. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1162–1167. doi: 10.1073/pnas.58.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHS S., MUKHERJEE A. K. Action of acetylcholine, choline and D-tubocurarine on the membrane of frog sartorius muscle fibers. Am J Physiol. 1959 Jun;196(6):1191–1196. doi: 10.1152/ajplegacy.1959.196.6.1191. [DOI] [PubMed] [Google Scholar]
- Portela A., Perez R. J., Vaccari J., Perez J. C., Stewart P. Muscle membrane depolarization by acetylcholine, choline and carbamylcholine, near and remote from motor end-plates. J Pharmacol Exp Ther. 1970 Nov;175(2):476–482. [PubMed] [Google Scholar]
- Rang H. P. Drug receptors and their function. Nature. 1971 May 14;231(5298):91–96. doi: 10.1038/231091a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Ras R., den Hertog A., Lammers W. The effect of suxamethonium on the striated muscle fibre outside the endplate region. Pflugers Arch. 1972;333(3):187–196. doi: 10.1007/BF00592682. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. The effects of acetylcholine, decamethonium and succinylcholine on neuromuscular transmission in the rat. Acta Physiol Scand. 1955 Oct 12;34(4):386–392. doi: 10.1111/j.1748-1716.1955.tb01257.x. [DOI] [PubMed] [Google Scholar]
- THESLEFT S. The mode of neuromuscular block caused by acetylcholine, nicotine, decamethonium and succinylcholine. Acta Physiol Scand. 1955 Oct 27;34(2-3):218–231. doi: 10.1111/j.1748-1716.1955.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Werman R. An electrophysiological approach to drug-receptor mechanisms. Comp Biochem Physiol. 1969 Sep 15;30(6):997–1017. doi: 10.1016/0010-406x(69)91038-x. [DOI] [PubMed] [Google Scholar]


