Abstract
1 The possibility of a vasodilator innervation to the isolated and perfused central artery of the rabbit ear was examined.
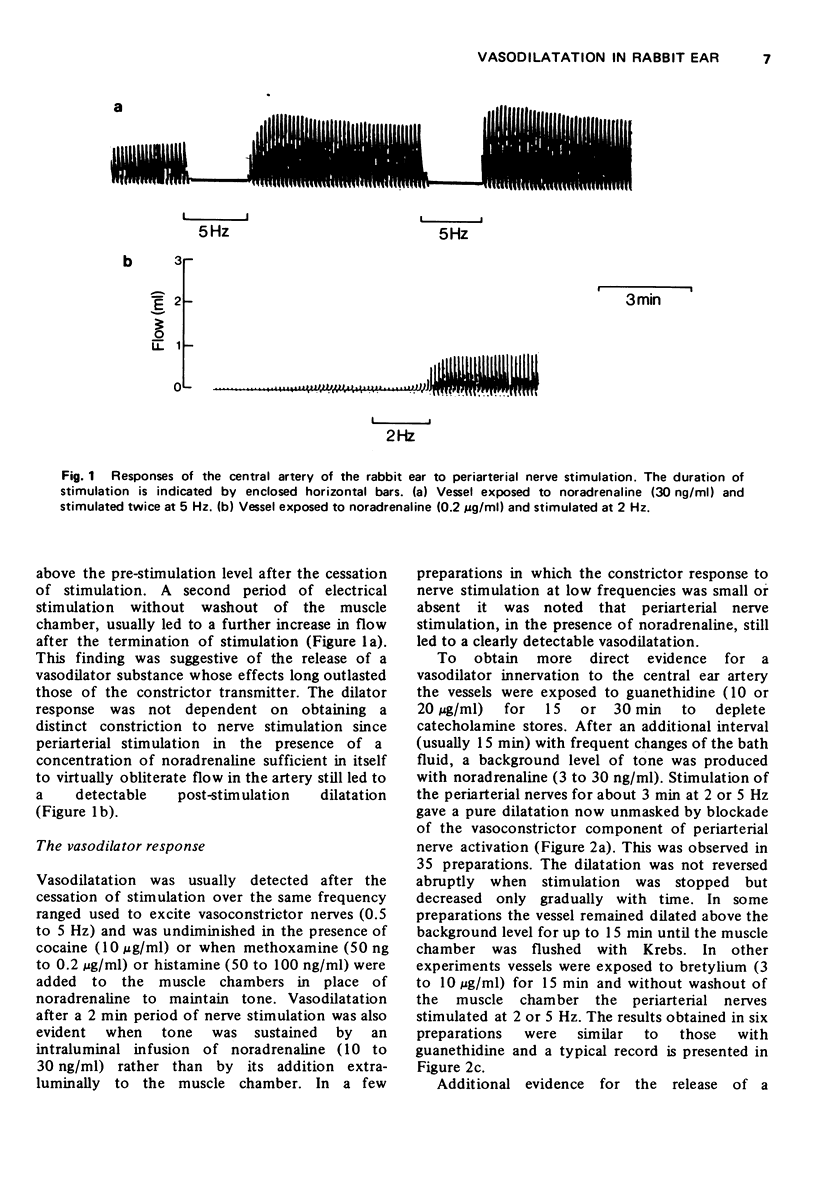
2 Stimulation of the periarterial nerves in the presence of noradrenaline or other agonist used to maintain a partial constriction of the ear artery, led to a decrease in intraluminal flow followed after the cessation of stimulation by an increase in flow beyond the pre-stimulation level.
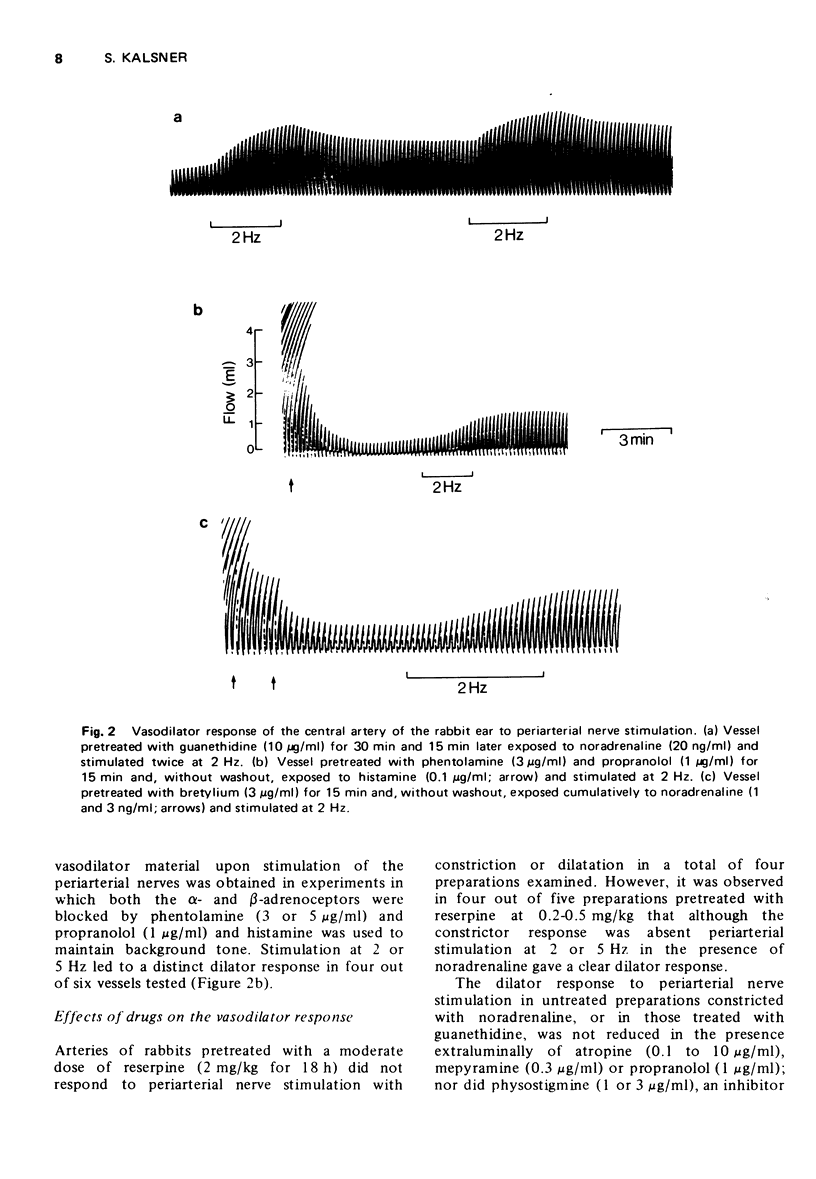
3 After blockade of adrenergic transmission with bretylium or guanethidine or of the α- and β-adrenoceptors with phentolamine and propranolol, stimulation of the periarterial nerves in the presence of a background tone, led to a clearly detectable vasodilation. This dilatation was not blocked by treatment with atropine or mepyramine; nor was it enhanced by physostigmine.
4 Pretreatment of rabbits with reserpine (2 mg/kg) to deplete catecholamine stores, eliminated both the vasoconstrictor and vasodilator responses to nerve stimulation. However, a lower dose of reserpine (0.2 to 0.5 mg/kg) selectively eliminated the vasoconstrictor component of periarterial nerve activation.
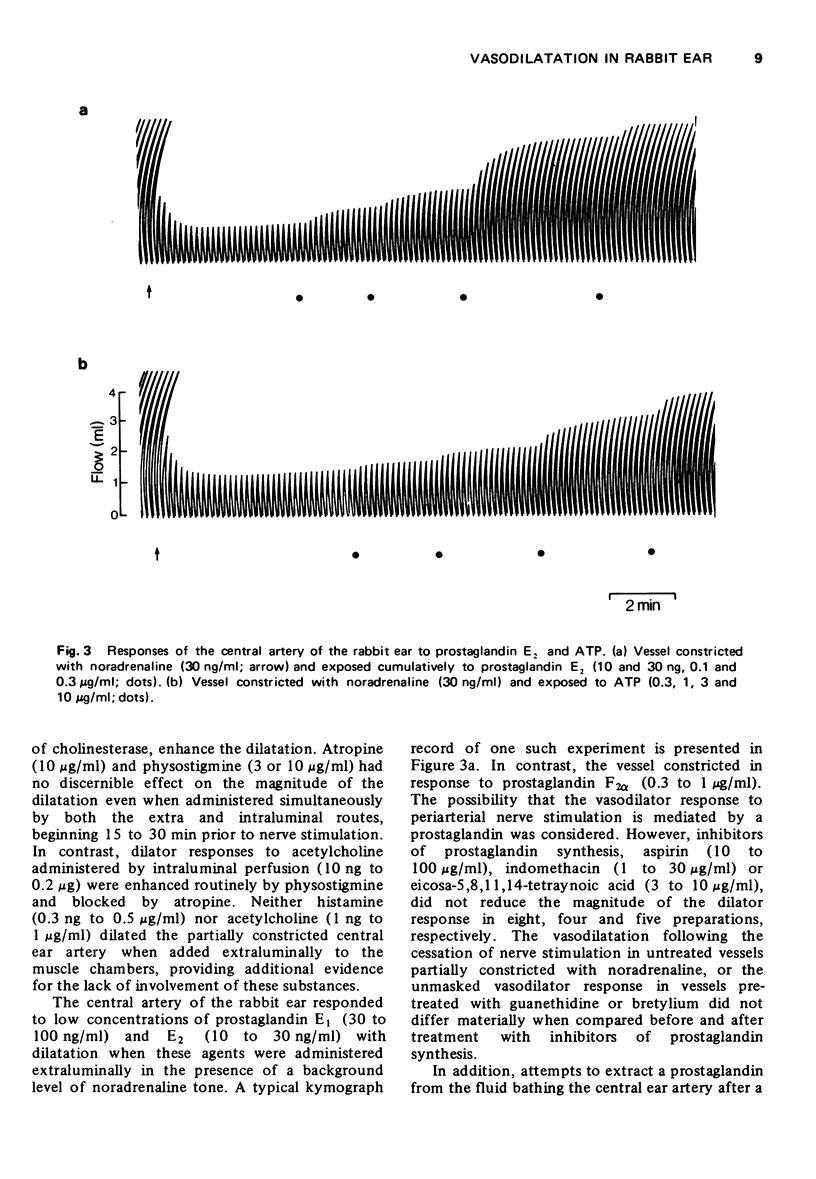
5 The ear artery dilated in response to low concentrations of prostaglandin E1, and E2, in the presence of noradrenaline, but treatment with inhibitors of prostaglandin synthesis, indomethacin, aspirin or eicosa-5,8,11,14-tetraynoic acid did not reduce the vasodilator response. Attempts to extract a prostaglandin in the bathing medium were unsuccessful.
6 The involvement of a purine nucleotide appeared unlikely since the ear artery dilated only in response to fairly high concentrations of adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP) and adenosine 5′-monophosphate (AMP). Furthermore, dipyridamole, an inhibitor of adenosine uptake, enhanced dilation due to exogenous ATP but not to periarterial nerve stimulation.
7 It is concluded that the central artery of the rabbit ear has a vasodilator innervation but the identity of the transmitter remains to be established.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK L., BRODY M. J. The physiology of vasodilation. Angiology. 1961 Jun;12:202–222. doi: 10.1177/000331976101200602. [DOI] [PubMed] [Google Scholar]
- Ballard D. R., Abboud F. M., Mayer H. E. Release of a humoral vasodilator substance during neurogenic vasodilatation. Am J Physiol. 1970 Nov;219(5):1451–1457. doi: 10.1152/ajplegacy.1970.219.5.1451. [DOI] [PubMed] [Google Scholar]
- Beck L., Pollard A. A., Kayaalp S. O., Weiner L. M. Sustained dilatation elicited by sympathetic nerve stimulation. Fed Proc. 1966 Nov-Dec;25(6):1596–1606. [PubMed] [Google Scholar]
- Brody M. J. Neurohumoral mediation of active reflex vasodilatation. Fed Proc. 1966 Nov-Dec;25(6):1583–1592. [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- De la Lande I. S., Frewin D., Waterson J. G. The influence of sympathetic innervation on vascular sensitivity to noradrenaline. Br J Pharmacol Chemother. 1967 Sep;31(1):82–93. doi: 10.1111/j.1476-5381.1967.tb01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Lande I. S., Rand M. J. A simple isolated nerve-blood vessel preparation. Aust J Exp Biol Med Sci. 1965 Oct;43(5):639–656. doi: 10.1038/icb.1965.48. [DOI] [PubMed] [Google Scholar]
- Downing D. T., Ahern D. G., Bachta M. Enzyme inhibition by acetylenic compounds. Biochem Biophys Res Commun. 1970 Jul 13;40(1):218–223. doi: 10.1016/0006-291x(70)91069-7. [DOI] [PubMed] [Google Scholar]
- Feldberg W. The peripheral innervation of the vessels of the external ear of the rabbit. J Physiol. 1926 Aug 6;61(4):518–529. doi: 10.1113/jphysiol.1926.sp002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- HOLTON P., PERRY W. L. M. On the transmitter responsible for antidromic vasodilatation in the rabbit's ear. J Physiol. 1951 Jun;114(1-2):240–251. doi: 10.1113/jphysiol.1951.sp004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON P., RAND M. J. Sympathetic vasodilatation in the rabbit ear. Br J Pharmacol Chemother. 1962 Dec;19:513–526. doi: 10.1111/j.1476-5381.1962.tb01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959 Mar 12;145(3):494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedqvist P. Prostaglandin-induced inhibition of vascular tone and reactivity in the cat's hindleg in vivo. Eur J Pharmacol. 1972 Jan;17(1):157–162. doi: 10.1016/0014-2999(72)90282-8. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Stjärne L., Wennmalm A. Facilitation of sympathetic neurotransmission in the cat spleen after inhibition of prostaglandin synthesis. Acta Physiol Scand. 1971 Nov;83(3):430–432. doi: 10.1111/j.1748-1716.1971.tb05099.x. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., von Euler U. S. Prostaglandin controls neuromuscular transmission in guinea-pig vas deferens. Nat New Biol. 1972 Mar 29;236(65):113–115. doi: 10.1038/newbio236113a0. [DOI] [PubMed] [Google Scholar]
- Hopkins S. V. The potentiation of the action of adenosine on the guinea-pig heart. Biochem Pharmacol. 1973 Feb 1;22(3):341–348. doi: 10.1016/0006-2952(73)90415-2. [DOI] [PubMed] [Google Scholar]
- Hughes J., Vane J. R. Relaxations of the isolated portal vein of the rabbit induced by nicotine and electrical stimulation. Br J Pharmacol. 1970 Jul;39(3):476–489. doi: 10.1111/j.1476-5381.1970.tb10356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Differential activation of the inner and outer muscle cell layers of the rabbit ear artery. Eur J Pharmacol. 1972 Oct;20(1):122–124. doi: 10.1016/0014-2999(72)90226-9. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Effects of the inhibition of noradrenaline uptake and synthesis on the maintenance of the response to continuous nerve stimulation in the central artery of the rabbit ear. Br J Pharmacol. 1972 May;45(1):1–12. doi: 10.1111/j.1476-5381.1972.tb09570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa N., Pfleger K., Rummel W. Specificity of adenosine uptake into the heart and inhibition by dipyridamole. Eur J Pharmacol. 1970 Mar;9(3):265–268. doi: 10.1016/0014-2999(70)90221-9. [DOI] [PubMed] [Google Scholar]
- Pollard A. A., Beck L. The distinct nature of the sustained dilator system: evidence against the pseudotransmitter hypothesis. J Pharmacol Exp Ther. 1971 Oct;179(1):132–143. [PubMed] [Google Scholar]
- Samuelsson B., Wennmalm A. Increased nerve stimulation induced release of noradrenaline from the rabbit heart after inhibition of prostaglandin synthesis. Acta Physiol Scand. 1971 Oct;83(2):163–168. doi: 10.1111/j.1748-1716.1971.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Tothill A., Rathbone L., Willman E. Relation between prostaglandin E2 and adrenaline reversal in the rat uterus. Nature. 1971 Sep 3;233(5314):56–57. doi: 10.1038/233056a0. [DOI] [PubMed] [Google Scholar]
- Waterson J. G., Smale D. E. Location of noradrenergic structures in the central artery of the rabbit ear. Aust J Exp Biol Med Sci. 1967 Jun;45(3):301–308. doi: 10.1038/icb.1967.25. [DOI] [PubMed] [Google Scholar]
- Zimmerman B. G. Comparison of sympathetic vasodilator innervation of hindlimb of the dog and cat. Am J Physiol. 1968 Jan;214(1):62–66. doi: 10.1152/ajplegacy.1968.214.1.62. [DOI] [PubMed] [Google Scholar]
- Zimmerman B. G. Influence of sympathetic stimulation on segmental vascular resistance before and after adrenergic neuronal blockade. Arch Int Pharmacodyn Ther. 1966 Mar;160(1):66–82. [PubMed] [Google Scholar]


