Abstract
In contrast to the immunosuppressive potential of UVB (280–320 nm) radiation in experimental animals and humans, UVA (320–400 nm) radiation at environmentally relevant doses appears to be immunologically inert. However, such exposure to UVA radiation has been observed unexpectedly to induce resistance to UVB-induced immunosuppression in mice, by a mechanism resulting in the inactivation of cis-urocanic acid (UCA), an epidermal immunosuppressive UV photoproduct. In this study in mice, we show that the immunoprotective activity of UVA radiation, against the effects of both UVB radiation and cis-UCA, can be attributed to the induction of cutaneous heme oxygenase (HO; EC 1.14.99.3). Cell-mediated immune function was assessed in vivo by the contact hypersensitivity response induced to oxazolone at an unirradiated skin site, and HO enzyme activity was measured in cutaneous microsomal preparations from treated mice. There was a progressive increase in HO enzyme activity for at least 3 days after UVA irradiation. However HO activity, both constitutive and UVA radiation-induced, was sensitive to the effects of injecting mice with the specific HO inhibitor, tin protoporphyrin (Sn [IV] protoporphyrin IX; SnPP). We observed, in addition, that in SnPP-injected mice, the immunoprotective effect of UVA radiation against either UVB radiation or cis-UCA was abrogated. Because SnPP injection did not affect normal contact hypersensitivity responsiveness but did inhibit the constitutive HO enzyme activity, it appeared that only the inducible HO was active in modulating immune function. This finding indicates that UVA-induced HO activity is a major player in the skin defenses against UVB immunosuppression.
T cell-mediated immune function, of which contact hypersensitivity (CHS) is a classical reaction, is readily suppressed by moderate exposure of mice to UVB radiation, or by topical application of cis-urocanic acid (UCA), a molecule normally produced in the skin as a direct epidermal UV photoproduct. Exogenous cis-UCA is capable of mimicking photoimmunosuppression in experimental animals, and thus cis-UCA is believed to play the role of one UVB-induced immunosuppressive mediator (1, 2). Other UVB-induced mediators also have been described, e.g., DNA lesions, prostaglandins, histamine, and anti-inflammatory cytokines (3–6); however, it remains uncertain whether cutaneous conditions dictate which mediators might predominate, and whether they might interact or provide independent pathways to the photoimmunosuppressed state. In contrast to these effects of UVB radiation, the effect of UVA radiation on immune function is less clear. In spite of several reports indicating an immune-suppressive role for UVA radiation under varying experimental conditions (7–9), we have reported previously that filtered UVA radiation was immunologically innocuous to mice at suberythemal doses. However, exposure to such UVA radiation induced a state of resistance in the mice to the immunosuppressive effects of either UVB radiation or cis-UCA (10), both of which could be applied before, immediately after, or up to 24 h after the UVA radiation. We postulated that UVA exposure may induce a relatively long-lived, but as-yet-unidentified, immunoprotective photoproduct that can inhibit the activity of cis-UCA and thus provide protection from UVB-induced photoimmunosuppression.
The search for a cutaneous factor that is preferentially induced by UVA, but not UVB, radiation, is daunting in the face of the array of UV-inducible genes identified to date (11). Unlike UVB radiation, UVA radiation acts on cells primarily via oxygen-dependent photosensitization reactions, resulting in the damaging presence of reactive oxygen species (ROS). However, UVA radiation also induces a number of genes encoding proteins with antioxidant and free radical scavenging properties, apparently in defense against the presence of such ROS. Several of such proteins have been identified as stress and heat shock proteins (hsp). Some, such as hsp72, have been shown to provide protection from UVB damage in heat-stimulated cultured human keratinocytes (12) and to reduce the number of sunburn cells in UVB-exposed human skin explants pretreated with hyperthermia (13). Whether ROS play a part in photoimmunosuppression is not clear, although high levels of polyunsaturated fatty acids incorporated into the epidermal lipids certainly predispose hairless mice to immunosuppression by UV radiation (14) and would be a prime source of ROS formation after UV irradiation. Furthermore, photoimmunoprotection by antioxidants such as vitamin E (15), green tea polyphenols (16), garlic (17), carnosine (18), and vitamin C (19), in studies where either UVB or cis-UCA was the immunosuppressive inducer, also suggest that oxidant states play a role.
Although UVB radiation also may cause some oxidant damage to cells, it is minor compared with the oxidative stress inflicted by UVA radiation (11). The major stress protein induced by UVA radiation is identified as heme oxygenase (HO; EC 1.14.99.3) (20). HO is the rate-limiting microsomal enzyme that catalyses the catabolism of heme to biliverdin, releasing Fe and carbon monoxide, and in most tissues the cytosolic NADPH-dependent enzyme biliverdin reductase rapidly converts biliverdin to the more stable bilirubin. HO-1 is strongly induced in fibroblasts by oxidant stress (21), but not in epidermal keratinocytes that express high levels of the constitutive isoform HO-2. Increases in HO activity appear to protect tissues from oxidative stress (22, 23). UVB is only a weak inducer of HO-1, perhaps because of its small oxidative component (11).
In recent studies, certain stress proteins have been shown to have immunologically related activities (24, 25). However, an immunological role for HO is a new concept, and most recently a correlation was demonstrated between HO-1 gene expression and the prevention of allograft rejection in mice (26, 27). In addition, the modulation of HO-1 induction with tin protoporphyrin IX (SnPP) in carrageenin-induced pleural inflammation in rats has been shown to modify the inflammatory process, with peak HO activity of infiltrating inflammatory cells coinciding with a striking resolution of the inflammation (28) and SnPP-inhibited HO induction with potentiation of the inflammation. Consequently, in this study we examine the hypothesis that the immunoprotective action of UVA radiation against both UVB radiation and cis-UCA results from the induction of HO-1. Hairless mice have been irradiated with environmentally relevant suberythemal doses of UVA radiation, preceded or followed by immunosuppressive treatment with UVB radiation or topical cis-UCA, and the immune function measured by the CHS reaction induced at an unirradiated skin site. The effect of inhibition of HO activity by injecting SnPP was used to indicate the relevance of HO to the photoimmunoprotective phenomenon.
METHODS
Mice.
Female inbred albino Skh:HR-1 hairless mice were provided from the Veterinary Pathology breeding colony. They were housed in treatment groups of six, in wire-topped plastic cages on vermiculite bedding and were maintained at 25°C under gold lighting (GEC F40GO) that does not emit UVB radiation, on a 12-h on/off cycle. The mice were 12–15 weeks old at the start of the study and were fed stock laboratory mouse cubes (Norco Stockfeeds, Lismore, Australia) and tap water ad libitum. All procedures were approved by the University of Sydney Animal Ethics Committee and complied with the state Animal Research Act 1985.
UV Radiation.
The UVA source comprised a planar bank of seven 120-cm fluorescent UVA tubes (Hitachi 40W F40T 10BL) held in a reflective batten at 19 cm above the irradiation table, incorporating a selected sheet of 6-mm window glass as filter (10). This source emitted radiation >320 nm, providing 2.7 × 10−3 W/cm2 UVA and 2.3 × 10−8 W/cm2 UVB. The UVB source consisted of a single UVB tube (Phillips TL-40W/12 RS, Eindhoven, The Netherlands) emitting 2.5 × 10−4 W/cm2 UVA and 4.1 × 10−4 W/cm2 UVB. Irradiance was measured with an International Light (Newburyport, MA) IL1500 radiometer with two detectors (SEE 015/UVA and SEE 240/UVB) that had been calibrated to the relevant spectral irradiances of the sources (F. Wilkinson, National Standards Laboratories, Commonwealth Scientific and Industrial Research Organization, Australia). Groups of mice, unrestrained but with the wire cage tops removed, were exposed to either radiation source. The boxes of mice were gently shaken regularly during the irradiation, to prevent shielding of mice by their sleeping cage mates, especially during the lengthy UVA exposures (4 h). Temperature was controlled with an electric fan.
UCA.
trans-UCA (Sigma) was photoisomerized in DMSO solution to an equilibrium mixture of 52% trans- and 48% cis-isomers as described (18). Lotions containing 0.2% (wt/vol) of trans- or UV-irradiated UCA, referred to here as cis-UCA, were prepared in a simple cosmetic oil-in-water base lotion and stored in the dark at 4°C. The control base lotion was identical in composition, without added UCA. Aliquots of 0.1 ml (200 μg UCA) were spread evenly over the mouse dorsum and allowed to be absorbed for 30 min.
HO Inhibition.
SnPP (Porphyrin Products, Logan, UT) was solubilized in 0.1 M NaOH and diluted 1:1 in 0.15 M sodium chloride/10 mM potassium phosphate, pH 7.4 (PBS), according to Willis et al. (28), and 20 μmol/kg body weight was injected s.c. in 0.1 ml into the left lower abdomen immediately after, and into the right lower abdomen again at 19 h after, the UVA exposure. This dose of SnPP administered similarly had been shown to effectively reduce inflammation in the carrageenin-treated rat (28) without causing toxicity. Control treatment consisted of injection of 0.1 ml of PBS.
Treatment Regime.
Groups of six mice were exposed on the dorsum to UVA radiation (387 kJ/m2 UVA). This dose is suberythemal and approximates to the UVA content of 6× minimal erythemal dose of sunlight in humans (29). A single exposure of UVB radiation (5.5 kJ/m2 UVB; approximately 3× minimal erythemal dose in the hairless mouse) immediately before or after UVA exposure, or three topical applications of cis-UCA lotion during the 24 h before or 24 h immediately after UVA exposure, constituted the immunosuppressive treatments applied to the dorsal skin. Control treatment groups received topical base lotion without UCA, or trans-UCA lotion. Injection of SnPP (or PBS) followed the UVA or UVB irradiations, thus avoiding protoporphyrin photosensitization. Experiments were repeated at least twice with similar results. The data shown are from treatments performed simultaneously, because the control CHS response and the effect of the immunosuppressive treatments vary slightly between experiments.
Induction of CHS.
CHS was induced in groups of six mice as described (18) by sensitization on the ventral nonirradiated skin with 0.1 ml 2% (wt/vol) oxazolone (Sigma) in ethanol, on days 8 and 9 after UVA exposure, to provide a measure of the systemic effect of irradiating the dorsal skin. Mice were challenged on day 15 by the application of 5 μl of 2% oxazolone/ethanol to each surface of both pinnae, and the group average maximum ear swelling was obtained by the increase in ear thickness measured before (prechallenge) and repeatedly between 18 and 24 h postchallenge. Statistical significance of the difference between treatments was assessed by Student’s t test.
HO Activity.
Enzyme activity was assayed by a modification of the method of Lincoln et al. (30) in microsomal preparations from dorsal skin. Mice were killed by cervical dislocation at 48 h post-UVA exposure (peak enzyme activity after inflammatory insult in rats) (28), and the dorsal skin was excised, chilled on ice, partially frozen in aluminum foil, and cut into small fragments with scissors, followed by homogenization in cold PBS using a Heidolph (Kelheim, Germany) variable-speed homogenizer equipped with a rotating stainless steel blade. Dorsal skins from 4–7 mice were pooled to obtain sufficient measurable microsomal activity. The homogenates were centrifuged sequentially, retaining the supernatants, at 10,000 × g and 20,000 × g (Sorvall RC5B refrigerated centrifuge), and the 100,000 × g (Beckman L7–55 Ultracentrifuge) final microsomal pellet was resuspended with brief homogenization (Dounce glass homogenizer) to an equivalent of a 200% (wt/vol) suspension (of original tissue wet weight) in cold PBS. Microsomal homogenates were assayed immediately whenever possible. Microsomes stored frozen at −10°C retained more than half the HO activity for at least several days.
The HO activity was measured by the rate of bilirubin formation at 37°C, indicated by the increase in absorbance at 470 nm versus 540 nm (30). The 1.0-ml incubation mixture (in duplicate) contained 0.15 M PBS, 42 μM hemin (ferroprotoporphyrin-IX; Sigma), 5 mM desferrioxamine (Sigma), 2–3 mg microsomal protein, a 1:5 dilution of the cytosolic fraction (100,000 × g supernatant) of normal mouse liver 30% homogenate as a source of biliverdin reductase, 100 μM NADPH (Sigma), and an additional NADPH-generating system consisting of 1 mM glucose 6-phosphate (Sigma) and 0.2 units of glucose-6-phosphate dehydrogenase (Sigma). For controls, the microsomal enzyme was inactivated at 100°C for 10 min. Incubation was for 30 min in a shaking water bath, and the reaction was stopped by centrifuging at 2°C for 20 min at 14,000 × g (Beckman Microfuge). Absorbance of the supernatant was measured at the two wavelengths by using a Perkin–Elmer spectrophotometer, and the absorption coefficient of 66 mM−1⋅cm−1 was used to calculate bilirubin concentration produced (30). Protein was measured by the method of Lowry et al. (31) with BSA (Sigma) as standard. Statistical significance of the differences between treatments for mean activities from three experiments was assessed by Student’s t test.
RESULTS
Photoimmunoprotective Effect of UVA Radiation.
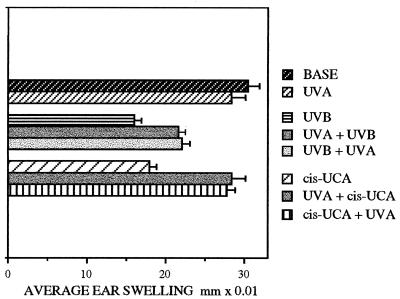
The systemic suppression of the CHS response to oxazolone resulting from UVB irradiation (48% suppression) or the topical application of cis-UCA (41% suppression) is illustrated in Fig. 1, whereas irradiation with UVA had no significant effect. However, if the UVB or cis-UCA treatment was preceded by, or followed by, UVA exposure, the degree of immunosuppression was significantly reduced. UVA totally prevented cis-UCA suppression, whether applied before (UVA+cis-UCA) or after (cis-UCA+UVA) the cis-UCA treatment. UVA was less effective in protecting from UVB suppression, although this protection that reduced the suppression from 48% to 28% (UVA+UVB) or 26% (UVB+UVA) was also significant (P < 0.01). The reduced UVA protection from UVB is consistent with the existence of other immunosuppressive pathways in addition to cis-UCA, perhaps histamine release or prostaglandin production, which may contribute to UVB-induced immunosuppression. Thus, as established in earlier studies (10), the photoimmunoprotection afforded by UVA irradiation appeared to be mediated via the inactivation of cis-UCA and to function equally whether UVA was administered before or after the immunosuppressive insult.
Figure 1.
CHS responses to oxazolone measured as average ear swelling ±SEM at 24 h postchallenge (average difference between prechallenge and postchallenge ear thickness) in groups of six mice, after exposure to UVA or UVB radiation or topical application of cis-UCA (cis-UCA) alone, and combinations of UVB or cis-UCA before (UVB+UVA, cis-UCA+UVA) or after (UVA+UVB, UVA+cis-UCA) the UVA exposure.
Effect of SnPP on UVA Immunoprotection.
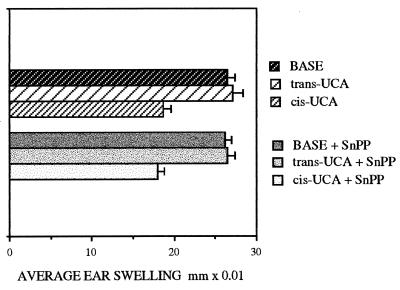
In unirradiated mice, the injection of SnPP did not alter normal CHS responsiveness, nor did SnPP have any immunological effect on either inactive trans- or suppressive cis-UCA (Fig. 2). Injection of SnPP after UVA exposure also had no effect on normal CHS (Fig. 3B).
Figure 2.
CHS responses to oxazolone in groups of six nonirradiated mice, measured as average ear swelling ±SEM, after topical treatment with cis- or trans-UCA (UCA) lotions. Injection of SnPP had no effect on these responses.
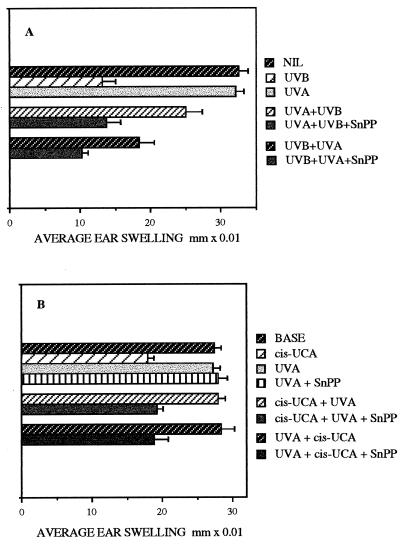
Figure 3.
CHS responses to oxazolone measured as average ear swelling ±SEM in groups of six mice. (A) Suppression of CHS by UVB radiation, and the reduction in this suppression by UVA exposure both before (UVA+UVB) and after UVB treatment (UVB+UVA). SnPP injection inhibited the protective effect of UVA exposure in both treatment regimes. (B) Suppression of CHS by cis-UCA and the protection from this suppression by UVA exposure both after (cis-UCA+UVA) and before (UVA+cis-UCA) cis-UCA treatment. Injection of SnPP prevented the protective effect of UVA exposure in both treatment regimes, but had no effect on CHS in mice exposed to UVA alone (UVA+SnPP).
However, under conditions where UVB exposure suppressed CHS by 60%, but this suppression was significantly (P < 0.001) reduced to only 23% by prior UVA irradiation (UVA+UVB), the subsequent injection of SnPP prevented the photoimmunoprotective effect of UVA, and CHS remained suppressed by 58% (UVA+UVB+SnPP; Fig. 3A). Similarly, UVA exposure after UVB exposure (UVB+UVA) reduced the immunosuppression from 60% to 44% (P < 0.001), but injected SnPP caused the suppression to persist (UVB+UVA+SnPP; 68% suppression of CHS).
Likewise, in mice in which subsequent UVA exposure should have provided total protection from the immunosuppressive effect (34% suppression) of treatment with cis-UCA, postirradiation injection of SnPP prevented the restoration by UVA, and CHS remained suppressed to 30% of the control response (cis-UCA+UVA+SnPP; Fig. 3B), not significantly different from the suppression resulting from cis-UCA alone. When prior UVA irradiation also resulted in abrogation of the cis-UCA suppression, again the injection of SnPP resulted in the retention of suppressed CHS (UVA+cis-UCA+SnPP; 38% suppression). Thus under these conditions, SnPP appeared to negate totally the UVA immunoprotection against both UVB and cis-UCA, whether the UVA preceded or followed the immunosuppressive treatment.
Time Course of Cutaneous HO Induction by UVA Radiation.
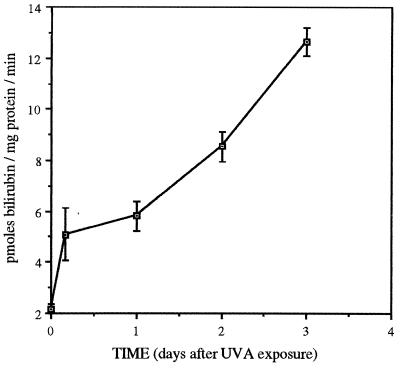
Dorsal skin microsomal HO activity was assayed before and at various time intervals between 4 h and 3 d post-UVA irradiation, after storing the preparations at −10°C. The HO activity increased steadily from the constitutive level of 2.1 pmol bilirubin/mg protein per min to a 6-fold increase (12.6 pmol/mg protein per min) by 3 d (Fig. 4), similar to the time course of the increase in HO-1-positive inflammatory cells described in the rat model of carrageenin inflammation (28). Thus prolonged elevated HO activity persisted after UVA exposure.
Figure 4.
Average cutaneous HO activity ±SEM measured in frozen stored microsomes as bilirubin production per mg microsomal protein per minute, and the time course of the increasing HO activity induced by UVA exposure (n = 3).
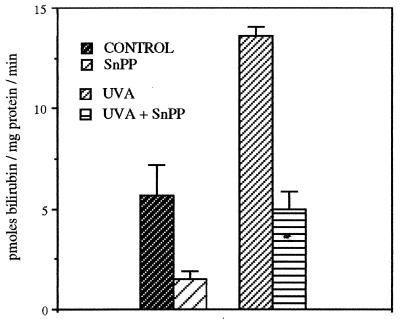
Effect of SnPP on Cutaneous HO Activity.
The efficacy of injected SnPP in inhibiting dorsal skin HO activity was measured at 48 h after UVA irradiation (Fig. 5). These assays were performed in freshly prepared microsomes in which the measurable constitutive HO activity can be seen to be higher than in frozen preparations (Fig. 4). SnPP injection inhibited the constitutive cutaneous HO activity by 73%, from 5.7 to 1.5 pmols bilirubin/mg microsomal protein per min. Exposure to UVA radiation increased the HO activity to 13.6 pmols/mg protein per min, approximately 2.4 times the constitutive activity, but SnPP also strongly inhibited this induced activity by 63% (5.0 pmols/mg protein per min). Thus both the constitutive and the UVA-induced HO activities were sensitive to SnPP inhibition.
Figure 5.
Average cutaneous HO activity ±SEM measured in freshly prepared microsomes as bilirubin production per mg microsomal protein per minute, from normal (control) mice and at 48 h post-UVA irradiation, and the inhibitory effect of injected SnPP (n = 3).
DISCUSSION
We have speculated that the mechanism of UVA photoimmunoprotection involves a UVA photoproduct that is relatively stable and persists for at least 24 h in the cutaneous environment, perhaps localized in the dermis where it would be preferentially produced by UVA rather than UVB radiation (10). This study has tested the hypothesis that induced HO-1 acts in this role and has shown that, whereas there is measurable HO enzyme activity present constitutively in hairless mouse skin, cutaneous HO activity is also UVA inducible and remains elevated for a period of at least 3 d after UVA irradiation. The study also has demonstrated the inhibition of cutaneous HO activity with systemically injected SnPP. The concurrent abrogation, by the injected SnPP, of the capacity for UVA radiation to protect from photoimmunosuppression or cis-UCA-induced immunosuppression, has provided evidence that the induction of this enzyme may have an immune regulating function.
Both UVA protection from the effects of UVB or cis-UCA on the immune system and the inhibition of this protection by SnPP occurred whether the immunosuppressive treatments preceded or followed the UVA exposure, suggesting that the mechanism of UVA protection is independent of cis-UCA formation. The rapid induction of HO, evident by 4 h, and the persistence of elevated HO activity after UVA irradiation, is consistent with this enzyme antagonizing the actions of newly formed cis-UCA or of cis-UCA formed 24 h later, affording immunoprotection in both treatment regimes.
Because SnPP did not affect the normal CHS response but did significantly inhibit constitutive HO enzyme activity, it was evident that the constitutive HO activity in the skin is inert in such an immune regulating role, although both enzyme isoforms have been reported to be similarly susceptible to SnPP inhibition (32). Our data suggest that only the inducible HO can modulate the effects of cis-UCA. There is currently no definitive evidence for HO isoform-specific localization in the cutaneous strata. However, from the demonstration of the in vitro cellular specificities for HO activity and inducibility by UVA radiation, we speculate that the constitutive enzyme might be localized in the epidermal keratinocytes, whereas the inducible HO-1 might be found in the dermal fibroblasts (21). Under these circumstances, the potential for the inducible HO-1 to modulate the effects of cis-UCA would be dermally located, consistent with the deeper penetration into this stratum of UVA radiation, which, unlike UVB, has been shown to have numerous dermal targets (11).
We suggest two possible mechanisms by which induced HO activity might antagonize cis-UCA in the dermis. First, systemic photoimmunosuppression via cis-UCA may depend on oxidative reactions, because a variety of antioxidants have been found to be photoimmunoprotective (15–19). Thus oxidant states, whether arising as a result of UVB or UVA irradiation, may be requisites for both the immunosuppressive response to UVB radiation and the UVA inducibility of HO-1 (11), and these states may in turn be reversed by the up-regulated antioxidant HO activity. Second, photoimmunosuppression is sensitive to histamine receptor antagonists (5, 33, 34), and recent studies indicate an immunosuppressive synergism between cis-UCA and cutaneous histamine originating from UVB-stimulated infiltrating mast cells in the dermis (35). It therefore is interesting that UVA irradiation of mast cells in the presence of protoporphyrin has suppressed their degranulation and mediator release in culture (36), suggesting that HO-1 also may inhibit histamine pathways, and thus prevent the functional synergy with cis-UCA.
The initiation of the immune suppressive effects of cis-UCA occurs in the stratum corneum where UCA concentration is the highest (37). The subsequent impairment of the normal T helper lymphocyte balance that underlies photoimmunosuppression is the result of cytokine activity generated in the epidermis (6), which rapidly leads to a systemic imbalance and can be demonstrated, as in these studies, by defective CHS to sensitizers presented through unirradiated skin sites. Sequential events involving the interaction between cis-UCA and the epidermal cytokine array, or the passage of cytokines or cis-UCA, which is produced in abundance in UVA-irradiated epidermis (38), via the dermis into the lymph or the circulation, have not been clarified. Both cis-UCA and keratinocyte-derived cytokines appear post-UVB in serum (39–41). The location of induced HO activity in the dermis therefore may provide an obstructive mechanism for secondary immunogenic reactions occurring also in the dermal compartment, e.g., oxidant-dependent cis-UCA activity or transport, cytokine release or access to extracutaneous receptors, or release of mast cell histamine for synergism with cis-UCA. It therefore will be important to identify whether there are significant cytokine alterations in the skin in response to UVA radiation in comparison with UVB radiation or cis-UCA and how these correlate with HO activity.
In summary, we have identified cutaneous HO induction as a mechanism to which the immunoprotective function of UVA radiation may in large part be attributed. The study illustrates one potent interaction of immunological significance between different UV spectral wavebands and suggests that this interaction might be harnessed by selective photoprotection strategies to reduce solar radiation-induced photoimmunosuppression and its long-term consequences in humans.
Acknowledgments
We thank Drs. Peter R. Sinclair and Judith M. Jacobs, Veterans Administration, White River Junction, VT for helpful discussions concerning the HO enzyme assay, and Ms. Chandrika Abeywardana and Mr. John Fisher for careful mouse husbandry. This study received financial support from the University of Sydney Cancer Research Fund. The research work of R.M.T. is supported by the Association for International Cancer Research, United Kingdom and the United Kingdom Department of Health.
ABBREVIATIONS
- CHS
contact hypersensitivity
- HO
heme oxygenase
- SnPP
tin protoporphyrin IX
- UCA
urocanic acid
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Kripke M L. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 2.Norval M, Gibbs N K, Gilmour J. Photochem Photobiol. 1995;62:209–217. doi: 10.1111/j.1751-1097.1995.tb05261.x. [DOI] [PubMed] [Google Scholar]
- 3.Kripke M L, Fox P A, Alas L G, Yarosh D B. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung H-T, Burnham D K, Robertson B, Roberts L K, Daynes R A. J Immunol. 1986;137:2478–2484. [PubMed] [Google Scholar]
- 5.Gilmour J W, Norval M, Simpson T J, Neuvonen K, Pasanen P. Photodermatol Photoimmunol Photomed. 1992/93;9:250–254. [PubMed] [Google Scholar]
- 6.Ullrich S. Photochem Photobiol. 1996;64:254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 7.Bestak R, Halliday G M. Photochem Photobiol. 1996;64:969–974. doi: 10.1111/j.1751-1097.1996.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 8.LeVee G J, Oberhelman L, Anderson T, Koren H, Cooper K D. Photochem Photobiol. 1997;65:622–629. doi: 10.1111/j.1751-1097.1997.tb01903.x. [DOI] [PubMed] [Google Scholar]
- 9.Damian D L, Halliday G M, Barnetson R S. J Invest Dermatol. 1997;109:146–151. doi: 10.1111/1523-1747.ep12319200. [DOI] [PubMed] [Google Scholar]
- 10.Reeve V E, Bosnic M, Boehm-Wilcox C, Nishimura N, Ley R D. Int Arch Allergy Immunol. 1998;115:316–322. doi: 10.1159/000069463. [DOI] [PubMed] [Google Scholar]
- 11.Tyrrell R. BioEssays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 12.Trautinger F, Kindasmugge I, Barlan B, Neuner P, Knobler R M. J Invest Dermatol. 1995;105:160–162. doi: 10.1111/1523-1747.ep12317003. [DOI] [PubMed] [Google Scholar]
- 13.Trautinger F, Knobler R M, Honigsmann H, Mayr W, Kindasmugge I. J Invest Dermatol. 1996;107:442–443. doi: 10.1111/1523-1747.ep12365498. [DOI] [PubMed] [Google Scholar]
- 14.Cope R B, Bosnic M, Boehm-Wilcox C, Mohr D, Reeve V E. J Nutr. 1996;126:681–692. doi: 10.1093/jn/126.3.681. [DOI] [PubMed] [Google Scholar]
- 15.Yuen K S, Halliday G M. Photochem Photobiol. 1997;65:587–592. doi: 10.1111/j.1751-1097.1997.tb08610.x. [DOI] [PubMed] [Google Scholar]
- 16.Katiyar S K, Elmets C A, Agarwal R, Mukhtar H. Photochem Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 17.Reeve V E, Bosnic M, Rozinova E, Boehm-Wilcox C. Photochem Photobiol. 1993;58:813–817. doi: 10.1111/j.1751-1097.1993.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 18.Reeve V E, Bosnic M, Rozinova E. Immunology. 1993;78:99–104. [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Pinnell S R, Darr D, Kurimoto I, Itami S, Yoshikawa K, Streilein J W. J Invest Dermatol. 1997;109:20–24. doi: 10.1111/1523-1747.ep12276349. [DOI] [PubMed] [Google Scholar]
- 20.Keyse S M, Tyrrell R M. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Applegate L A, Noel A, Vile G, Frenk E, Tyrrell R M. Photochem Photobiol. 1995;61:285–291. doi: 10.1111/j.1751-1097.1995.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 22.Vile G F, Basu-Modak S, Waltner C, Tyrrell R M. Proc Natl Acad Sci USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi A, Santoro M G. Biochem J. 1995;308:455–463. doi: 10.1042/bj3080455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon M, Reikerstorfer A, Schwarz A, Krone C, Luger T A, Jaattela M, Schwarz T. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peetermans W E, Raats C J I, Vanfurth R, Langermans J A M. Infect Immun. 1995;63:3454–3458. doi: 10.1128/iai.63.9.3454-3458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock W W, Buelow R, Sayegh M H, Turka L A. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 27.Soares M P, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey S T, Colvin R B, Choi A M, Poss K D, Bach F H. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 28.Willis D, Moore A, Frederick R, Willoughby D. Nat Med. 1996;2:87–89. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 29.Diffey B L. In: Biological Responses to Ultraviolet A Radiation. Urbach F, editor. Overland Park, KN: Valdenmar; 1992. pp. 321–328. [Google Scholar]
- 30.Lincoln B C, Healey J F, Bonkovsky H L. Biochem J. 1988;250:189–196. doi: 10.1042/bj2500189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Mark J A, Maines M D. Pediatr Res. 1992;32:324–329. doi: 10.1203/00006450-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Matheson M J, Reeve V E. Photochem Photobiol. 1991;53:639–642. doi: 10.1111/j.1751-1097.1991.tb08491.x. [DOI] [PubMed] [Google Scholar]
- 34.Jaksic A, Finlay-Jones J J, Watson C J, Spencer L K, Santucci I, Hart P H. Photochem Photobiol. 1995;61:303–309. doi: 10.1111/j.1751-1097.1995.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 35.Hart P H, Grimbaldeston M A, Swift G J, Jaksic A, Noonan F P, Finlay-Jones J J. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen A, Mathieu-Costello O, Gigli I, Barrett K. J Allergy Clin Immunol. 1994;93:909–918. doi: 10.1016/0091-6749(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 37.De Fabo E C, Noonan F P. J Exp Med. 1983;157:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeve V E, Boehm-Wilcox C, Bosnic M, Cope R, Ley R D. Photochem Photobiol. 1994;60:268–273. doi: 10.1111/j.1751-1097.1994.tb05103.x. [DOI] [PubMed] [Google Scholar]
- 39.Moodycliffe A M, Norval M, Kimber I, Simpson T J. Immunology. 1993;79:667–672. [PMC free article] [PubMed] [Google Scholar]
- 40.Urbanski A, Schwarz T, Neuner P, Krutmann J, Kirnbauer R, Kock A, Luger T. J Invest Dermatol. 1990;94:808–811. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura N, Tohyama C, Satoh M, Nishimura H, Reeve V. Immunology. 1999;97:77–83. doi: 10.1046/j.1365-2567.1999.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]